272 OOCYTE SPONTANEOUS ACTIVATION IN DIFFERENT RAT STRAINS
P. Ross A , A. Yabuuchi A and J. Cibelli ACellular Reprogramming Laboratory, Michigan State University, East Lansing, MI 48824, USA. Email: rosspabl@msu.edu
Reproduction, Fertility and Development 17(2) 285-286 https://doi.org/10.1071/RDv17n2Ab272
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
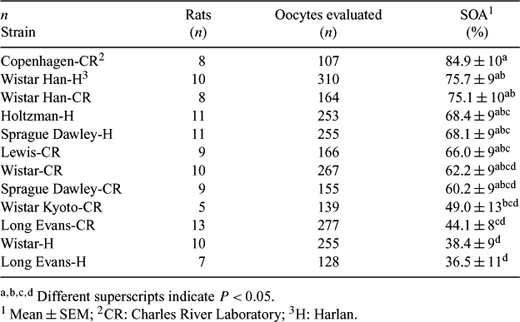
Oocyte spontaneous activation (OSA) has been reported to occur during in vitro culture of ovulated rat eggs. Approximately 1.5 h after isolation and culture, unfertilized oocytes extrude the second polar body and enter a metaphase arrest, and by 3 h individual chromatids separate and scatter throughout the egg's cytoplasm. The objective of this study was to compare the proportion of OSA and the level of maturation promoting factor (MPF) activity in oocytes from different strains. Twelve strains were selected from two commercial sources based on litter size. Mature female rats (9 to 12 weeks old) were superovulated using 20 IU eCG s.c. followed by 30 IU eCG s.c. 5 h later. After 48 h, a dose of 50 IU hCG was administered intraperitonealy and females were mated with vasectomized males. Oocytes were collected 17 h after hCG injection and cumulus cells were removed by transfer to M2 medium containing hyaluronidase (1 mg mL−1). Denuded oocytes were cultured in 50 μÂL drops of M16 medium under oil at 37°C and 5% CO2 in air. In order to assess OSA, oocytes were mounted in glycerol containing Hoechst 33342 (10 μg mL−1) on a glass slide after 6 h of in vitro culture. The proportion of activated oocytes was determined by epifluorescence microscopy. Oocytes were considered to be in metaphase II if they presented a compact group of chromosomes at the metaphase plate, and were considered activated when chromosome dispersion at different degrees was observed. Data were analyzed by ANOVA, considering each animal as an experimental unit. Significant differences were observed between strains (P < 0.01, Table). In order to determine MPF activity of each strain, 10 oocytes were removed from culture at 0, 1.5, and 3 h after oocyte collection and immediately stored in kinase buffer in LN2 for posterior analysis using an ELISA based kit (MESACUP cdc2 Kinase Assay Kit, MBL, Nagoya, Japan). The log ratio of the MPF activity at 1.5 and 3 h relative to 0 h for each animal (5 per strain) was analyzed by ANOVA. There were no MPF differences between or within strains (P > 0.3 and P > 0.05, respectively). We did not observe the expected decrease in MPF activity that allows for the exit of metaphase II arrest. This could imply that OSA is not associated with a decrease in MPF, or that MPF decreased rapidly and returned to metaphase levels by 1.5 h after culture. In conclusion, different levels of OSA were observed between strains, however, no differences in MPF activity were detected at the analyzed time points.
|


