147 GENE KNOCKDOWN OF MATERNAL- AND EMBRYONIC-EXPRESSED GREEN FLUORESCENT PROTEIN IN MURINE EMBRYOS BY THE INJECTION OF A SHORT INTERFERING RNA (siRNA)
K. Iqbal A , W. A. Kues A , J. W. Carnwath A and H. Niemann AAInstitute for Animal Breeding, Neustadt D-31535, Germany
Reproduction, Fertility and Development 19(1) 191-191 https://doi.org/10.1071/RDv19n1Ab147
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
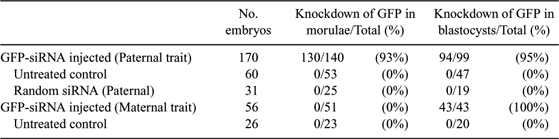
RNA interference (RNAi) is now widely used for gene silencing in various biological systems. Injection of long double-stranded RNAs has been shown to specifically knock down gene expression in mammalian embryos. The utilization of short interfering RNAs (siRNA) to target specific embryonic genes would make this approach flexible and efficient enough for studying physiological functions in development. To demonstrate the feasibility of a single class of siRNA molecules for achieving long-lasting effects after injection into mammalian zygotes, we used siRNAs to knock down expression of the green fluorescent protein (GFP) in transgenic murine embryos of the OG2 transgenic line. Homozygous OG2-animals, carrying the Oct4-GFP transgene, were mated with NMRI animals to produce OG2 hemizygous zygotes, which show a parentally dependent expression pattern of the marker gene. Hemizygous zygotes with a maternally inherited Oct4-GFP gene continuously express the GFP marker; hemizygous zygotes with a paternally inherited Oct4-GFP start transcription of the GFP gene at the 4–8 cell stage, i.e., after onset of embryonic genomic activation. Thus efficacy and duration of gene silencing could be tested under 2 different conditions where (i) GFP mRNA was already present at the time point of injection, and (ii) GFP transcription started 2–3 cell cycles after siRNA injection. Zygotes were microinjected with either a 22-basepair GFP-siRNA or a control siRNA and then cultured in vitro. The siRNAs were conjugated with the fluorochome rhodamine to allow monitoring of injection and subsequent degradation of siRNAs. At the end of the in vitro culture, the developmental stage, the number of nuclei, GFP fluorescence (Table 1), and the GFP mRNA levels were determined by RT-PCR. In conclusion, results demonstrate that injection of a synthetic siRNA is sufficient to knock down a target gene transcript with either maternal or embryonic expression in mammalian embryos. Importantly, maternally derived GFP proteins showed a delayed functional knockdown of GFP for 2 days. The advantages of this approach are that (i) off-target effects of long double-stranded RNAs can be avoided, (ii) siRNAs against any known transcript can be rapidly designed and synthesized, and (iii) developmentally important genes can be silenced in embryos for at least 5 days following injection.
|


