224 CHARACTERIZATION OF EQUINE ENDOMETRIAL-DERIVED MESENCHYMAL STROMAL CELLS
E. Rink A B , H. French A , E. Watson A , C. Aurich C and F. X. Donadeu BA Ross University School of Veterinary Medicine, Basseterre, St. Kitts, West Indies;
B The Roslin Institute, Edinburgh, United Kingdom;
C University of Veterinary Medicine, Vienna, Austria
Reproduction, Fertility and Development 28(2) 243-244 https://doi.org/10.1071/RDv28n2Ab224
Published: 3 December 2015
Abstract
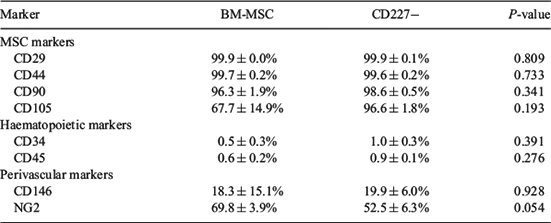
Equine mesenchymal stromal cells (MSC) are mainly harvested from bone marrow and adipose tissue, requiring surgical procedures. Although human endometrium is known to harbor mesenchymal precursor cells, the presence of MSC in equine endometrium, a dynamic tissue, has not been investigated. This study reports for the first time the culture and characterisation of MSC from equine endometrium compared with equine bone marrow (BM)-derived MSC. Samples of equine endometrium (n = 6) and BM (n = 3) were collected postmortem. Endometrial tissue was digested using a dissociation medium containing collagenase I and DNase type I, and CD227 (mucin-1)-bound magnetic beads were utilised to separate epithelial (CD227+) from stromal (CD227–) cell fractions. Red blood cells from BM samples were excluded using a density gradient. All cell fractions were cultured in DMEM/F-12 containing 10% fetal bovine serum. After expansion, colony-forming unit (CFU) assay at passage 2, trilineage differentiation (adipogenic, chondrogenic, osteogenic), and flow cytometry analysis at passage 3/4 were performed for CD227– fractions and BM-MSC. Descriptive statistical analysis and 2-tailed t-test was performed with IBM SPSS Statistics 22 (SPSS Inc./IBM, Chicago, IL, USA). Both isolated cell fractions were plastic adherent and grew well under standard MSC culture conditions, although endometrial CD227– cells attached quicker to culture plasticware than did BM-MSC. The CFU assay at passage 2 showed no significant difference in cloning efficiency (CE) between BM-MSC (20.78 ± 2.86%) and CD227– (24.89 ± 3.04%) cell lines (P = 0.36). Flow cytometry showed the expression of MSC markers (CD29, CD44, CD90, CD105) and perivascular markers (CD146, NG2) but almost no expression of haematopoietic markers (CD34, CD45) in both cell lines (Table 1). No statistically relevant difference was seen except for the higher expression of NG2 in BM-MSC (P = 0.054). Trilineage differentiation was successfully induced in both cell lines. In conclusion, we showed the presence of putative MSC in equine endometrium. We successfully isolated and cultured these cells, which display comparable characteristics in MSC criteria as well-established BM-derived MSC. These endometrial-derived MSC may provide a convenient source for veterinary regenerative therapies in equine reproduction.
|


