85 Anti-Müllerian hormone in Holstein heifers and reproductive performance after fixed-time embryo transfer
J. C. Lemos Motta A , R. V. Sala B , V. A. Absalón-Medina B A , E. R. Canadas A , B. J. Duran A , C. Hayden A , J. F. Moreno C and A. Garcia-Guerra AA Department of Animal Sciences, The Ohio State University, Columbus, OH, USA
B STgenetics, South Charleston, OH, USA
C STgenetics, Navasota, TX, USA
Reproduction, Fertility and Development 34(2) 279-280 https://doi.org/10.1071/RDv34n2Ab85
Published: 7 December 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS
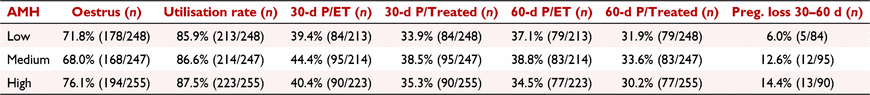
Anti-Müllerian hormone (AMH) has been associated with improved embryo production and fertility outcomes after AI in cattle. Its association with fertility in embryo transfer recipients, however, has not been explored. The aim of this study was to investigate the relationship between circulating AMH and reproductive performance of recipient heifers. Holstein heifers (n = 750), 15.7 ± 0.1 months of age, with moderate body condition score (3.2 ± 0.1; scale 1 to 5) were synchronised using a modified 5-day CO-Synch protocol as follows: Day −8: an intravaginal progesterone (P4) implant (CIDR®, 1.38 g P4, Zoetis) was inserted; Day −3: CIDR was removed followed by administration of prostaglandin F2α analogue (PGF; 500 μg cloprostenol, Parnell); Day 0: administration of gonadotrophin-releasing hormone (GnRH; 100 μg gonadorelin, Parnell). On Day −3, all heifers received an Estrotec® patch, which was checked for evidence of mounting activity on Day 0. On Day 5, heifers were examined by ultrasonography to determine the presence and size of the corpus luteum (CL). On Day 7 ± 1, heifers with a CL received an embryo, and pregnancy was determined using ultrasonography 30 and 60 days after GnRH. Blood samples were collected on Day −8 from all heifers and on Day 5 from a subset (n = 222) of heifers, for AMH (Bovine AMH ELISA, AnshLabs®) and P4 (ImmuChemTM RIA; MP Biomedicals) measurements, respectively. Heifers were classified into low (129.0 ± 2.7 pg mL−1; n = 248), medium (258.1 ± 2.5 pg mL−1; n = 247), and high (554.4 ± 22.8 pg mL−1; n = 255) AMH based on tercile distribution. Data were evaluated using generalised linear mixed models (SAS 9.4; SAS Institute Inc.). Corpus luteum volume tended (P = 0.08) to be larger in high AMH heifers (3669 ± 133 mm3) than in low (3320 ± 112 mm3) and medium (3384 ± 132 mm3) AMH heifers. Conversely, serum P4 was not different (P = 0.59) between heifers with low (2.2 ± 0.1 ng mL−1), medium (2.0 ± 0.1 ng mL−1), or high (2.0 ± 0.1 ng mL−1) AMH. There was no effect of AMH group on the percentage of heifers that expressed oestrus (P = 0.13) nor in the recipient utilisation rate (transferred/treated; P = 0.87; Table 1). Pregnancies per embryo transfer (P/ET) at Days 30 and 60 were not different between AMH groups (P > 0.50; Table 1). Similarly, pregnancy loss between Days 30 and 60 was not different between AMH groups (P = 0.20; Table 1). As a result, pregnancies per treated (P/treated) heifer at 30 and 60 days were not different (P > 0.50) between AMH groups (Table 1). In conclusion, the overall reproductive performance of recipient heifers submitted to fixed-time embryo transfer is not associated with circulating AMH concentration.
|


