181 XENOTRANSPLANTATION OF BOVINE OVARIAN CORTEX IN SEVERE COMBINED IMMUNODEFICIENT MICE TO STUDY PRE-ANTRAL FOLLICULAR DEVELOPMENT: DETERMINATION OF THE OPTIMAL GRAFT SITE
A. Langbeen A , E. P. A. Jorssen A , S. Andries A , E. Merckx A , J. L. M. R. Leroy A and P. E. J. Bols AUniversity of Antwerp, Wilrijk, Antwerp, Belgium
Reproduction, Fertility and Development 25(1) 239-240 https://doi.org/10.1071/RDv25n1Ab181
Published: 4 December 2012
Abstract
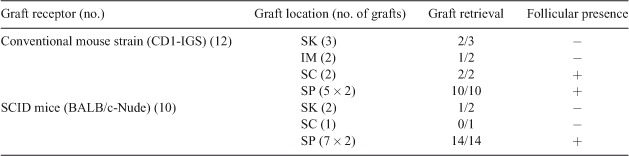
Nowadays, a lot of attention is given to female reproductive preservation strategies. However, opportunities to study the dynamics of pre-antral folliculogenesis in vitro are not readily available because routine procedures for culture of (ruminant) isolated follicles are still lacking. One of the current techniques to study follicular dynamics and activation is xenografting bovine ovarian cortex into immune-deficient mice. Several transplantation sites have been described (Bols et al. 2010 Theriogenology 73, 740–747), such as subcutaneously (SC), underneath the kidney capsule (SK), intramuscularly (IM), and underneath the peritoneum (SP). In the present study, our objective was to determine the optimal host type and graft location in order to maximize the success of graft retrieval and follicular development. In total, 22 mice [12 conventional, 10 severe combined immunodeficient (SCID)] were used as graft recipients. All mice were anesthetized with an intraperitoneal injection of ketamine and xylazine and subsequently sterilized. Small pieces (maximum dimension of 9 mm3) of adult bovine ovarian cortex, retrieved from slaughterhouse ovaries, were then grafted at 4 different sites: SC grafts were localised at the left-hand side of the neck of the host, IM at the left hamstring (between the semimembranosus and semitendinosus muscle), SK under the left kidney capsule or SP, namely on the left and right-hand side, retroperitoneal. Blood vessels were macroscopically localised and stimulated by curettage before the cortex piece was grafted. Fourteen days later, mice were killed and the graft was localised and retrieved (if possible), after which the presence of follicles was assessed by visualisation following hematoxilin-eosin staining of histological slides. Data (Table 1) show that graft retrieval rates were highest when cortex fragments were grafted underneath the peritoneum (SP site). Although the extent of follicular presence and quality assessment of the detected follicles surely require additional experiments, our data do not support a difference between SCID or a conventional mouse strain as an optimal host type when it comes to graft retrieval rates and the determination of follicular activity when grafts are left in place for a limited period of 14 days.
|


