59 The positive effect of the use of recombinant equine chorionic gonadotrophin for ovarian stimulation for in vitro embryo production in buffaloes
A. Bandeo B A , J. L. Konrad B A , J. Berdugo C , P. Ibaña D , N. Vallejos A , P. Maldonado Vargas A and G. Crudeli EA Catedra de Teriogenología, Facultad de Ciencias Veterinarias, Universidad Nacional del Nordeste, Corrientes, Argentina
B Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
C Gentechbiosciences, Envigado, Envigado, Antioquia, Colombia
D In Vitro Corrientes (IVC), Corrientes, Argentina
E Universidad Nacional del Chaco Austral (UNCAUS), Saenz Peña, Chaco., Argentina
Reproduction, Fertility and Development 34(2) 265-265 https://doi.org/10.1071/RDv34n2Ab59
Published: 7 December 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the IETS
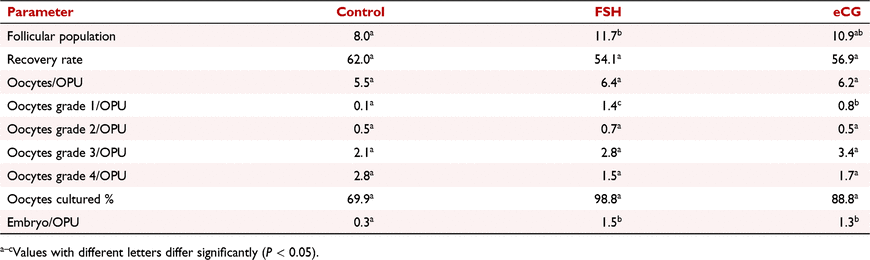
Buffalo in vitro embryo production (IVP) needs to be improved. The objective of this project was to compare the use of FSH or equine chorionic gonadotrphin (eCG) for stimulating follicle growth before oocyte aspirations (ovum pick-up, OPU) and in vitro embryo production in buffalo (Bubalus bubalis). This work was performed during the reproductive season of 2020–2021 in a Mediterranean and Murrah buffalo herd located in Corrientes Province, Argentina. Two different protocols to stimulate follicular development were used after follicle ablation of the ovary. Treatment 1 (n = 10): Day 0: insertion of an intravaginal device (IVD) + 2 mg of oestradiol benzoate i.m. (EB), Day 4: start FSH (Folltropin-V®; Vetoquinol) injections with 160 mg distributed in four decreasing doses 12 h apart (50 mg, 50 mg, 30 mg, 30 mg, respectively) IM; 36 h after the last FSH injection, OPU were performed. Treatment 2 (n = 10): Day 0: IVD insertion + EB iIM; Day 4: administration of 1050 IU of recombinant eCG (Foli-rec®; Zoovet) IM; OPU 72 h later. Control (n = 23) was no hormonal treatment before OPU. Before OPU, antral follicle counts were performed. Oocytes obtained were classified according to the layers of granulosa cells and the appearance of the cytoplasm (grades 1, 2, 3, or 4; Stringfellow and Givens, 2010; Manual of the International Embryo Transfer Society, Champaign, IL). Embryo production was performed according to Konrad et al. (2017 Anim. Reprod. Sci. 183, 39-45). Total oocytes entered fertilization, and only quality 1 or exellent embryos (quality 1 excellent, 2 regular, 3 bad, or 4 degenerated; Palma, 2008 Biotecnología de la Reproducción, 2nd ed., p. 699) were vitrified. Data were analysed with ANOVA with P < 0.05 considered significant. Results are recorded in Table 1. Hormonal treatments produced more follicles and grade 1 oocytes than control. There were no statistical differences among grade 2, 3, and 4 oocytes and culture percentage. eCG and FSH produced more embryos per OPU session than the control. These data support evidence from other authors that hormonal stimulation increases the number of follicles compared with nonstimulated controls and helps to produce better quality oocytes. To the best of our knowledge, this is the first time that recombinant eCG has been used to stimulate follicular development in buffaloes. This is valuable information because of the low cost of eCG relative to FSH. Further studies are necessary to confirm these findings and determine optimal doses and coasting periods for maximising IVP potential after OPU in buffalo.
|


