161 PREGNANCY RATES OBTAINED AFTER EMBRYO TRANSFER AT FIXED TIME OF IN VIVO-, IVF- AND CLONED-DERIVED EMBRYOS
J. Lagioia A , M. Panarace A , M. Marfil A , M. Basualdo A , J. Gutierrez A , M. Révora A and M. Medina AACentro de Investigaciones Reproductivas Perez Companc, Goyaike, Ea. San Joaquín, Argentina. Email: jlagioia@goyaike.com.ar
Reproduction, Fertility and Development 17(2) 231-232 https://doi.org/10.1071/RDv17n2Ab161
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
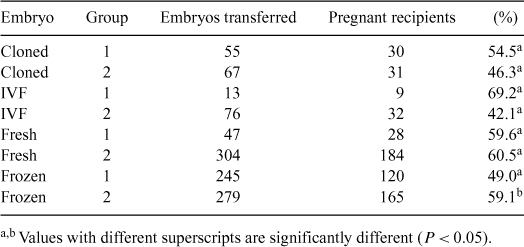
The most important factor in bovine embryo transfer programs is the low efficiency in the utilization of the recipients; this low efficiency is associated with low response to synchronization protocols and failures in estrus detection. It has been shown that cows transferred at fixed time with in vivo-derived embryos resulted in high rates of recipients selected for transfer and high overall pregnancy rates (recipients pregnant/recipients treated) (Tribulo et al. 2002 Theriogenology 57, 563). An experiment was designed to evaluate the pregnancy rate in recipients transferred with in vivo (fresh and frozen), IVF, and cloned-derived embryos without estrus detection. A total of 1555 non-lactating Bos Taurus crossbred beef cows was divided into two groups. Cows from group 1 (n = 421) were synchronized with a progesterone intravaginal releasing device (1 g P4; DIB, Syntex®, Buenos Aires, Argentina) plus 2 mg of estradiol benzoate (EB) i.m. (Syntex®) on Day 0. On Day 5, they received 400 IU of eCG (Novormon 5000, Syntex®) i.m. and 150 μg of D-Cloprostenol (PGF2α) (Bioprost-D, Biotay®, Buenos Aires, Argentina). The DIB devices were removed on Day 8 and on Day 9, 1 mg of EB was injected. Day 10 was arbitrarily considered as the day of estrus. Cows from group 2 (n = 1134) received 2 doses of PGF2α 14 days apart and were checked for heat during 5 days after the second PGF2α dose. Cows of both groups were examined 7 days after estrus by ultrasonography (Pie Medical Scanner 200®) and those with a corpus luteum >10 mm of diameter were transferred nonsurgically with in vivo (fresh and frozen), IVF, and cloned-derived embryos. In group 1, 360 cows were transferred, and in group 2, 726 cows were transferred (Table 1). Pregnancy was diagnosed 23 days later by ultrasonography (Pie Medical Scanner 200®). The pregnancy rates were compared statistically between groups 1 and 2 by analysis of variance (Infostat, LSD Fisher). There was no significant statistic difference (P > 0.05) between pregnancy rate in group 1 and 2 with in vivo (fresh), IVF, and cloned-derived embryos. However, pregnancy rate of frozen in vivo-derived embryos was lower in group 1 than in group 2 (P < 0.05). Results showed that treatment using DIB combined with EB, PGF2α, and eCG associated with embryo transfer without estrus detection (group 1) had no difference in pregnancy rate when compared with the treatment where synchronization with PGF2α and heat detection were used (group 2). Another important advantage is the use the group 1 treatment for increasing the flexibility and efficiency in the management of the recipients of in vivo, IVF, and cloned-derived embryo transfer programs.
|


