321 DEVELOPMENT IN VIVO AND IN VITRO OF PORCINE OOCYTES FERTILIZED BY INTRACYTOPLASMIC INJECTION OF A FREEZE-DRIED SPERM HEAD
M. Nakai A , K. Kikuchi B , A. Takizawa A , M. Ozawa B , J. Noguchi B , H. Kaneko B , M. Shino A and N. Kashiwazaki AA Graduate School, Azabu University, Kanegawa 229-8501, Japan
B National Institute of Agrobiological Sciences, Ibaraki 305-8602, Japan. Email: da0304@azabu-u.ac.jp
Reproduction, Fertility and Development 17(2) 311-311 https://doi.org/10.1071/RDv17n2Ab321
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
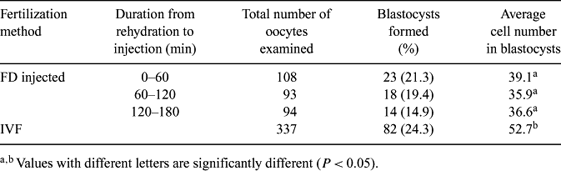
The present study investigated the development in vivo and in vitro of in vitro matured porcine oocytes injected with a freeze-dried (FD) boar sperm head. In mice, DNA damage was induced during the holding period after rehydration and before sperm injection (Wakayama, T. and Yanagimachi, R. 1998, Nat. Biotechnol., 16, 639–641). Here, we examined the relationship between duration of rehydration of FD sperm and in vitro development of FD sperm-injected porcine oocytes. We also assessed the in vivo developmental competence of the injected oocytes after embryo transfer. Ejaculated boar spermatozoa were suspended in Pig-FM (Suzuki, K. et al. 2002, Int. J. Androl. 25, 84–93) and sonicated for 1 min to separate sperm heads from the tails. An aliquot (100 μL) of the sperm suspension was put into a glass tube and then pre-cooled at −40°C for 6 h. Each tube was attached to a freeze-dry system (DuraDry μP, FTS Systems, Stone Ridge, NY, USA) for 12 h. The ampules were closed and stored at 4°C for more than 7 days before use. For rehydration, 100 μL of distilled water was added into the ampules. In Experiment I, we injected FD sperm heads which were kept for 0–60, 60–120, or 120–180 min after rehydration. At 1 h after the injection, the injected oocytes were stimulated with a DC pulse and cultured for 6 days. The rate of blastocyst formation and the number of cells in the blastocysts were examined. Embryos after in vitro fertilization (IVF) were evaluated as a control. As shown in Table 1, the rates of blastocyst formation were not different (by χ2 test) for duration of rehydration and the control. However, the cell numbers of FD groups were lower (P < 0.05; by Student's t-test) than that in the control. In Experiment II, oocytes injected with a single FD sperm head and stimulated were transferred to both oviducts of a total of ten recipient gilts. Two recipients were diagnosed as pregnant at Day 30 of gestation. At Day 39, one of the pregnant recipients had an abortion, and two fetuses were recovered. The other pregnancy was not maintained. The results suggest that oocytes fertilized with a single FD sperm head have competence to be implanted and to develop to the early fetal stage, and also that the duration for rehydration does not influence in vitro developmental ability in pigs.
|


