35 USE OF METAPHASE DONOR CELLS AND ACTIVATION WITH ROSCOVITINE FOR SOMATIC CELL NUCLEAR TRANSFER IN BOVINE
G. V. Landschoot A B , V. Savy A , N. Canel A , S. Ferraris B and D. Salamone AA Laboratorio de Biotecnología Animal, Facultad de Agronomía, Universidad de Buenos Aires, Buenos Aires, Argentina;
B Laboratorio de Clonación y Transgénesis, Centro de Investigación y Desarrollo en Medicina Experimental (CIDME), Universidad Maimónides, Buenos Aires, Argentina
Reproduction, Fertility and Development 29(1) 125-125 https://doi.org/10.1071/RDv29n1Ab35
Published: 2 December 2016
Abstract
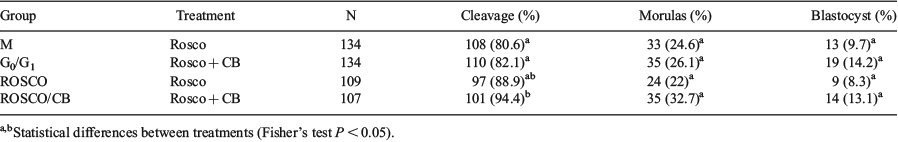
Cloning of domestic species by somatic cell nuclear transfer (SCNT) continues to be inefficient, probably due to an incomplete reprogramming of the reconstituted embryo. The ability of the embryonic cytoplasm to support reprogramming fluctuates within the cell cycle (Egli et al. 2007 Nature 447, 679–85). In this context, we compared the development capability and second polar body (2PB) extrusion of embryos produced by metaphase (M) cells, in comparison with G0/G1 cells, which are commonly used as nuclear donors. Because M cells have 2 sets of chromosomes (in contrast with G0/G1 cells, which have only 1 set), an activation protocol that impedes 2PB extrusion is required to produce reconstituted embryos with the correct ploidy. Therefore, we performed SCNT with M or G0/G1 cells, followed by different activation protocols, and evaluated in vitro development and 2PB extrusion of the reconstituted embryos. Cow oocytes were in vitro matured and enucleated as described by Gambini et al. (2014 PLoS One 14, 9). A group of cells at 70 to 80% confluence was synchronized in M stage using 0.05 μg mL−1 demecolcine for 3 to 4 h and used as nuclear donors for SCNT (M group). Another group of cells was induced into quiescence by serum starvation for 3 to 4 days before SCNT (G0/G1 group). For activation, reconstituted embryos were treated with 5 µM ionomycin (Io) for 4 min followed by 5-h incubation in 50 μM roscovitine for M group, or in 50 μM roscovitine and 5 μg mL−1 cytochalasin B for G0/G1 group. Parthenogenetic controls were activated with Io followed by 50 μM roscovitine alone (ROSCO) or with 5 μg mL−1 cytochalasin B (ROSCO/CB). Hoescht 33342 staining was performed 16 h post-Io to evaluate 2PB extrusion. Other activated oocytes were cultured in SOFaa medium and rates of cleavage, morulas, and blastocysts were evaluated at Days 2, 5 and 7 of in vitro development, respectively. Data were analysed by Fisher’s exact test (P < 0.05). Rates of 2PB extrusion were 72.72 (n = 33), 65.63 (n = 32), 80 (n = 15), and 42.86 (n = 14) for M, G0/G1, ROSCO, and ROSCO/CB, respectively. Results of in vitro development are shown in Table 1. In conclusion, somatic M cells can be used as donors to produce cloned embryos. The M and G0/G1 groups were able to induce cloned blastocysts, even though rates did not differed statistically from controls groups (ROSCO and ROSCO/CB). The M group was as effective as G0/G1. Although further analysis is required to establish the quality of the embryos, our results are encouraging for use in SCNT.
|


