360 EVALUATION OF QUALITY OF IN VITRO-MATURED BOVINE OOCYTES BY SEDIMENTATION WITH PERCOLL
K. Yotsushima A , M. Shimizu A , H. Kon A and Y. Izaike BA Toyama Prefectural Agricultural Research Center, Livestock Experiment Station, Toyama, 939-2622, Japan
B Iwate University, Morioka, 020-8550, Japan
Reproduction, Fertility and Development 19(1) 295-296 https://doi.org/10.1071/RDv19n1Ab360
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
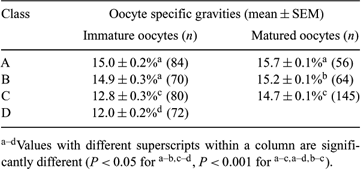
A simple method to evaluate the quality of in vitro-matured bovine oocytes is available for development of an in vitro embryo production system. Oocyte quality relates closely to oocyte fatty acid composition and mitochondrial distribution. The purpose of this study was to examine the influence of the quality of cumulus–oocyte complexes (COCs) and serum supplementation in IVM medium on the distribution of bovine oocyte specific gravities by sedimentation with Percoll before and after IVM. COCs were aspirated from abattoir-derived ovaries and were classified as classes A to D by the morphology of their cumulus cell layers as follows: class A, compact and more than 3 layers thick; class B, compact but <3 layers; class C, partially naked and <3 layers; and class D, naked or expanded. The classified COCs were cultured in TCM-199 supplemented with 0.1% BSA, 5 µg mL−1 insulin, 10 µg mL−1 transferrin, and 10 ng mL−1 transforming growth factor-α (M199-BITT) for 22–24 h. To evaluate the influence of serum supplementation, oocytes from classes A and B were also incubated in M199-BITT as serum-free culture or TCM-199 supplemented with 10% fetal calf serum as serum-supplemented culture. Percoll solutions were prepared by diluting Percoll with PBS supplemented with 0.3% BSA, 1 mg mL−1 glucose, and 0.2 mM sodium pyrvate to 20, 17.5, 15, 12.5, 10, 7.5, and 5% solutions. After removal of cumulus cells, denuded oocytes were put on the surface of Percoll solution for 3 min, and the precipitated oocytes were transferred to stepwise high density solution. The percent of Percoll solution just before buoyancy was considered as the oocyte specific gravity value. Statistical analysis was performed by one-way ANOVA. Oocytes from class A had the highest specific gravities before and after IVM in all classes (Table 1). After IVM, oocyte specific gravities from classes A and C were higher than those of oocytes before IVM (class A: P < 0.05, class C: P < 0.001). The specific gravities of in vitro-matured oocytes cultured in serum-free medium were higher than those cultured in serum-supplemented medium (15.3 ± 0.3%, n = 71, and 14.0 ± 0.3%, n = 58; P < 0.01). These results show that the specific gravity was affected by the morphological quality of COC, and the culture conditions for IVM may profile the metabolic activity of oocytes during IVM.
|


