101 OVARIAN RESPONSE AND DEVELOPMENTAL COMPETENCE OF OOCYTES COLLECTED BY OPU IN SHEEP TREATED WITH GnRH ANTAGONIST
F. Berlinguer A , A. Gonzalez-Bulnes B , S. Succu A , G. Leoni C , I. Rosati A , A. Veiga-Lopez B , R.M. Garcia Garcia D , M.J. Cocero B and S. Naitana AA Department of Animal Biology, University of Sassari, Sassari, Italy
B Department of Animal Reproduction, INIA, Madrid, Spain
C Department of Animal Biology, Department of Phys. Bioch. and Cell Science, University of Sassari, Sassari, Italy
D Department of Science and Agrarian Technologies, University of Castilla-La Mancha, 02071 Albacete, Spain. Email: vetfis@uniss.it
Reproduction, Fertility and Development 17(2) 201-201 https://doi.org/10.1071/RDv17n2Ab101
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
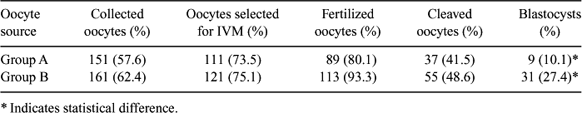
The use of a single dose of GnRH antagonists during the progestagen treatment prior to superovulatory treatment protocols in sheep increases the number of smaller follicles able to grow and ovulate in response to the exogenous FSH treatment (Lopez-Alonso C et al. 2004 Reprod. Fertil. Dev. 16, 233). The aim of our study was to test if such treatment affects the in vitro developmental competence of oocytes collected by ovum pick up (OPU) from GnRH-antagonist treated sheep during an ovarian by perstimulation protocol. Adult Sarda sheep (n = 18) were synchronized by the insertion of intravaginal sponges (Day 0) which were left in situ for 12 days; on Day 7, group A (n = 10) received a single dose of 3 mg of Antarelix (Teverelix, Europeptides, France) s.c., while group B (n = 8) served as control. All animals received 96 IU of FSH (Ovagen, ICP, New Zealand) administered in 4 equal doses given i.m. every 12 h starting on Day 10. Twelve hours after the last FSH administration oocytes were collected by OPU technique. Follicular growth was monitored by transrectal ultrasonography from Day 7 to Day 11. Collected oocytes were matured, fertilized, and cultured in vitro up to blastocyst stage under standard conditions used in our laboratory (Berlinguer F et al. 2004 Theriogenology 61, 1477–1486). After IVF, uncleaved oocytes were stained with acetolacmoid to evaluate chromatin configuration, while the cleaved ones were cultured in SOF + 0.4% BSA up to the blastocyst stage. Data were analyzed by ANOVA statistical analysis after arcsine transformation of the value percentages. Ultrasonographic monitoring showed a significant increase in the number of follicles (mean ± SEM) present in the ovaries from Day 8 to Day 11 of treatment in group A compared to group B (Day 8: 19 ± 5.1 vs. 13 ± 3.4, P > 0.05; Day 9: 20.1 ± 4.6 vs. 14.1 ± 2.4, P > 0.001; Day 10: 22.5 ± 6.1 vs. 14.7 ± 2.7, P > 0.001; Day 11: 25.3 ± 5.1 vs. 20.5 ± 4.1, P > 0.05), thus confirming that GnRH antagonist administration enhances ovarian response to exogenous FSH stimulation. On the other hand, oocytes collected from untreated sheep lead to a higher blastocyst output (P = 0.014), as illustrated in the table. These results indicated that although GnRH antagonist administration caused a significant increase in the ovarian response to the hormonal treatment, the final blastocyst output was significantly lower compared to that of the control group. This finding seems to suggest an impairment in the developmental competence of treated sheep oocytes.
|
This work was supported by funds from the Spanish MEC (projects SC 00-051-C3.1 and HI2002-0004) and the Italian MIUR (cofin).


