23 EFFECT OF HISTONE ACETYLATION LEVELS ON THE IN VITRO DEVELOPMENT OF CLONED BOVINE EMBRYOS
L. Chacón A B , J. A. Jenkins D , S. P. Leibo B C , G. Wirtu C , B. L. Dresser B C , C. E. Pope C and M. C. Gómez C AA Colombian National University, Bogotá, Colombia;
B University of New Orleans, New Orleans, LA, USA;
C Audubon Center for Research of Endangered Species, New Orleans, LA, USA;
D Geological Survey, National Wetlands Research Center, Lafayette, LA, USA
Reproduction, Fertility and Development 21(1) 111-112 https://doi.org/10.1071/RDv21n1Ab23
Published: 9 December 2008
Abstract
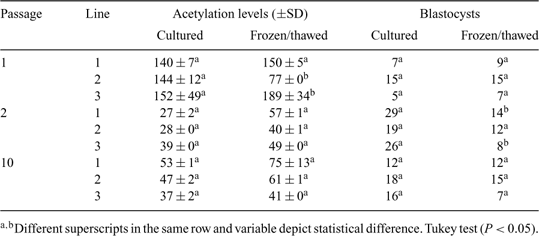
The epigenetic status of donor cells is an important factor for their successful reprogramming during somatic cell nuclear transfer (SCNT). Environmental factors partly influence DNA methylation and histone modifications (Fraga et al. 2005 PNAS USA 102, 10 604–10 609; Ke et al. 2006 Carcinogenesis 27, 1481–1488; Rodenhiser and Mann 2006 CMAJ 174, 341–348); low temperatures have altered epigenetic events in plants (Amasino 2004 Plant Cell; Hao et al. 2002 Cryo Letters 23, 37–46). Because cryopreservation alters histone acetylation levels in donor cells and subsequent viability of cloned embryos (Gómez et al. 2008 Cloning Stem Cells, in press), similar alterations may occur in bovine cloned embryos reconstructed with donor cells thawed immediately before SCNT. The objectives of the present study were (1) to measure the relative levels of nuclear histone acetylation in bovine fibroblasts immediately after thawing (frozen/thawed) or following a period of culturing (cultured) and (2) to determine the influence of the epigenetic status of donor cells on the in vitro development of reconstructed, cloned bovine embryos by gauging blastocyst development. Cell cultures lines were derived from the skin of 3 adult cows and analyzed at passage 1 (P1), 2 (P2), and 10 (P10). For each of 3 passages, cells were cultured until reaching 100% confluence, followed by an additional 3 days of culture during which time acetylation levels were measured in cultured and frozen/thawed cells. For cryopreservation, cells at P1, P2, and P10 were disaggregated and resuspended in CryoStor™ (CS10; BioLife Solutions, Bothell, WA, USA) and cooled at 1.0°C min–1 to –80°C prior to storage in liquid nitrogen. Cells were fixed with ethanol for 12 h and incubated for 30 min with antibody directed against acetylated lysine 9 on histone 3 (H3K9). The cells were then incubated with a fluorescein isothiocyanate conjugated secondary antibody and DNA stain and evaluated by flow cytometry. Cloned embryos were reconstructed with cultured or frozen/thawed cells at P1, P2, and P10 as described by Vajta et al. 2005 (Reprod. Fertil. Dev. 17, 791–797). Derived embryos were cultured until Day 8, and cleavage and development to the blastocyst stage were evaluated. Histone acetylation levels for all 3 cell lines, either fresh or frozen/thawed, were significantly higher at P1 than at P2 and P10 (Table 1), and cryopreservation reduced histone acetylation levels only in cell culture line 2 at P1. Higher development to the blastocyst stage (25%) was observed when embryos were reconstructed with cultured cells at P2 and with cells that had lower histone acetylation levels (Pearson correlation, r = –0.55; P = 0.01)
|


