203 ASSESSMENT OF OVARIAN RESERVE. IS THERE A ROLE FOR OVARIAN BIOPSY?
R. De Roover A and C. Hanzen BA Veterinary Science Unit, Institute of Life Sciences, Catholic University of Louvain, Louvain-la-Neuve, Belgium
B Institute of Biostatistics and Animal Selection, University of Liege, Liege, Belgium. Email: rderoover@msn.com
Reproduction, Fertility and Development 17(2) 252-252 https://doi.org/10.1071/RDv17n2Ab203
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
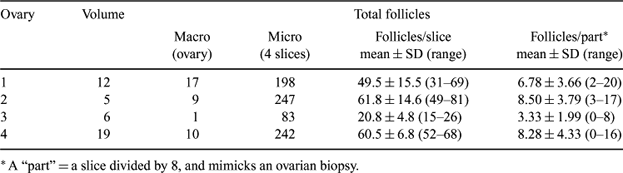
The pool of primordial follicles in the ovary or ovarian reserve is a major factor in human fertility potential. In bovine medicine as well, this ovarian reserve has been linked to the results of superovulation procedures (Cushman et al. 1999 Biol. Reprod. 60, 349–354). These authors suggested a biopsy to assess the level of this reserve. Whether the biopsy(ies) is(are) a true reflection of the follicular distribution in the ovarian cortex, is (to the best of our knowledge) a factor never investigated until now in bovine medicine. In human medicine, this procedure has been critically examined for that particular use and found not to be suited (Lass et al. 2004 Hum. Reprod. 19, 467–469). Indeed, randomized or “blind” sampling of one biopsy is adequate only if follicles are evenly spread in the ovarian cortex; in any case they are not deeper than a few mm from the surface. Moreover, the quantitative counting of follicles does not provide any information about the quality of the oocytes embedded in them. Taking a biopsy of a bovine ovary in a minimally invasive way is technically feasible (Aerts 2004 Reprod. Fertil. Dev. 16, 229–230). Therefore, the aim of this study was to examine the natural distribution of primordial follicles in the ovarian cortex of bovine ovaries. Slaugtherhouse ovaries were collected at random. The volume (mL) was measured and the macroscopically visible follicles were counted. Then the ovaries were cut in slices of 5Âμm, and every 8th (8 × 5 = 40 μm interval) slice was subjected to fixation in formalin and hematoxylin-eosin staining. Before counting of the primordial follicles, the ovarian cortex was subdivided into 8 equal parts. These “parts” were supposed to mimick a (single) ovarian biopsy. The 8 parts of a slice represent here multiple biopsies. For each of these parts, the number of primordial follicles was counted; only follicles with a visible oocyte were included. The results of the parts containing the ligament of the ovary were excluded. Results are shown in Table 1. The results show that the distribution of primordial follicles between small parts of the bovine ovarian tissue was extremely uneven. A large variation was observed between samples obtained from the same ovary. Moreover, an extrapolation of follicle numbers found in biopsies to entire ovaries were hampered by the uneven size and morphology of these ovaries. Therefore, we conclude that the use of single biopsies of ovarian cortex for a quantitative evaluation of the ovarian reserve has limited value; an empty cortex or a cortex with very few follicles might be just incidental and meaningless. Even the use of multiple biopsies, although less variable, does not solve the problem of extrapolation of these data to entire ovaries.
|


