105 VITRIFICATION OF BOVINE EMBRYOS USING THE CLV METHOD
W. Lindemans A , L. Sangalli A , A. Kick A , C.R. Earl B and R.C. Fry BA CryoLogic Pty Ltd, 54 Geddes St., Mulgrave, Victoria 3170 email: bill@cryologic.com;
B Animal Reproduction Company, Sneydes Rd., Werribee, Victoria 3030, Australia.
Reproduction, Fertility and Development 16(2) 174-174 https://doi.org/10.1071/RDv16n1Ab105
Submitted: 1 August 2003 Accepted: 1 October 2003 Published: 2 January 2004
Abstract
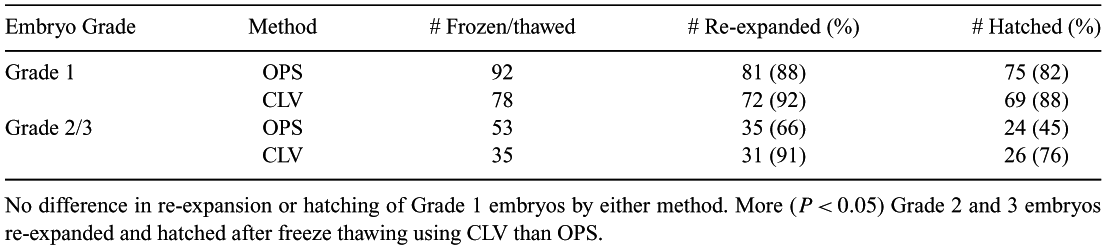
Vitrification has become the preferred method for cryopreserving in vitro-produced bovine embryos (IVP). Here we introduce a technique for vitrification developed at CryoLogic (the CLV Method), in conjunction with a study comparing the post-thaw viability of IVP embryos frozen by the widely used open pulled straw method (OPS—Vajta et al., 1997 Cryo-Letters 18, 191) and the new CLV Method. Vitrification on thin metal surfaces has been explored and demonstrated previously (Le Gal & Massip 1999, Cryobiology 38, 290), and Dinnyes presented a Solid Surface Vitrification (SSV) (Dinnyes et al., 2000, Biol. Reprod. 63, 513). The CLV Method utilizes vitrification on the surface of a solid metal block. This surface has been custom shaped and treated to enhance vitreous formation. The method also includes handling, storage and thawing protocols designed to avoid damage from crystallization of the unstable glass. Briefly, the block is precooled in LN2 to −196°C. Up to 10 embryos are collected into a droplet of medium (3 μL), on the end of a fibre carrier attached to a handle. The droplet is presented to the vitrification surface, where it is converted into a glass bead by cooling rates comparable to that of plunging into solid/liquid phase nitrogen (−210°C) (Arav et al., 2001 Theriogenology 55, 313). The glass bead, fibre and handle are transferred quickly into a half-sealed, precooled straw, and the handle seals the open end. A bead is thawed very rapidly by removing the handle, fibre and bead from the straw and transferring the bead into washing medium. COCs collected from bovine ovaries obtained from abattoirs were matured, fertilized and cultured for 6 days (Fry et al., 2003 Theriogenology 59, 446). Embryos reaching the blastocyst or expanding blastocyst stage of development were graded (Grades 1, 2, and 3), equilibrated for 5 min in HEPES-199 medium with 20% FCS (HFm), placed in HFm with 10% EG, and 10% DMSO (VS1) for 2 min, and then transferred to HFm with 20% EG, 20% DMSO (VS2) for 30 s (Vajta). Between 5 and 10 IVP embryos were processed and collected for vitrification, either in an OPS plunged into LN2, or in a 3 μL droplet vitrified by the CLV Method. The two sets of specimens were stored in LN2, and later thawed. Both OPS tips and beads were thawed in 0.5 mL of HFm with 0.2 M sucrose at 39°C. Embryos were maintained at 39°C, examined after 5 min for contraction, and again after 6 h for re-expansion. They were then transferred to culture medium, incubated and examined at 24 and 48 h to assess hatching. As shown in Table 1, the CLV method appears to be satisfactory for maintaining membrane integrity (expansion) and developmental potential (hatching) for even poorer grade embryos, that might be more sensitive to the stresses of cryopreservation.
|


