248 Evaluation of two cryoprotectants for the cryopreservation of bovine embryos produced in vitro
S. Rodríguez-Maldonado A , H. Álvarez-Gallardo B , A. Velázquez-Roque C , M. Kjelland D E , F. Villaseñor-González F and S. Romo AA Facultad de Estudios Superiores Cuautitlán, UNAM, Cuautitlán, México, México
B Centro Nacional de Recursos Genéticos, INIFAP, Tepatitlán, Jalisco, México
C Private practice, H&A Biotecnologías en Reproducción Animal, Tepatitlán, Jalisco, México
D Conservation, Genetics & Biotech, LLC, Valley City, North Dakota, USA
E Mayville State University, Mayville, North Dakota, USA
F Campo Experimental Centro Altos de Jalisco, INIFAP, Tepatitlán, Jalisco, México
Reproduction, Fertility and Development 35(2) 253-254 https://doi.org/10.1071/RDv35n2Ab248
Published: 5 December 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS
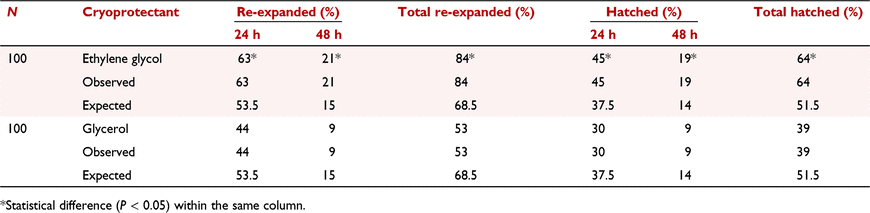
In vitro embryo production (IVP) has had a great impact for the genetic improvement in cattle, and most embryos produced worldwide are generated by this technology. The transfer of cryopreserved embryos is very important for commercialisation and for disseminating valuable genetics. A more efficient IVP protocol is still being sought for bovine embryos. The objective of this research was to evaluate slow-freezing cryotolerance of IVP bovine embryos using ethylene glycol (EG) and glycerol (GLY) as cryoprotectants. The research was carried out in the reproduction laboratory at Palominos Ranch (Jalisco, México). The IVP was performed with a continuous in vitro culture system based on IVF-Bioscience™ media, using ovaries (cross-bred beef cattle) collected in a slaughterhouse and fertilised with the same bull (Angus) in five replicates. On Day 7 of in vitro culture, expanded blastocyst stage embryos (n = 200; 100 for EG, 100 for GLY) Quality 1 and 2 (according to the fourth edition of the International Embryo Transfer Society Manual) were subjected to a controlled-rate freezing curve after equilibration for 8 to 10 min in freezing medium with EG (Ethylene Glycol Freeze Plus Vigro™, Vetoquinol USA Inc.) and GLY (Glycerol Freeze plus Vigro™, Vetoquinol USA Inc.), starting in −6°C (seeding) and decreasing 0.5°C min−1 and 0.3°C min−1 for EG and GLY respectively, and ending at −32°C and then plunging directly into liquid nitrogen. The embryos frozen in EG were thawed 10 s in air and then 20 s in water at 30°C and cultured for 48 h at 38.5°C, 5% CO2, 5% O2, and 90% N2 at 100% humidity. The embryos frozen in GLY were thawed 10 s in air and then 20 s in water at 30°C and were rehydrated (One Step Thaw Plus Vigro™, Vetoquinol USA Inc.) for 15 min; at the end of this period, embryos were cultured for 48 h at 38.5°C, 5% CO2, 5% O2, and 90% N2 at 100% humidity. Re-expansion and hatching rates were evaluated at 24 and 48 h. The statistical analysis was carried out using the chi-squared procedure on the Jamovi software (version 1.2; The Jamovi project). Re-expansion rates at 24 h were 63% and 44%, and at 48 h, were 21% and 9%, respectively, for EG and GLY. The percentages of hatching were 45% and 30% at 24 h and 19% and 9% at 48 h, respectively, for EG and GLY. There were significant differences (P < 0.05) for all the variables analysed, being higher for EG concerning to GLY, perhaps due to the toxicity of GLY on the IVP embryos, because of the increased freezing time compared to EG. In conclusion, under the conditions of this research, slow freezing with EG had better survival post-thawing for IVP bovine embryos compared to slow freezing with GLY (Table 1).
|


