249 INTRACYTOPLASMIC SPERM INJECTION OF ELAND (TAUROTRAGUS ORYX) AND BONGO (TRAGELAPHUS EURYCERUS)ANTELOPE OOCYTES
G. Wirtu A B , C. E. Pope A , M. C. Gomez A , A. Cole A , D. L. Paccamonti B and B. L. Dresser A CA Audubon Center for Research of Endangered Species, New Orleans, LA 70131, USA
B Veterinary Clinical Sciences, Louisiana State University School of Veterinary Medicine, Baton Rouge, LA 70803, USA
C Department of Biological Sciences, University of New Orleans, New Orleans, LA 70148, USA
Reproduction, Fertility and Development 19(1) 241-241 https://doi.org/10.1071/RDv19n1Ab249
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
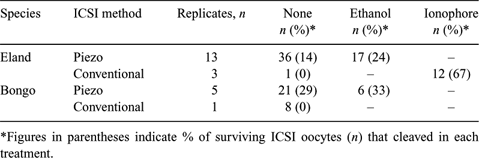
Intracytoplasmic sperm injection (ICSI) is an assisted reproductive technique applicable in cases of limited male gamete availability. Moreover, it bypasses barriers of the oocyte, thus avoiding poorly understood species-specific capacitation events affecting sperm–egg interaction. In the present study, we evaluated the application of conventional and piezo drill-assisted ICSI and whether subsequent chemical activation is required for initiating embryonic development in eland (Taurotragus oryx) and bongo (Tragelaphus eurycerus) oocytes. Oocytes were collected using transvaginal ultrasound-guided follicular aspiration after gonadotropin-induced ovarian stimulation and incubated in modified TCM-199 medium (Gomez et al. 2000 Reprod. Fertil. Dev. 12, 423) containing 10% FBS. After 3 to 24 h, the cumulus cell layers were removed either by repeated mouth-pipetting and/or by using hyaluronidase. Oocytes with an extruded first polar body were used for ICSI and the other oocytes were returned to culture and evaluated every six hours Piezo drill-assisted (Kimura and Yanagimachi 1995 Biol. Reprod. 52, 709) or conventional (Gomez et al.) ICSI were done as described previously using glass pipettes with internal tip diameters of 9–10 µm. We used frozen–thawed or freshly collected spermatozoa that were kept in HEPES-buffered Tyrode's medium (Gomez et al.) for up to 24 h. Four to 6 h after ICSI, 3 activation treatments were examined: (1) none; (2) 7% ethanol, 5 min; or (3) calcium ionophore (5 µM, 5 min) followed by DMAP (2 mM, 4 h). Then we cultured oocytes in a humidified atmosphere of 5% O2, 5% CO2, and 90% N2 at 38.5°C in one of 3 media: SOF, α-MEM, or CR1aa containing essential and nonessential amino acids and FBS. Fifty-three of 70 (76%) eland oocytes survived after piezo-ICSI, and 13 of 16 (81%) survived after conventional ICSI. For bongo oocytes, 27 of 30 (90%) survived piezo-ICSI and all (n = 8) survived after conventional ICSI. Table 1 outlines cleavage data on Day 2. Generally, embryonic development was arrested at about 10 cells. In summary, eland and bongo oocytes can survive both conventional and piezo drill-assisted ICSI. Activation treatments do not appear to be a prerequisite for initiating cleavage after ICSI in eland and bongo antelope oocytes.
|


