80 GENE EXPRESSION PROFILES AND IN VITRO DEVELOPMENT FOLLOWING VITRIFICATION OF PRONUCLEAR AND 8 CELL-STAGE MOUSE EMBRYOS
D. Boonkusol A , A. Baji Gal B , S.Z. Bodo B , B. Gorhony B , K. Pavasuthipaisit A D and A. Dinnyes B CA Department of Anatomy, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
B Institute of Animal Biology, Agricultural Biotechnology Center, Godollo, Hungary
C Research Group on Applied Animal Genetics and Biotechnology, Hungarian Academy of Sciences and Szent Istvan University, Godollo, Hungary
D Institute of Science and Technology for Research and Development, Mahidol University, Bangkok 10400, Thailand. Email: ngamsomd@yahoo.com
Reproduction, Fertility and Development 17(2) 190-190 https://doi.org/10.1071/RDv17n2Ab80
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
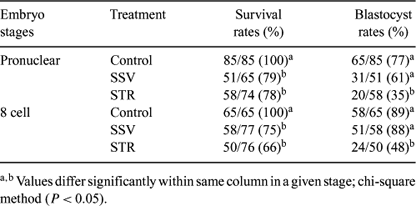
The analysis of differences in gene expression of embryos in response to cryopreservation may explain some of the observed differences in further development. Experiments were conducted to study effects of two vitrification methods, solid surface (SSV) and in-straw vitrification (STR), for pronuclear and 8 cell-stage mouse embryos with regard to gene expression and in vitro development. Both stages of embryos were vitrified by SSV (Dinnyes et al. 2000 Biol. Reprod. 63, 513–518) using 35% ethylene glycol (EG) for vitrification solution (VS) and STR using 40% EG for VS. Warming and rehydration of embryos in the SSV method was performed by placing vitrified droplets into a 37°C 0.3 M trehalose solution for 1 min, and then for 2 min in each of 0.15 M trehalose, 0.075 M trehalose, and base medium at room temperature before culture in CZB medium. Warming of embryos for the in-straw method was performed by holding straws in air for 10 s and then plunging them into 37°C water for 15–20 s. The contents of the straws were expelled into 37°C 0.3 M trehalose solution and embryos were treated as above. No significant differences were found between immediate survival rates of embryos vitrified by SSV and STR in both stages. Blastocyst rates were significantly higher with SSV than with STR and not significantly different from those of the control (Table 1). These results show that SSV was more efficient than STR. Quantification of selected gene transcripts from single embryo (6 embryos/treatment group) was carried out by quantitative real-time RT-PCR. Genes related to oxidative stress (MnSOD and CuSOD), cold stress (CIRP, RBM3, and p53), and β-actin as reference gene were amplified. We found up-regulation of all stress genes at 3 h post-warming in all treatment groups. At 10 h post-warming, a low level of gene expression was found in SSV-treated embryos, but gene expression remained at high level in STR-treated embryos. However, no differences in gene expression were found between blastocysts developed from fresh and vitrified embryos. In conclusion, the real-time RT-PCR method from single embryos opened new opportunities for understanding molecular events following cryopreservation. The continuous up-regulation of stress-related genes at 10 h post-warming might have been an early indicator of reduced viability following STR, as was also indicated by the developmental rate to the blastocyst stage.
|
This work was supported by the Thailand Research Fund (Royal Golden Jubilee Ph.D. scholarship) and a Bio-00017/2002 grant of the Hungarian National Office of Research and Technology.


