Implication of transcriptome profiling of spermatozoa for stallion fertility
Yara Suliman A , Frank Becker A C and Klaus Wimmers BA Institute for Reproductive Biology, Leibniz Institute for Farm Animal Biology Dummerstorf, D-18196 Dummerstorf, Wilhem-Stahl-Allee 2, Germany.
B Institute for Genome Biology, Leibniz Institute for Farm Animal Biology Dummerstorf, D-18196 Dummerstorf, Wilhelm-Stahl-Allee 2, Germany.
C Corresponding author. Email: becker@fbn-dummerstorf.de
Reproduction, Fertility and Development 30(8) 1087-1098 https://doi.org/10.1071/RD17188
Submitted: 9 July 2016 Accepted: 6 December 2017 Published: 14 March 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Poor fertility of breeding stallions is a recognised problem in the equine industry. The aim of the present study was to detect molecular pathways using two groups of stallions that differed in pregnancy rates as well as in the proportion of normal and motile spermatozoa. RNA was isolated from spermatozoa of each stallion and microarray data were analysed to obtain a list of genes for which transcript abundance differed between the groups (P ≤0.05, fold change ≥1.2). In all, there were 437 differentially expressed (DE) genes between the two groups (P ≤ 0.05, fold change ≥1.2). Next, the DE genes were analysed using Database for Annotation, Visualisation, and Integrated Discovery (DAVID). Finally, ingenuity pathways analysis (IPA) was used to identify top biological functions and significant canonical pathways associated with the DE genes. Analysis using the DAVID database showed significant enrichment in the gene ontology (GO) term ‘RNA binding’ (P = 0.05) and in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway cytokine–cytokine receptor interaction (P = 0.02). Furthermore, IPA analysis showed interconnected biological functions and canonical pathways involved in the regulation of spermatogenesis and male fertility. In addition, significantly enriched metabolic pathways were identified. In conclusion, the present study has identified, for the first time, molecular processes in stallion spermatozoa that could be associated with stallion fertility.
Additional keywords: microarray, sperm RNA, spermatogenesis.
Introduction
Stallion fertility is of high economic importance for the horse industry. Among farm animals, horses have the lowest reproductive rate. This is reflected in lower per cycle pregnancy rates, which range from 43% to 60% (Morris and Allen 2002), compared with 85–90% in boars (Colenbrander et al. 1993) and 80–90% in rams (Menzies 1999). Unlike other domestic species, the selection of equines for breeding is based on their pedigree, athletic prowess and conformation characteristics, with no consideration of fertility potential during selection (Colenbrander et al. 2003). Breeding values for different breeds are calculated for important traits such as sportive results, movement, conformation and health. Stallion fertility can be determined in different ways, including considering parameters reflecting breeding or reproduction success rates, such as pregnancy rate, foaling rate and non-return rate, which show immense individual variation. Male fertility may also be assessed using sperm characteristics, which are also highly variable (Hamann et al. 2005). Attempts to find a relationship between stallion fertility and sperm characteristics have reported inconsistent results. For example, Jasko et al. (1990a) and Hirano et al. (2001) found a significant correlation between sperm features and fertility in stallion, but Dowsett and Pattie (1982) and Voss et al. (1981) reported no correlation between sperm characteristics and fertility in the stallion. Moreover, animal-based intrinsic, genetic and physiological data sets associated with male fertility are largely unknown. In recent years, increased attention has focused on the analysis and characterisation of sperm RNAs and their role in the regulation of spermatogenesis, fertilisation and early embryo development (Boerke et al. 2007). Spermatozoa contain a large population of RNAs, including mRNA, interference RNA, antisense RNA and micro-RNA (Dadoune 2009; Hosken and Hodgson 2014). In the study of Zhao et al. (2006) a large number of RNAs was detected in human spermatozoa. The quality and quantity of these RNAs, as well as their potential roles in male fertility, are still largely unknown. It was first believed that RNAs in spermatozoa are remnants and that their sole purpose was to transfer the paternal genome to the oocyte during fertilisation (Krawetz 2005). Recently, Das et al. (2013) characterised the global transcriptome in the spermatozoa of fertile stallions and explored the important role of these transcripts in male fertility. These findings provided a better concept of the biological importance of sperm RNAs, allowing the identification of biomarkers of stallion fertility. The sperm transcriptome has been studied in humans (Ostermeier et al. 2002), boars (Kempisty et al. 2008; Yang et al. 2009) and bulls (Gilbert et al. 2007; Bissonnette et al. 2009; Feugang et al. 2010), and the utility of sperm RNA as a marker of infertility has been explored (Miller 2000; Steger 2001), whereby differences in transcript levels in spermatozoa with different motilities (Bissonnette et al. 2009; Lambard et al. 2004), as well as between normal and abnormal sperm samples (Platts et al. 2007; Steger et al. 2003), have been reported. Krawetz (2005) demonstrated that some of the sperm RNAs can be delivered to the oocyte during fertilisation. Martins and Krawetz (2005) identified protamine2 and clusterin in zygotes using zona-free hamster egg and human spermatozoa, which were incubated together in culture medium. These transcripts were detected in human spermatozoa and zygotes, but not in unfertilised oocytes. These transcripts could have a role in the oocyte at the time of fertilisation and during early embryonic development (Boerke et al. 2007; Carrell 2008; Lalancette et al. 2008; Bukowska et al. 2013). Despite the advances achieved in transcriptional analysis of spermatozoa in several species, genetic studies of stallion spermatozoa are still limited. Therefore, the aim of the present study was to identify molecular processes that are associated with male fertility by comparing microarray-derived sperm transcriptomes of stallions that were clearly assigned to either a fertile or subfertile group based on reproductive success and sperm characteristics. The results of the present study may help predict stallion fertility, as well as in the selection of future breeding stallions.
Materials and methods
Animals and semen collection
All animal procedures were performed in accordance with the modified Council Directive 92/65/EEC (https://ec.europe.eu/food/animals/semen/equine_en, assessed 15 December 2017). No particular ethical issues had to be considered because the semen samples used in the present study had been collected as part of routine breeding procedures. The stallions were properly housed and fed, and their surroundings were kept in sanitary conditions in accordance with the requirements to operate an AI station within the EU. Six warmblood stallions (Hanoverian, Mecklenburg and Oldenburg Warmblood) were used in the present study. The age of the stallions ranged from 7 to 12 years. Stallions were selected from more than 150 active stallions with a minimum covering number of 10 mares per season. Available seasonal breeding reports were analysed by the stud manager examining results after AI with extended fresh semen. All stallions were part of a regular semen collection regimen (four times from April until July) during the breeding period. In all, 24 ejaculates were collected (four per animal) for the present study using an artificial vagina (Hannover model; Minitüb). After semen collection, the volume of each gel-free ejaculate was measured using a graduated cylinder, and the sperm concentration was determined using a photometer (Minitüb). The semen samples were then diluted using an appropriate extender (Minitüb) to a concentration of 100 × 106 spermatozoa mL−1. The extended semen was divided into 10-mL tubes, loaded into a transport container and transported at 4°C to the laboratory.
Semen quality assessment
Stallion ejaculates were assessed individually for routine semen quality parameters such as progressive motility and normal morphology. Progressive motility was estimated using computer-aided sperm analysis (CASA; Minitüb). First, the diluted semen was diluted further in the laboratory to a concentration of 25–50 × 106 spermatozoa mL−1 and kept at room temperature for 10–15 min. Samples were analysed in a 10-µm Leja chamber at 37°C. The percentage of spermatozoa with normal morphology was determined using eosin–nigrosin staining (Nidacon). Semen smears were prepared by mixing 20 µL semen with the same volume of stain. The mixture was then smeared on a glass slide and air dried. A total of 100 spermatozoa in each ejaculates was examined and classified under phase contrast microscopy oil immersion (×1000). After assessing sperm quality, 1 mL fresh semen from each ejaculate (containing 100 × 106 spermatozoa) was washed three times at 1200g for 10 min at 4°C with phosphate-buffered saline (PBS), which was prepared by dissolving 1 tablet of PBS (Sigma Aldrich) in 200 µL diethylpyrocarbonate (DEPC)-treated water. After 30 min to allow the table to dissolve, the PBS medium was filtered through a Syringe-filter (SARSTEDT) with a pore size of 0.20 μm and stored at 4°C until use. Washed sperm pellets were then stored at −80°C until RNA extraction.
RNA isolation and purity assessment
An RNA isolation protocol was developed to extract pure sperm RNA without contamination by somatic cell RNAs. In this protocol frozen sperm samples were placed on ice for 5–10 min to thaw, after which they were centrifuged at 5000g for 5 min at 4°C. After the supernatant had been removed, 3 mL hypotonic solution (99 mL DECP water supplemented with 0.5 mL of 0.1% sodium dodecyl sulfate (SDS) and 0.5 mL of 0.5% Triton X-100) was added to the sperm pellet and the mixture was pipetted up and down five to 10 times and then placed on ice for 15 min to lyse the somatic cells. After centrifugation of samples at 5000g for 15 min at 4°C, the hypotonic solution was removed and the pellet was washed three times with PBS. Then, 1 mL TRIzol (1 mL for 108 cells in suspension) was added to each sperm pellet to isolate total RNA from the spermatozoa. The pellet was lysed by aspirating the solution and releasing it 15–20 times with a 27-gauge needle connected to a 5-mL syringe. After 15 min incubation at room temperature (with vortexing every 5 min), the homogenised samples were centrifuged at 12 000g for 10 min at 4°C. The resulting pellet represents insoluble material, such as polysaccharides, extracellular membranes and high molecular weight DNA. The supernatant, containing the RNA, was transferred to a fresh tube with 1 mL TRIzol. Then, 200 μL chloroform was added to each sample and the samples were homogenised by vortexing for 30 s, followed by incubation at room temperature for 5 min and then centrifugation at 12 000g for 15 min at 4°C. During centrifugation, the mixture separates into an organic phase, an interphase and an upper aqueous phase that contains the RNA. The aqueous phase was transferred to a fresh tube and mixed with 1 volume isopropanol (100%) and 1 μL glycogen. The samples were then incubated for 30 min at room temperature to precipitate the RNA and then centrifuged at 20 000g for 30 min at 4°C. The supernatant was removed and the pellets washed three times with 75% ethanol. The RNA was sedimented by centrifugation at 12 000g for 5 min at 4°C, the ethanol was removed and the pellets dried for 5–10 min at room temperature. The RNA pellet was then dissolved in 20 μL water and the sample was heated for 15 min at 58°C. The RNA concentration was determined by measuring absorbance at 260 nm using a NanoDrop 1000A spectrophotometer. The absence of DNA contamination in RNA samples was verified by real-time PCR using a set of primers specific to protamine2 (Table 1) with isolated RNA as a template. After assessment of RNA purity, all RNAs were stored at −80°C until further analysis.

|
Microarray experiments and data analysis
For the microarray experiments, individual RNA samples of each of the six stallions were pooled and used to prepare microarray probes. Labelled cRNA was prepared from 500 ng total RNA using the GeneChip WT PLUS Reagent Kit (Affymetrix) according to the manufacturer’s instructions. The cRNA targets were fragmented and individually hybridised onto a catalogue genome-wide Equine Gene 1.0 ST Array (Affymetrix) covering 30 559 transcript clusters. After staining and washing, the arrays were scanned and raw data were obtained using Affymetrix GCOS 1.1.1 software. First, the raw microarray data were subjected to quality control using Microarray Suite 5 (MAS5; present and absent) and quantitative expression levels of the transcripts were estimated using Probe Logarithmic Intensity Error (PLIER) for normalisation using Affymetrix Expression Console 1.1 software. Then, one-way analysis of variance (ANOVA) was used to detect transcripts with significant differences in abundance between fertile and subfertile stallions. According to the Minimum Information About a Microarray Experiment (MIAME) standards (Brazma et al. 2001) the microarray data have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo, accessed 5 January 2017) under the Accession number GSE75350.
Functional annotation and pathway analysis to identify significantly overrepresented functions
The list of genes with significantly different abundance (P ≤ 0.05 and fold change (FC) ≥1.2 based on Affymetrix microarray technology sensitivity; corresponding to a false discovery rate (FDR) of <0.4) in the stallion groups was submitted to the Database for Annotation, Visualisation and Integrated Discovery (DAVID; http://david.abcc.ncifcrf.gov/, accessed 16 January 2017) and the web-based ingenuity pathways analysis (IPA; www.Ingenuity.com, accessed 13 January 2017) to identify gene ontology (GO) terms, canonical pathways and biological functions associated with these genes. The significance of gene enrichment was determined by Fisher’s exact test, with a cut-off set at 0.05. P-values from Fisher’s exact test were adjusted for multiple testing using Benjamini–Hochberg multiple testing corrections.
Synthesis of cDNA
In preparation for first-strand synthesis, 1 μL total RNA was incubated with 1 μL oligo (dt) 13 primer (0.5 µg µL−1), 1 µL RNAse inhibitor (40 U µL−1; Promega), 2.5 µL random hexamer primer (0.2 µg µL−1; Fermentas) and 7 µL RNAse-free water at 68°C for 5 min. The samples were then placed on ice for 5 min. The reaction mix for first strand synthesis consisted of 4 μL of 5× First Strand buffer (Invitrogen), 1 μL dithiothreitol (DTT; 0.1 M; Invitrogen), 1 μL dNTPs Mix (10 mM; Invitrogen), 0.5 µL RNAse-free water and 1 μL SuperScript III Reverse Transcriptase (200 units µL−1; Invitrogen). Samples were incubated at 25°C for 5 min, 50°C for 1 h and then heated to 75°C for 15 min to inactivate the enzyme. Finally, samples were cooled at 4°C. The cDNA was then stored at −20°C until use.
Quantitative real-time PCR
Genomic DNA contamination of the samples was evaluated using real-time PCR (Light cycler 480; Roche) using a set of primers specific to protamine2 (Table 1) and spanning an intron; different PCR products were amplified from RNA (167 bp) and genomic DNA (351 bp), as described previously (Das et al. 2010). Reactions for real-time PCR were performed in a final volume of 12 µL using 6 µL Kapa SYBR Fast (KAPA BIOSYSTEMS), 0.6 µL each primer, 2.8 µL distilled water and 2 µL cDNA, which was replaced with distilled water as a negative control and with equine genomic DNA as a positive control. The thermal cycling conditions consisted of an initial denaturation step at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 10 s and extension at 72°C for 15 s. The real-time PCR products were subjected to melting curve analyses and gel electrophoresis to prove the absence of any non-specific product.
Four different genes from the most affected biological functions, namely 2′,5′-oligoadenylate synthetase 1, 40/46 kDa (OAS1), 2′,5′-oligoadenylate synthetase 2, 69/71kDa (OAS2), interleukin-13 (IL13) and interleukin-22 receptor, α1 (IL22RA1) were selected and validated using real-time PCR. The primers were designed using mRNA accession numbers and the NCBI website (http://www.ncbi.nlm.nih.gov/, accessed 20 January 2017). The list of primer sequences is given in Table 1. In order to determine real-time PCR efficiency, a gene-specific standard curve was generated for each gene using a real-time PCR amplicon quantified by spectrophotometry (absorbance at 260 nm/280 nm), converted into the number of molecules and serially diluted to produce the six reference points of the calibration curve (101–106 copies). Two common housekeeping genes, namely ribosomal protein L32 (RPL32) and hypoxanthine phosphoribosyltransferase (HPRT) were used to normalise the data, and t-tests were used to compare gene expression between the two groups using normalised Ct values (i.e. ΔCtGroup 1 vs ΔCtGroup 2) for each stallion in both groups, calculated as follows:

where CtReference median was calculated as the mean of Ct values for RPL32 and HPRT. Differences between groups were considered significant at two-sided P ≤ 0.05.
Results
Stallion fertility and semen quality
Four ejaculates were collected from each stallion during the breeding season. Motility and morphology measurements for each stallion, as well as seasonal pregnancy rate for each group, are presented in Table 2. The stallions in the fertile group had a better semen profile (progressive motility and percentage of spermatozoa with normal morphology) compared with stallions in the subfertile group.

|
Functional annotation and KEGG pathway analysis of differentially expressed genes
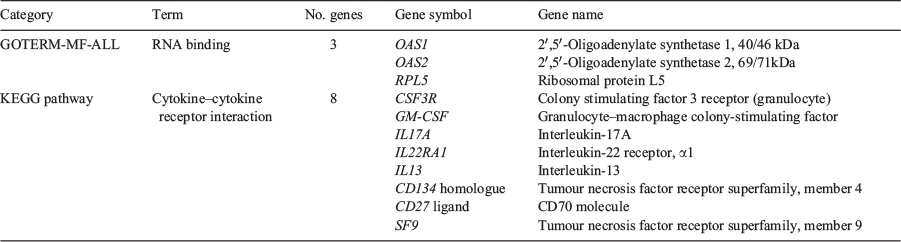
In all, 437 genes were found to be significantly differentially expressed (DE) between the two groups of stallions (P ≤ 0.05, FC ≥ 1.2). To explore and view functionally related genes together, the list of DE genes was evaluated using DAVID, and DE genes were assessed using GO and the KEGG pathway database. DAVID assigned 216 transcripts on the gene list to GO terms and KEGG pathways. Of these, three genes (OAS1, OAS2, RPL5) belonged to GO term ‘RNA binding’ and had a P-value of 0.05. Another eight genes from the gene list, namely colony-stimulating factor 3 receptor (granulocyte) (CSF3R), granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-17A (IL17A), IL13, IL22RA1, tumour necrosis factor receptor superfamily, member 4 (CD134), CD27 and tumour necrosis factor receptor superfamily, member 9 (SF9), were found to be significantly enriched in the KEGG pathway cytokine–cytokine receptor interaction (P = 0.02), as summarised in Table 3.
|
Biological functions and canonical pathways of DE genes
IPA was used to select the main biological functions and canonical pathways represented in the gene list generated in the present study. The top 20 significantly enriched IPA biological functions are shown in Fig. 1. Most of the DE genes were associated with biological functions related to cellular processes, including cellular assembly and organisation, cellular function and maintenance, cellular development and cellular growth and proliferation. IPA also identified canonical pathways from the DE genes, which included G1/S checkpoint regulation, cyclin and cell cycle regulation, ATM (ataxia-telangiectasia mutated) signalling and netrin signalling (Fig. 2). Significantly enriched metabolic pathways were related to phosphatase and D-myoinositol phosphate metabolism The enriched signalling pathways are shown in Fig. 3.
Quantitative real-time PCR validation of microarray results
To validate the microarray results, four genes from the gene list (OAS1, OAS2, IL13 and IL22RA1) that exhibited differential expression in the microarray were selected for real-time PCR analysis. These genes were chosen according to their biological function and, in all cases, were validated as having high transcript abundance in the fertile stallion group (Table 4).

|
Discussion
Research into human fertility, as well as that of other mammals, has advanced considerably in recent years, but research into the molecular mechanisms affecting stallion fertility remains in its infancy. Genetic markers associated with stallion fertility may be useful in selection and breeding management. Analysis of stallion sperm transcriptome by microarray analysis in the present study allowed comparison of transcript abundance between fertile and subfertile stallions. Analysis of the sperm transcriptome by gene expression microarray is typically affected by the RNA isolation technique used. RNA degradation is one of the most crucial problems in the RNA-extraction process. The concentration of RNA in spermatozoa is lower than in somatic cells; therefore, it is essential to avoid any RNA degradation during the extraction process. Furthermore, global microarray analysis of the low quantity of RNA in samples obtained from spermatozoa compared with the quantity of RNA obtained from somatic cells has traditionally required either one or more amplification step(s) or mixing of samples depending on the RNA concentration to obtain sufficient nucleic acid material for subsequent detection. In the present study, the RNA samples for each stallion were pooled, glycogen was used as a coprecipitant for the small amounts of RNA and the purity of the RNA samples was checked using real-time PCR and a set of primers specific to protamine2.
In several previous studies, many genes and proteins were analysed for their association with fertility in the stallion (Schambony et al. 1998; Hamann et al. 2007; Zaabal and Ahmed 2010). For example, equine cysteine-rich secretory protein (CRISP) genes were found to be significantly associated with male fertility (Schambony et al. 1998). The CRISP proteins represent the major equine fraction of seminal plasma proteins and were found to be involved in sperm–oocyte fusion (Töpfer-Petersen et al. 2005). Furthermore, Giesecke et al. (2011) chose three genes, namely angiotensin-converting enzyme (ACE), sperm autoantigenic protein 17 (SPA17) and FSH beta subunit (FSHB), to test as candidates for determining Hanoverian stallion fertility, finding that haplotypes of all three genes significantly contributed to the paternal and embryonic fertility components of the pregnancy rate per oestrus. In addition, Gamboa and Ramalho-Santos (2005) investigated the presence of soluble N-ethylmaleimide-sensitive factor activating protein receptor (SNARE) proteins in equine spermatozoa and found that, stallions with fertility problems had the worst sperm and acrosomal membrane quality, as well as fewer sperm cells that stained positive for SNAREs and caveolin1 compared with spermatozoa from fertile donors. In the present study, we used the microarray approach to identify molecular pathways relevant to stallion fertility. In the present study we chose six stallions with differing fertility, as evaluated in terms of semen profile (progressive motility and percentage of spermatozoa with normal morphology) and percentage of pregnancies per season, calculated by dividing the number of all pregnant mares (for each stallion individually) by the number of mares bred during the year. Recently, many developments have been achieved in the field of gene expression analysis, providing new molecular detection tools to aid our understanding of stallion fertility (Bright et al. 2009; Novak et al. 2010). RNA in the spermatozoa could be used in genomic analysis to evaluate male fertility. Currently, several studies have emerged focusing on an analysis of transcripts or proteins in spermatozoa that could be associated with male fertility. Feugang et al. (2010) identified 415 DE transcripts from high- and low-fertility Holstein bulls. These transcripts were associated with different cellular functions and biological processes. Kropp et al. (2017) focused in their study on bull fertility and demonstrated that bulls with different fertility status delivered embryos with different transcriptome profiles despite similar morphology and adequate development to blastocyst stage. In addition, the identification of paternal components, which are delivered into the oocyte during fertilisation, could lead to a better understanding of male fertility and suitable selection of breeding sires. Several genes and molecular pathways involved in spermatogenesis and male fertility were identified by Bansal et al. (2015). In that study, analysis of 2081 transcripts that were differentially expressed between three groups of men with known fertility, showed that some of these transcripts were related to heat shock proteins, testis-specific genes and Y chromosome genes, which suggested that sperm RNA has considerable potential as a marker in the evaluation of fertility. Proteomics has also been used to identify putative biomarkers associated with male fertility. In particular, Ashrafzadeh et al. (2013) identified several proteins that differed in abundance between two groups of fertile and subfertile zebu cattle using frozen semen samples. These proteins were involved in energy metabolism, glycolysis, sperm motility and male fertility. Similarly, in the present study we compared the RNA profile of spermatozoa obtained from fertile and subfertile stallions using Affymetrix microarray technology. The analysis identified 437 transcripts that differed significantly between fertile and subfertile stallions. These transcripts were associated with different biological functions and molecular processes known to be fundamental for spermatogenesis and male fertility.
RNA-binding proteins
RNA-binding proteins (RBPs) are a class of proteins that are expressed in the nucleus and are defined by their ability to bind RNA. RBPs play important roles in the organism. They are involved in many cellular processes that occur during tissue development. Moreover, these proteins regulate immune response, the formation of dendrites and the differentiation of embryonic stem cells (Colegrove-Otero et al. 2005; Idler and Yan 2012). RBPs are highly expressed during spermatogenesis and play an essential role during all stages of germ cell development. During mammalian spermatogenesis, chromatin structure is markedly modified, resulting in the shutting down of nuclear gene expression in mature spermatozoa (Miller and Ostermeier 2006). In elongating and condensing spermatids, the histones are largely removed and replaced first by the translation proteins, which are subsequently displaced with sperm-specific protamines (Balhorn et al. 1984). The resulting chromatin is highly condensed and the sperm nucleus is transcriptionally inert and does not contain sufficient rRNA to support translation (Miller et al. 1999). In post-meiotic spermatozoa, de novo transcription is silenced and the synthesis and storage of sufficient mRNA is very important to compensate for the lack of mRNAs during the subsequent transcriptionally inactive stage of spermatogenesis (Sassone-Corsi 2002). Germ cells have been shown to express high levels of RBPs throughout spermatogenesis, which are very essential to post-transcriptional events during all stages of spermatogenesis (Idler and Yan 2012). According to DAVID, in the present study three genes from the DE genes were involved in GO term ‘RNA binding’ (with P = 0.05; Table 3). This result highlights the importance of RBPs in the regulation of spermatogenesis and male fertility. Bettegowda and Wilkinson Miles (2010) reported the role of four RBPs, namely gonadotrophin-regulated testicular RNA helicase (GRTH), Src-associated in mitosis 68 kDa (SAM68), mouse Y-box protein 2 (MSY2) and deleted in azoospermia-associated protein-1 (DAZAP1), that were shown to be critical for spermatogenesis by promoting the translation of a subset of germ cell mRNAs, indicating the importance of translation control for normal spermatogenesis. Furthermore, Idler and Yan (2012) reported that in mice, the inactivation of many of the RBPs studied caused infertility and spermatogenic arrest at various steps of germ cell differentiation.
Cytokine–cytokine receptor interaction
The results of the present study also indicated that eight genes from the gene list created (i.e. CSF3R, GM-CSF, IL17A, IL13, IL22RA1, CD134, CD27 and SF9) were significantly enriched in the KEGG pathway cytokine–cytokine receptor interaction (P = 0.02; Fig. 4). Cytokines are a broadly defined group of regulatory proteins that have a wide range of biological activities in addition to their original functions in the immune system (Hales et al. 1999; Ihsan 2014). Several cytokines have direct effects on testicular cell function, and several of these are involved in normal reproductive physiology and fertility regulation (Hales et al. 1999; Hedger and Meinhardt 2003). Cytokines act as growth and differentiation factors during pathological states, as well as under normal physiological conditions (Hales et al. 1999). Various immunological factors, cytokines, chemokines and growth factors have been documented in human semen (Poltich et al. 2007), as well as that from rodents (Ingman and Rebecca 2008) and other livestock species (Paulesu et al. 2010). These molecules in semen affect vaginal immunology and female fertility following insemination and play an important role in regulating the proliferation, viability and differentiation of blastomeres in embryos (Kane et al. 1997). However, in the present study we investigated the presence of cytokines in stallion spermatozoa whose quantitative expression levels were higher in fertile than subfertile stallions. It has been reported that cytokines and growth factors play an essential role in both the implantation and development of embryos (Sharkey 1998). Robertson et al. (2001) reported the important role of GM-CSF in murine preimplantation embryos by promoting blastocyst formation and increasing the number of viable blastomeres by reducing the incidence of apoptosis and increasing the uptake of glucose. In addition, the in vitro culture of human embryos in GM-CSF demonstrated that GM-CSF has a positive effect on the development of embryos to the blastocyst stage by increasing the number of cells in the inner cell mass and in the trophectoderm (Sjöblom et al. 1999). Many other cytokines have been studied extensively in mice and other mammalian species, including IL-1, IL-2, IL-6, IL-8, IL-10, colony-stimulating factor 1 (CSF1) and anti-inflammatory cytokines of the transforming growth factor β family, because there is strong evidence that some of them are directly involved in the production of male and female gametes, embryo implantation and development (Sharkey 1998; Simón et al. 1998; Hedger and Meinhardt 2003; Robertson et al. 2007).
Biological functions and canonical and metabolic pathways of DE genes
IPA was used to obtain a deeper understanding of the significantly altered biological functions and canonical pathways associated with the DE genes that were identified in the microarray analysis. IPA of DE genes showed several different associated biological functions. The target genes were significantly enriched for diverse cellular functions and biological processes know to be fundamental for spermatogenesis and male fertility, including cellular assembly and organisation, cellular function and maintenance, cellular development, cellular growth and proliferation, tissue development, cell morphology and organismal injury and abnormalities. Further analysis was also conducted in IPA to understand the enriched and significant canonical pathways of DE genes identified in this dataset. In all, 17 significantly enriched canonical pathways were identified, using threshold values of P < 0.05 and FC ≥ 1.2 (corresponding to an FDR <0.4). Of these pathways, significance was higher (based on P-values) for pathways associated with G1/S checkpoint regulation, cyclin and cell cycle regulation, ATM signalling, netrin signalling and natural killer cell signalling. Furthermore, IPA identified significantly enriched metabolic pathways that were related to phosphatase and D-myoinositol phosphate metabolism. Sperm formation refers to the differentiation of diploid spermatogonia into mature spermatozoa. During this transformation, spermatogonia grow in the first gap phase (G1), synthesise DNA in the synthesis phase (S), prepare for mitosis in the second gap phase (G2) and undergo mitosis and meiosis in the mitosis phase (M) (Ruwanpura et al. 2010). The G1 phase is the longest phase in the cell cycle, during which the cell grows continuously and undergoes most of its physiological activity. The next phase of the cell cycle is the S phase, during which DNA replication occurs. Therefore, passing the G1 phase is tightly controlled by both cell cycle machinery and checkpoint pathways (Neganova and Lako 2008). The G1/S checkpoint is essential to ensure that all conditions of cell division are successfully met and to guarantee the possibility of replicating the DNA and producing healthy daughter cells. This transition is signalled and controlled by cyclins and cyclin-dependent kinases (CDKs) (Neganova and Lako 2008; Wolgemuth et al. 2013). Cyclins are the principal cell cycle regulatory proteins, and have been identified in simple as well as higher-order organisms (Wolgemuth et al. 2002). Cyclins can be divided into eight classes (cyclin A–H) according to their amino acid similarity and the timing of their appearance during the cell cycle (Wolgemuth et al. 2002). Although the function of cyclins during mitosis and meiosis has been studied in somatic cells, their function during mitosis and meiosis in the germ line is poorly understood. Wolgemuth et al. (2013) reported that cyclin A2 is highly expressed in differentiated Type A and B spermatogonia and plays an important role during both meiosis and mitosis in the germ cell line. Based on the IPA in the present study, G1/S checkpoint regulation and cyclin and cell cycle regulation are the most highly significantly enriched canonical pathway identified for the DE genes. These pathways are known to be the principle regulatory elements involved in cell cycle regulation and therefore play an important role during sperm proliferation and male fertility. Using IPA of DE genes, several significantly enriched metabolic pathways related to phosphatase and D-myoinositol phosphate metabolism signalling pathways were identified. Inositol is a cyclic polyol that is considered as a member of vitamin B complex. Myoinositol is the most available form of inositol in nature. Myoinositol is distributed in the tissues of many organisms and plays an essential role in the signal transduction system in cells (Condorelli et al. 2011). In addition, inositol is involved in several activities and pathways, such as mRNA transcription, cytoskeleton and chromatin remodelling and P53 activity (Bizzarri et al. 2016). In conclusion, myoinositol plays a key role in the male reproductive system by regulating the osmolality of the seminal plasma, as well as sperm motility and acrosome reaction (Condorelli et al. 2011). Condorelli et al. (2012) suggested that myoinositol significantly increased the percentage of spermatozoa with progressive motility based on 2 h incubation of human spermatozoa with 2 mg mL−1 myoinositol. The increase in progressive motility of spermatozoa was associated with a significant increase in the percentage of spermatozoa with high mitochondrial membrane potential. In addition, inositols have been reported to affect different processes involved in oocyte fertilisation, which improve the penetration of the ovum cumulus oophorus, binding with the zona pellucida and the acrosome reaction (Calogero et al. 2015). Furthermore, high concentrations of myoinositol have been found in the fluid of the seminiferous tubule in many mammals (Setchell et al. 1968; Hinton et al. 1980; Chauvin and Griswold 2004). Myoinositol is produced by two enzymatic steps from glucose-6-phosphate, and its synthesis is regulated by myoinositol-1-phosphate synthase and myoinositol monophosphatase-1. These activity of these two enzymes is high in the testes (Chauvin and Griswold 2004). These findings support our identification of myoinositol within the top metabolic pathways in the present study.
Conclusion
In summary, prediction of stallion fertility is one of the most important problems in horse breeding and the analysis of semen characteristics has limited capacity for predicting fertility. In addition, there are only a few genetic studies on stallion fertility, which may provide possibilities and new tools that would help in predicting male fertility. In the present study, comparison of transcript abundance between fertile and subfertile stallions provided a glimpse into the various functional pathways involved in stallion fertility. Furthermore, this analysis confirmed the involvement of the immune response in stallion fertility. These results identify molecular processes in spermatozoa that could be associated with stallion fertility.
Conflicts of interest
The authors declare no conflicts of interest.
References
Ashrafzadeh, A., Nathan, S., and Anuar Karsani, S. (2013). Comparative analysis of Mafriwal (Bos taurus × Bos indicus) and Kedah Kelantan (Bos indicus) sperm proteome identifies sperm proteins potentially responsible for higher fertility in a tropical climate. Int. J. Mol. Sci. 14, 15860–15877.| Comparative analysis of Mafriwal (Bos taurus × Bos indicus) and Kedah Kelantan (Bos indicus) sperm proteome identifies sperm proteins potentially responsible for higher fertility in a tropical climate.Crossref | GoogleScholarGoogle Scholar |
Balhorn, R., Weston, S., Thomas, C., and Wyrobek, A. (1984). DNA packaging in mouse spermatids. Synthesis of protamine variants and four transition proteins. Exp. Cell Res. 150, 298–308.
| DNA packaging in mouse spermatids. Synthesis of protamine variants and four transition proteins.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhtVSktbs%3D&md5=e4ef8ae04ec845d904daf2ea3ad3142eCAS |
Bansal, S. K., Gupta, N., Sankhwar, S. N., and Rajender, S. (2015). Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis. PLoS One 10, e0127007.
| Differential genes expression between fertile and infertile spermatozoa revealed by transcriptome analysis.Crossref | GoogleScholarGoogle Scholar |
Bettegowda, A., and Wilkinson Miles, F. (2010). Transcription and post-transcriptional regulation of spermatogenesis. Phil. Trans. R. Soc. B. 365, 1637–1651.
| Transcription and post-transcriptional regulation of spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVaisrrI&md5=a171a274dd2603810749bf6e6a4dbdd8CAS |
Bissonnette, N., Le’vesque-Sergerie, J. P., Thibault, C., and Boissonneault, G. (2009). Spermatozoal transcriptome profiling for bull sperm motility: a potential tool to evaluate semen quality. Reproduction 138, 65–80.
| Spermatozoal transcriptome profiling for bull sperm motility: a potential tool to evaluate semen quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXovFemsb0%3D&md5=a32b4746c7290058912952df52903d2eCAS |
Bizzarri M.Dinicola S.Bevilacqua A.Cucina A.(2016) . Broad spectrum anticancer activity of myo-inositol and inositol hexakisphosphate. Int. J. Endocrinol. 2016, 5616807.
Boerke, A., Dieleman, S. J., and Gadella, B. M. (2007). A possible role for sperm RNA in early embryo development. Theriogenology 68, S147–S155.
| A possible role for sperm RNA in early embryo development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotlaitLc%3D&md5=65478b39c78a5c4b6ce4c086e7162853CAS |
Brazma, A., Hingamp, P., Quackenbush, J., Sherlock, G., Spellman, P., Stoeckert, C., Aach, J., Ansorge, W., Ball, C. A., Causton, H. C., et al. (2009). Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat. Genet. 29, 365–371.
| Minimum information about a microarray experiment (MIAME)-toward standards for microarray data.Crossref | GoogleScholarGoogle Scholar |
Bright, L. A., Burgess, S. C., Chowdhary, B., Swiderski, C. E., and McCarthy, F. M. (2009). Structural and functional-annotation of an equine whole genome oligoarray. BMC Bioinformatics 10, S8.
| Structural and functional-annotation of an equine whole genome oligoarray.Crossref | GoogleScholarGoogle Scholar |
Bukowska, D., Kempisty, B., Piotrowska, H., Sosinska, B., Wozna, M., Ciesiolka, S., Antosik, P., Jaskowski, J. M., Brüssow, K. P., and Nowicki, M. (2013). The structure and role of mammalian sperm RNA: a review. Vet Med (Praha) 2, 57–64.
Calogero, A. E., Gullo, G., La Vignera, S., and Condorelli, R. A. (2015). Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: a prospective double-blind randomized placebocontrolled study. Andrology 3, 491–495.
| Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: a prospective double-blind randomized placebocontrolled study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXpt1WqtL0%3D&md5=7f83b6fccc3cfe21d5cc332dea34903bCAS |
Carrell, D. T. (2008). Contributions of spermatozoa to embryogenesis: assays to evaluate their genetic and epigenetic fitness. Reprod. Biomed. Online 16, 474–484.
| Contributions of spermatozoa to embryogenesis: assays to evaluate their genetic and epigenetic fitness.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlsFOqs70%3D&md5=50b7c226ce9afb8f2d6f1c44599bbc99CAS |
Chauvin, T. R., and Griswold, M. D. (2004). Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol. Reprod. 70, 744–751.
| Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhs1Chsb4%3D&md5=4bc471ca670dd53c8db2ece25fcf9ea3CAS |
Colegrove-Otero, L. J., Minshall, N., and Standart, N. (2005). RNA-binding proteins in early development. Crit. Rev. Biochem. Mol. Biol. 40, 21–73.
| RNA-binding proteins in early development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXisl2ku7k%3D&md5=628245b16fde059ce49052efd32c8158CAS |
Colenbrander, B., Feitsma, H., and Grooten, H. J. (1993). Optimizing semen production for artificial insemination in swine. J. Reprod. Fertil. Suppl. 48, 207–215.
| 1:STN:280:DyaK2c7psVCltQ%3D%3D&md5=71b943a3f22e61fd1910f97f4234132dCAS |
Colenbrander, B., Gadella, B. M., and Stout, T. A. (2003). The predictive value of semen analysis in the evaluation of stallion fertility. Reprod. Domest. Anim. 38, 305–311.
| The predictive value of semen analysis in the evaluation of stallion fertility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3svis1Srtw%3D%3D&md5=4db49265c54f0524fb81c38702eedc0cCAS |
Condorelli, R. A., La Vignera, S., Di Bari, F., Unfer, V., and Calogero, A. E. (2011). Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 15, 129–134.
| 1:STN:280:DC%2BC3M3psV2mtQ%3D%3D&md5=359e882290fa686e40485fcc23531c3fCAS |
Condorelli, R. A., La Vignera, S., Bellanca, S., Vigari, E., and Calogero, A. E. (2012). Myoinositol: does it improve sperm mitochondrial function and sperm motility? Urology 79, 1290–1295.
| Myoinositol: does it improve sperm mitochondrial function and sperm motility?Crossref | GoogleScholarGoogle Scholar |
Dadoune, J. P. (2009). Spermatozoal RNAs: what about their functions? Microsc. Res. Tech. 72, 536–551.
| Spermatozoal RNAs: what about their functions?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVOnurrF&md5=937f88ba85e7e2e40dc6e1afdcaed394CAS |
Das, P. J., Paria, N., Gustafson-Seabury, A., Vishnoi, M., and Chaki, S. P. (2010). Total RNA isolation from stallion sperm and testis biopsies. Theriogenology 74, 1099–1106.e2.
| Total RNA isolation from stallion sperm and testis biopsies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFWqtLbN&md5=73339f428a0e56e3a31b759a537e8c90CAS |
Das, P. J., McCarthy, F., Vishnoi, M., Paria, N., Gresham, C., Li, G., Kachroo, P., Sudderth, A. K., Teague, S., Love, C. C., Varner, D. D., Chowdhary, B. P., and Raudsepp, T. (2013). Stallion sperm transcriptome comprises functionally coherent coding and regulatory RNAs as revealed by microarray analysis and RNA-seq. PLoS One 8, e56535.
| Stallion sperm transcriptome comprises functionally coherent coding and regulatory RNAs as revealed by microarray analysis and RNA-seq.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjtFKgsbc%3D&md5=6944150cea425b55af05ccf21956b158CAS |
Dowsett, K. F., and Pattie, W. A. (1982). Characteristics and fertility of stallion semen. J. Reprod. Fertil. Suppl. 32, 1–8.
| 1:STN:280:DyaL3s7mtl2gsg%3D%3D&md5=eea7914b9a6ec179e0b1db5448fd1c5aCAS |
Feugang, J. M., Rodriguez-Osorio, N., Kaya, A., Wang, H., and Page, G. (2010). Transcriptome analysis of bull spermatozoa: implications for male fertility. Reprod. Biomed. Online 21, 312–324.
| Transcriptome analysis of bull spermatozoa: implications for male fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlSgtbrJ&md5=582cfa217d99c76533cb9e7b5419ef07CAS |
Gamboa, S., and Ramalho-Santos, J. (2005). SNARE proteins and caveolin-1 in stallion spermatozoa: possible implications for fertility. Theriogenology 64, 275–291.
| SNARE proteins and caveolin-1 in stallion spermatozoa: possible implications for fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlt1Ggu7g%3D&md5=73db0db020a1f6d99d79e356b3304c53CAS |
Giesecke, K., Hamann, H., Stock, K. F., Klewitz, J., Martinsson, G., Distl, O., and Sieme, H. (2011). Evaluation of ACE, SP17, and FSHB as candidates for stallion fertility in Hanoverian warmblood horses. Anim. Reprod. Sci. 126, 200–206.
| Evaluation of ACE, SP17, and FSHB as candidates for stallion fertility in Hanoverian warmblood horses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpvFygurk%3D&md5=f3997bc60550cd924fe5a0bc01361fa7CAS |
Gilbert, I., Bissonnette, N., Boissonneault, G., Vallee, M., and Robert, C. (2007). A molecular analysis of the population of mRNA in bovine spermatozoa. Reproduction 133, 1073–1086.
| A molecular analysis of the population of mRNA in bovine spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsVGnu7k%3D&md5=e054e25b9956a92c0fc9496276748257CAS |
Hales, D. B., Diemer, T., and Hales, K. H. (1999). Role of cytokines in testicular function. Endocrine 10, 201–217.
| Role of cytokines in testicular function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlt1Olsrk%3D&md5=f1cf4420780f2d122eee4104e377d01cCAS |
Hamann, H., Mertens, U., Sieme, H., Klug, E., and Distl, O. (2005). Einfluss des Besamungsmanagements auf Fruchtbarkeitsmerkmale in der Population des Hannoverschen Warmbluts. Züchtungskunde 77, 194–205.
Hamann, H., Jude, R., Sieme, H., Mertens, U., Töpfer-Petersen, E., Distl, O., and Leeb, T. (2007). A polymorphism within the equine CRISP3 gene is associated with stallion fertility in Hanoverian warmblood horses. Anim. Genet. 38, 259–264.
| A polymorphism within the equine CRISP3 gene is associated with stallion fertility in Hanoverian warmblood horses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXnsVOisrg%3D&md5=e1c77cd96943e41b6bca30f4077a08a7CAS |
Hedger, M. P., and Meinhardt, A. (2003). Cytokines and the immune–testicular axis. J. Reprod. Immunol. 58, 1–26.
| Cytokines and the immune–testicular axis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhsVCntLo%3D&md5=42e27de69ac0c055c83b2825d588d97dCAS |
Hinton, B. T., White, R. W., and Setchell, B. P. (1980). Concentrations of myo-inositol in the luminal fluid of the mammalian testis and epididymis. J. Reprod. Fertil. 58, 395–399.
| Concentrations of myo-inositol in the luminal fluid of the mammalian testis and epididymis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXhslOqs7Y%3D&md5=0154c2aace91d5d1eaa617fa1f352b66CAS |
Hirano, Y., Shibahara, H., Obara, H., Suzuki, T., Takamizawa, S., and Yamaguchi, C. (2001). Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J. Assist. Reprod. Genet. 18, 215–220.
| Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro.Crossref | GoogleScholarGoogle Scholar |
Hosken, D. J., and Hodgson, D. J. (2014). Why do sperm carry RNA? Relatedness, conflict, and control. Trends Ecol. Evol. 29, 451–455.
| Why do sperm carry RNA? Relatedness, conflict, and control.Crossref | GoogleScholarGoogle Scholar |
Idler, R. K., and Yan, W. (2012). Control of messenger RNA fate by RNA-binding proteins: an emphasis on mammalian spermatogenesis. J. Androl. 33, 309–337.
| Control of messenger RNA fate by RNA-binding proteins: an emphasis on mammalian spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVOmt7%2FN&md5=e9f675eff118e9248531bc3d4dda6cbeCAS |
Ihsan, E. A. A. (2014). Evaluation of serum levels of pro-inflammatory cytokines (interleukins 2, 6, 8) in fertile and infertile men. DJMBR 1, 23–34.
Ingman, W. V., and Rebecca, L. J. (2008). Cytokine knockouts in reproduction: the use of gene ablation to dissect roles of cytokines in reproductive biology. Hum. Reprod. Update 14, 179–192.
| Cytokine knockouts in reproduction: the use of gene ablation to dissect roles of cytokines in reproductive biology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisVKmu74%3D&md5=353da1138dc856326553156eb0269060CAS |
Jasko, D. J., Lein, D. H., and Foote, R. H. (1990a). Determination of the relationship between sperm morphologic classifications and fertility in stallions: 66 cases (1987–1988). J. Am. Vet. Med. Assoc. 197, 389–394.
| 1:STN:280:DyaK3czmtFGktQ%3D%3D&md5=6ee8d80141802be0fe03bd5deb0ee70cCAS |
Kane, M. T., Morgan, P. M., and Coonan, C. (1997). Peptide growth factors and preimplantation development. Hum. Reprod. Update 3, 137–157.
| Peptide growth factors and preimplantation development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltVWmt70%3D&md5=f69492eb0dc82b6f1f52e80f0634c353CAS |
Kempisty, B., Antosik, P., Bukowska, D., Jackowska, M., and Lianeri, M. (2008). Analysis of selected transcript levels in porcine spermatozoa, oocytes, zygotes and two-cell stage embryos. Reprod. Fertil. Dev. 20, 513–518.
| Analysis of selected transcript levels in porcine spermatozoa, oocytes, zygotes and two-cell stage embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXksFagsrs%3D&md5=c8a9f1657b2026806fe2d418a3050453CAS |
Krawetz, S. A. (2005). Paternal contribution: new insights and future challenges. Nat. Rev. Genet. 6, 633–642.
| Paternal contribution: new insights and future challenges.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXntFeqtbo%3D&md5=505a7c9dcacf8cce716459f2dfb60659CAS |
Kropp, J., Carillo, J. A., Namous, H., Daniels, A., Salih, S. M., Song, J., and Katib, H. (2017). Male fertility status is associated with DNA methylation signatures in sperm and transcriptomic profiles of bovine preimplantation embryos. BMC Genomics 18, 280–296.
| Male fertility status is associated with DNA methylation signatures in sperm and transcriptomic profiles of bovine preimplantation embryos.Crossref | GoogleScholarGoogle Scholar |
Lalancette, C., Miller, D., Li, Y., and Krawetz, S. A. (2008). Paternal contributions: new functional insights for spermatozoal RNA. J. Cell. Biochem. 104, 1570–1579.
| Paternal contributions: new functional insights for spermatozoal RNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpvVOjt7w%3D&md5=19664600994c1098835d8850a26083bbCAS |
Lambard, S., Galeraud-Denis, I., Martin, G., Levy, R., Chocat, A., and Carreau, S. (2004). Analysis and significance of mRNA in human ejaculated sperm from normozoospermic donors: relationship to sperm motility and capacitation. Mol. Hum. Reprod. 10, 535–541.
| Analysis and significance of mRNA in human ejaculated sperm from normozoospermic donors: relationship to sperm motility and capacitation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltVWnsr8%3D&md5=32b4a2953d5f363e0db7d22e7e6bd457CAS |
Martins, R. P., and Krawetz, S. A. (2005). RNA in human sperm. Asian J. Androl. 7, 115–120.
| RNA in human sperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXos1aluro%3D&md5=47681c406fb3ac80b9f9ba3f3b624c46CAS |
Menzies, P. I. (1999). Reproductive health management programs. In ‘Current Therapy in Large Animal Theriogenology’. (Ed. R. S. Youngquist.) pp. 643–649. (W. B. Saunders and Co.: Philadelphia, PA.)
Miller, D. (2000). Analysis and significance of messenger RNA in human ejaculated spermatozoa. Mol. Reprod. Dev. 56, 259–264.
| Analysis and significance of messenger RNA in human ejaculated spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtFyrtrk%3D&md5=6902e779229ab96888eb7c689263cdf1CAS |
Miller, D., and Ostermeier, G. C. (2006). Towards a better understanding of RNA carriage by ejaculate spermatozoa. Hum. Reprod. Update 12, 757–767.
| Towards a better understanding of RNA carriage by ejaculate spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFeisbzF&md5=6da137dd68eadc3509bcc144a75eda70CAS |
Miller, D., Briggs, D., Snowden, H., Hamlington, J., Rollinson, S., Lilford, R., and Krawetz, S. A. (1999). A complex population of RNAs exists in human ejaculate spermatozoa: implications for understanding molecular aspects of spermiogenesis. Gene 237, 385–392.
| A complex population of RNAs exists in human ejaculate spermatozoa: implications for understanding molecular aspects of spermiogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvVWlt7o%3D&md5=9645bc51a39e28c5d8ed4ccf25558adaCAS |
Morris, L. H. A., and Allen, W. R. (2002). Reproductive efficiency of intensively managed thoroughbred mares in Newmarket. Equine Vet. J. 34, 51–60.
| Reproductive efficiency of intensively managed thoroughbred mares in Newmarket.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2Fptlequg%3D%3D&md5=93f3fdf6cacfa401d93f1026d2998a9bCAS |
Neganova, I., and Lako, M. (2008). G1 to S phase cell cycle transition in somatic and embryonic stem cells. J. Anat. 213, 30–44.
| G1 to S phase cell cycle transition in somatic and embryonic stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpslajsbw%3D&md5=f823863916467e8e9b7e6b9508ca1979CAS |
Novak, S., Smith, T. A., Paradis, F., Burwash, L., Dyck, M. K., Foxcroft, G. R., and Dixon, W. T. (2010). Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology 74, 956–967.
| Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFWqtLbL&md5=8bf52c0c61ea8acedad059afa4473bb7CAS |
Ostermeier, G. C., Dix, D. J., Miller, D., Khatri, P., and Krawetz, S. A. (2002). Spermatozoal RNA profiles of normal fertile men. Lancet 360, 772–777.
| Spermatozoal RNA profiles of normal fertile men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xmsl2nsLg%3D&md5=edba25b46292df4b7eb91e6cdaf6e2d4CAS |
Paulesu, L., Jantra, S., Ietta, F., Brizzi, R., Avanzati, A. M., and Bigliardi, E. (2010). Cytokines in vertebrate reproduction. Herpetol. Conserv. Biol. 5, 335–340.
Platts, A. E., Dix, D. J., Chemes, H. E., Thompson, K. E., Goodrich, R., Rockett, J. C., Rawe, V. Y., Quintana, S., Diamond, M. P., Strader, L. F., and Krawetz, S. A. (2007). Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum. Mol. Genet. 16, 763–773.
| Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmsVWnsbk%3D&md5=d9aa10f6ab2344ecceda3f3dfb3cce92CAS |
Poltich, J. A., Tucker, L., Bowman, F. P., and Anderson, D. J. (2007). Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum. Reprod. 22, 2928–2935.
| Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men.Crossref | GoogleScholarGoogle Scholar |
Robertson, S. A., Sjoblom, C., Jasper, M. J., Norman, R. J., and Seamark, R. F. (2001). Granulocyte–macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol. Reprod. 64, 1206–1215.
| Granulocyte–macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXit1aru7c%3D&md5=1cd37869bf155797fdf0b433a3e1c7ebCAS |
Robertson, S. A., Care, A. S., and Skinner, R. J. (2007). Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol. Reprod. 76, 738–748.
| Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXks1Ohs78%3D&md5=fabb94e785bd55750f0ad262c0e357f1CAS |
Ruwanpura, S. M., McLachlan, R. E., and Meachem, S. J. (2010). Hormonal regulation of male germ cell development. J. Endocrinol. 205, 117–131.
| Hormonal regulation of male germ cell development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvVCgs7s%3D&md5=7c028f2b82b89a6cdda67b75235caf3cCAS |
Sassone-Corsi, P. (2002). Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science 296, 2176–2178.
| Unique chromatin remodeling and transcriptional regulation in spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvFGhsb8%3D&md5=7ff9f57aa74032f802913d151ad6cdb1CAS |
Schambony, A., Gentzel, M., Wolfes, H., Raida, M., Neumann, U., and Töpfer-Petersen, E. (1998). Equine CRISP-3: primary structure and expression in the male genital tract. Biochim. Biophys. Acta 1387, 206–216.
| Equine CRISP-3: primary structure and expression in the male genital tract.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsl2gsbw%3D&md5=6075294d3e8fececbb0549b2fe147b10CAS |
Setchell, B. P., Dawson, R. M., and White, R. W. (1968). The high concentration of free myo-inositol in rete testis fluid from rams. J. Reprod. Fertil. 17, 219–220.
| The high concentration of free myo-inositol in rete testis fluid from rams.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF1MXis12jsQ%3D%3D&md5=c9e86ec0ced874993714b1d0e53e6516CAS |
Sharkey, A. (1998). Cytokines and implantation. Rev. Reprod. 3, 52–61.
| Cytokines and implantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXht1Wrsbw%3D&md5=787831dbd5ab906a17275ad93747c2ecCAS |
Simón, C., Valbuena, D., Krüssel, J. S., Bernal, A., and Murphy, C. R. (1998). Interleukin-1 receptor antagonist prevents embryonic implantation by a direct effect on the endometrial epithelium. Fertil. Steril. 70, 896–906.
| Interleukin-1 receptor antagonist prevents embryonic implantation by a direct effect on the endometrial epithelium.Crossref | GoogleScholarGoogle Scholar |
Sjöblom, C., Wikland, M., and Robertson, S. A. (1999). Granulocyte–macrophage colony-stimulating factor promotes human blastocyst development in vitro. Hum. Reprod. 14, 3069–3076.
| Granulocyte–macrophage colony-stimulating factor promotes human blastocyst development in vitro.Crossref | GoogleScholarGoogle Scholar |
Steger, K. (2001). Haploid spermatids exhibit translationally repressed mRNAs. Anat. Embryol. (Berl.) 203, 323–334.
| Haploid spermatids exhibit translationally repressed mRNAs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXosFChtrY%3D&md5=151cc34987058e181ffe5fcc91803c2bCAS |
Steger, K., Fink, L., Failing, K., Bohle, R. M., Kliesch, S., Weidner, W., and Bergmann, M. (2003). Decreased protamine1 transcript levels in testes from infertile men. Mol. Hum. Reprod. 9, 331–336.
| Decreased protamine1 transcript levels in testes from infertile men.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXovVKhsLk%3D&md5=cf44e61527e40ed5db9f3f9e4e1ec857CAS |
Töpfer-Petersen, E., Ekhlasi-Hundrieser, M., Kirchhoff, C., Leeb, T., and Sieme, H. (2005). The role of stallion seminal proteins in fertilization. Anim. Reprod. Sci. 89, 159–170.
| The role of stallion seminal proteins in fertilization.Crossref | GoogleScholarGoogle Scholar |
Voss, J. L., Pickett, B. W., and Squires, E. L. (1981). Stallion spermatozoal morphology and motility and their relationship to fertility. J. Am. Vet. Med. Assoc. 178, 287–289.
| 1:STN:280:DyaL3M7ptFOgtw%3D%3D&md5=8337f708b478d03e324f9fefa399d68aCAS |
Wolgemuth, D. J., Laurion, E., and Lele, K. M. (2002). Regulation of the mitotic and meiotic cell cycles in the male germ line. The Endocrine Society 365, 1653–1662.
| Regulation of the mitotic and meiotic cell cycles in the male germ line.Crossref | GoogleScholarGoogle Scholar |
Wolgemuth, D. J., Manterola, M., and Vasileva, A. (2013). Role of cyclins in controlling progression of mammalian spermatogenesis. Int. J. Dev. Biol. 57, 159–168.
| Role of cyclins in controlling progression of mammalian spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVersbjP&md5=3050e4b613b5ec818f5dc0048a23a344CAS |
Yang, C. C., Lin, Y. S., Hsu, C. C., Wu, S. C., and Lin, E. C. (2009). Identification and sequencing of remnant messenger RNAs found in domestic swine (Sus scrofa) fresh ejaculated spermatozoa. Anim. Reprod. Sci. 113, 143–155.
| Identification and sequencing of remnant messenger RNAs found in domestic swine (Sus scrofa) fresh ejaculated spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltFeltbo%3D&md5=bd553270ba9ca5cb88312716a9208c69CAS |
Zaabal, M. M., and Ahmed, W. M. (2010). Monitoring of gene markers associated with fertility in purebred Arabian stallions. J. Reprod. Infertil. 1, 41–44.
Zhao, Y., Li, Q., Yao, C., Wang, Z., Zhou, Y., Wang, Y., Liu, L., Wang, Y., Wang, L., and Qiao, Z. (2006). Characterization and quantification of mRNA transcripts in ejaculated spermatozoa of fertile men by serial analysis of gene expression. Hum. Reprod. 21, 1583–1590.
| Characterization and quantification of mRNA transcripts in ejaculated spermatozoa of fertile men by serial analysis of gene expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntF2gu70%3D&md5=557635bb3d6b89061876e230242d3477CAS |






