241 POLYADENYLATION STATUS OF MRNAs CODING FOR PRDX-6, GDF-9, G6PDH AND CYCLIN B1 BEFORE AND AFTER IN VITRO MATURATION OF BOVINE OOCYTES: REAL-TIME PCR RESULTS AFTER REVERSE TRANSCRIPTION WITH OLIGO(DT) OR WITH HEXAMERS
A.S. Lequarre A , J.M. Traverso A , J. Marchandise A and I. Donnay AUnité Vétérinaire, Institut des Sciences de la Vie, Université catholique de Louvain, B-1348 Louvain-la-Neuve, Belgium. email: lequarre@vete.ucl.ac.be
Reproduction, Fertility and Development 16(2) 241-241 https://doi.org/10.1071/RDv16n1Ab241
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
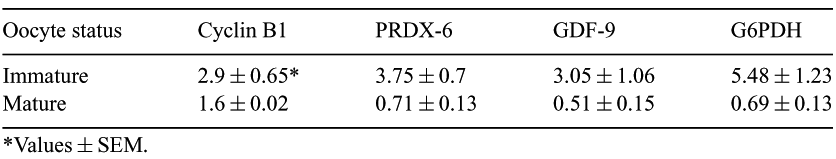
Changes in length of the polyA tail of an mRNA is determinant for its stability and translation. During oocyte maturation, although some genes are specifically polyadenylated, an important decrease of polyadenylated mRNA is generally observed. Both degradation and de-adenylation can be responsible for this decline but little information about these processes is available yet for in vitro-matured bovine oocytes. Total RNA was extracted from 2 pools of 50 immature and 2 pools of 50 in vitro matured bovine oocytes, after addition of 1 pg of polyadenylated rabbit globin mRNA to each pool. The RNA of each pool was then divided in 8 samples, half of them were reverse-transcribed using hexamers and the other half using oligo(dT). Six genes were amplified in every sample using real-time PCR and specific molecular beacon probes: (1) rabbit globin, an exogenous gene added as a reporter for the efficiency of extraction and RT;; (2) histone H2A, an endogenous gene reporter for RNA quantity and quality in each pool [This housekeeping gene, that is not adenylated, should be maintained at a constant level through the maturation process (Robert et al., 2002 Biol of Reprod. 67, 1425–1472)]; (3) two antioxidant enzymes, peroxyredoxin 6 (PRDX-6) and G6PDH preventing oxidative stress during maturation [PRDX-6 also has a phospholipase A2 activity that could be implicated in prostaglandins synthesis during maturation]; (4) GDF-9, an oocyte-specific gene involved in cumulus expansion during maturation;; and (5) cyclin B1, one subunit of the MPF factor whose activity is required for meiosis resumption. The PCR result of each gene was normalized with the globin value of the same sample. After this standardization, the variability of the results among the 4 samples of a same pool similarly reverse transcribed did not exceed 15%. Within a pool, the mean PCR signal obtained for a specific mRNA using oligo(dT) was compared with the mean signal obtained with hexamers. Results obtained for immature and mature oocytes are reported in the Table 1. Before maturation, the PCR signals were higher when reverse transcription was performed using oligo(dT) instead of hexamers but that was not the case after maturation. Most messengers coding for these genes probably lost their polyA tails during maturation. This loss was much less pronounced for cyclin B1 messengers. Furthermore, for all genes studied, the PCR results obtained after reverse transcription using hexamers and normalized with the histone H2A value did not show a decrease during the maturation process. In conclusion, deadenylation, more than degradation, would be responsible for the disappearance of maternal polyadenylated mRNA during maturation.
|


