44 IMPROVED DEVELOPMENT OF BOVINE NUCLEAR TRANSFER EMBRYOS BY THE TREATMENT OF NUCLEAR DONOR CELLS WITH APOPTOSIS INHIBITORS DURING SERUM STARVATION
C.-K. Lee A B , Bon-sik Koo A , C.-H. Park A B , S.-G. Lee A B , D.-H. Choi A B , J.-M. Lim A and H.-J. Yong AA School of Agricultural Biotechnology, College of Agriculture and Life Sciences, Seoul National University, Suwon, 441-744, South Korea
B Xenotransplantation Research Center, Seoul National University, Suwon, 441-744, South Korea. Email: leeck@snu.ac.kr
Reproduction, Fertility and Development 17(2) 171-172 https://doi.org/10.1071/RDv17n2Ab44
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
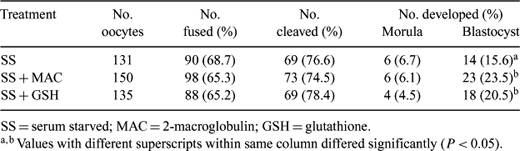
In somatic cell nuclear transfer, serum starvation is a widely used method to synchronize donor cells at the quiescent stage (Go) of the cell cycle. However, it has been shown that serum starvation during culture of mammalian cells could induce cell death via apoptosis by removing growth factors and increasing intracellular stress. Therefore, apoptosis caused by serum starvation in somatic cells could induce damages to nuclear DNA contributing to a lower efficiency of nuclear transfer. This study was performed to characterize apoptosis during serum starvation of bovine embryonic fibroblasts (BEFs) and to determine the effects of BEFs treated with apoptosis inhibitors on the development of bovine embryos after nuclear transfer. BEFs, collected from a fetus with a 3–4-cm crown-rump length, were cultured for 7 days in starvation medium consisting of Dulbecco's modified Eagle's medium containing 0.5% fetal bovine serum to induce quiescence. Cells were also placed in starvation medium containing the apoptosis inhibitors, β2-macroglobulin (broad-range protease inhibitor: MAC; 1.4 pM) and glutathione (GSH: reactive oxygen species scavenger; 2.0 mM). Apoptosis of serum starved BEFs with or without apoptosis inhibitors were analyzed morphologically with light and electron microscope, and biochemically using a TUNEL assay. Somatic cell nuclear transfer was performed by our standard procedure as follows. Bovine oocytes were matured in vitro and enucleated after 22 h. Nuclear donor cells were collected randomly before injection. The reconstructed embryos were placed into the fusion chamber in a solution containing 0.28 M mannitol and aligned manually. A double pulse of 1.8 kV/cm for 15 μs was used to fuse the cells and activate the embryos simultaneously. The fused embryos were cultured for 4 min in 5 μÂM ionomycin and 4 h in 2 mM 6-DMAP. Then, embryos were moved to culture media and cultured in 5% CO2 and 39°C in 100% humidity. Development of NT embryos was recorded at 120 h post NT (morulae) and 168 h (blastocysts) with experiments being repeated three times. Serum starved BEFs showed typical morphology of apoptotic cells such as chromatin condensation and membrane blebbing. Also, when stained for DNA fragmentation by TUNEL assay, 22.6% of BEFs showed apoptosis, in contrast to 0.1% in actively growing cells. However, when BEFs were cultured with MAC and GSH, the proportions of apoptotic BEFs were greatly reduced, 6.0% and 2.1%, respectively. After nuclear transfer with BEFs, embryos reconstructed with BEF treated with apoptosis inhibitors showed significant improvement in in vitro development compared to the controls (Table 1). In conclusion, while there are a number of factors affecting the nuclear transfer procedure, damage to the donor nuclei by serum starvation is likely to reduce the efficiency of the procedure; the addition of apoptosis inhibitors could reduce this unnecessary damage to donor nuclei and result in improvement in the development of nuclear transferred embryos. Further experiments are needed to assess the effect of apoptosis inhibitors on improvement of overall nuclear transfer efficiency.
|


