115 ADDITION OF ETHANOL AT SUB-STRESS CONCENTRATIONS DURING IN VITRO MATURATION OF BOVINE OOCYTES IMPROVES BLASTOCYST CRYOTOLERANCE
M. A. M. M. Shehab-El-Deen A , J. L. M. R. Leroy C , D. Maes A and A. Van Soom AA Ghent University, Merelbeke, Belgium;
B Suez Canal University, Ismailia, Egypt;
C Antwerp University, Wilrijk, Belgium
Reproduction, Fertility and Development 22(1) 216-216 https://doi.org/10.1071/RDv22n1Ab115
Published: 8 December 2009
Abstract
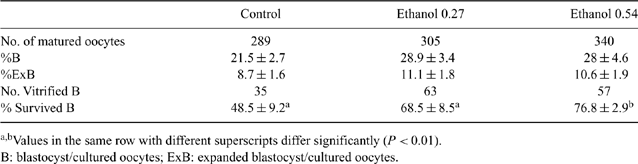
In in vitro experiments, the percentage of bovine oocytes that develops to the blastocyst stage is much lower compared with the in vivo counterparts. The quality of the oocyte is the main factor affecting blastocyst yield. Moreover, in vitro-produced bovine embryos are more sensitive to cryo-injuries than those produced in vivo. Exposure of oocytes to sub-lethal concentrations of stressors may enhance their quality through upregulation of intracellular shock proteins. We aimed to evaluate whether addition of ethanol at low concentrations (0.27 or 0.53%) during oocyte maturation could have a carry-over effect on embryo quality and could subsequently affect embryo cryotolerance. Cumulus-oocyte complexes (n = 934) were matured in serum-free TCM199 plus 20 ng mL-1 of epidermal growth factor (control), supplemented with ethanol 0.27% (treatment 1) or 0.54% (treatment 2), in 3 replicates. After fertilization, the presumptive zygotes were cultured for 6 days in modified SOF medium supplemented with 5% fetal calf serum (FCS); the number of blastocysts was recorded and classified. Then, expanded blastocysts were cryopreserved by open pulled straw vitrification using the 2-step approach described by Vajta G et al. (1998 Mol. Reprod. Dev. 51, 53-58). After 24 h, vitrified embryos were warmed and cultured in groups of <25 per 50 μL droplet of modified SOF medium with 5% FCS under mineral oil for 48 h and examined for re-expansion and hatching. Differences between the groups in blastocyst yield were analyzed by ANOVA; differences in survival rates between the groups were analyzed using logistic regression analysis. For all statistical models, group was included as fixed effect, and also the effect of replicate was included. Differences were considered to be statistically significant when P < 0.05. Addition of ethanol to in vitro maturation media had no significant effects on blastocyst yield on 7 dpi. Also, addition of ethanol at 0.27% did not affect blastocyst cryotolerance. However, addition of ethanol 0.54% to in vitro maturation media significantly increased the survival of bovine blastocysts after vitrification (P < 0.01; Table 1). The results of the present study indicate that maturation of oocytes under ethanol stress at low concentrations has carry-over effects on embryo quality, leading to improved cryotolerance.
|
The authors thank I. Lemahieu and P. Vandamme for their excellent technical support. This research was supported by the special research fund, Ghent University (Grant, BOF/DOS No. 01W05706).


