Transcriptomic analysis of luteal tissue supports the earlier onset of luteolysis in heifers with diminished ovarian reserve
Martim Kaps A C , Alexandria P. Snider A , Lacey K. Quail B D , Jeremy R. Miles A , George A. Perry
A , George A. Perry  B and Robert A. Cushman
B and Robert A. Cushman  A *
A *
A
B
C Present address:
D Present address:
Abstract
Antral follicle count (AFC) is reflective of ovarian reserve and linked to reproductive performance in mammalian females. We previously demonstrated earlier upregulation of endometrial oxytocin receptor transcription in heifers with low AFC, indicating an earlier onset of luteolysis.
We aimed to support the earlier onset of luteolysis in non-pregnant heifers with a low number of antral ovarian follicles (Open Low AFC) and hypothesized a reduced abundance of luteinizing hormone/choriogonadotropin receptor (LH/CG-R) and increased abundance of thrombospondin-1 (THBS1) in luteal tissue of those heifers. We further investigated if classical interferon stimulated genes (ISGs) are already upregulated in response to conceptus-derived interferon tau.
For 4 years, 10 heifers with the highest (High AFC) and 10 heifers with the lowest AFC (Low AFC) of the population (n = 120/year) were selected, synchronized and inseminated. On day 15 or 16 after insemination, reproductive tracts were collected, and pregnancy status was determined. Corpora lutea were isolated, weighed and underwent transcriptomic analysis.
Transcript abundance of LH/CG-R was lower in Open Low AFC heifers compared to all other subgroups. Transcript abundance of THBS1 was upregulated in Open vs Pregnant heifers and showed greater abundance in Low vs High AFC heifers. Luteal weights and abundance of ISGs did not differ between heifers of differing AFC groups or pregnancy status.
The patterns of luteal LH/CG-R and THBS1 abundance support the concept of earlier onset of luteolysis in Open heifers with diminished AFC.
This may result in a shorter window for maternal recognition of pregnancy and contribute to inferior reproductive performance.
Keywords: antral follicle count, corpus luteum, interferon stimulated genes, LH/CG receptor, luteolysis, maternal recognition of pregnancy, ovarian reserve, oxytocin receptor, thrombospondin 1, transcriptomic analysis.
Introduction
The antral follicle count (AFC) is well-established as a reliable measure of the ovarian reserve in cattle (Cushman et al. 1999, 2000; Ireland et al. 2008) and has been suggested for the selection of heifers with greater reproductive longevity in cow–calf operation systems (Cushman et al. 2013, 2014; McNeel and Cushman 2015). Although ovarian reserve was thought to be a major determinant of bovine fertility about a decade ago (Ireland et al. 2011), the relationship between AFC and fertility remains more controversial and complex than initially anticipated (Juengel et al. 2021; Cushman et al. 2023; Mossa and Evans 2023). Nonetheless, low AFC has repeatedly been associated with characteristics of suboptimal fertility, such as reduced progesterone concentrations (Jimenez-Krassel et al. 2009; Martinez et al. 2016; Santa Cruz et al. 2018), reduced protein and glucose concentrations in uterine luminal fluid (McNeel et al. 2017; Snider et al. 2022) and increased gonadotrophin concentrations (Singh et al. 2004; Burns et al. 2005).
In addition to the characteristics mentioned above, we recently investigated conceptus development and embryo–maternal signaling in High vs Low AFC heifers and demonstrated an earlier upregulation of oxytocin receptor (OXTR) gene transcription in the endometrium of non-pregnant (Open) Low AFC compared to Open High AFC heifers (Kaps et al. 2024). Onset of OXTR expression during diestrus is the prerequisite necessary for the induction of luteolysis and occurs between day 14 and 15 of the estrous cycle, with peak expression on day 18 (Flint et al. 1994; Mann and Lamming 1994; Robinson et al. 2001). The earlier increase in OXTR transcript abundance that was observed in Open Low AFC heifers thus indicates that the prerequisites for the onset of luteolysis are given earlier in Low AFC heifers compared to their High AFC counterparts.
Luteolysis is a dynamic process driven by pulsatile secretion of prostaglandin F2α (PGF2α) from the endometrium (McCracken et al. 1981). During the luteal phase, progesterone is secreted in an episodic pattern. The onset of luteolysis has been defined as the hour when peak progesterone concentration in consecutive progesterone episodes start to decrease (Ginther and Beg 2012; Shrestha 2021). This transition from pre-luteolytic phase is followed by functional luteolysis with a progressive decline of circulating progesterone concentration, and structural luteolysis, characterized by tissue degradation and remodeling (Pate 1994). The drop of progesterone concentration below 1 ng/mL is used to define the end of luteolysis (Mann and Lamming 2006). To clarify, if luteolysis is already ongoing in an individual animal, repeated blood sample collections at relatively short intervals are necessary to retrospectively determine the transition to initiation of luteolysis (Ginther and Beg 2012). As the main aim of the above-mentioned study was to investigate differences in conceptus development around maternal recognition, continuous blood sampling was not applicable. Further, the general variability in progesterone concentration and the intrinsic differences in progesterone production among heifers differing in AFC (Jimenez-Krassel et al. 2009; Martinez et al. 2016; Santa Cruz et al. 2018) would not allow a secure classification of luteolytic and non-luteolytic status using cut-off values of serum progesterone. We, therefore, aimed to identify endpoints associated with the onset of luteolysis other than progesterone concentration to support the hypothesis that luteolysis is initiated earlier in Open Low AFC compared to Open High AFC animals.
Binding capacity of luteinizing hormone/choriogonadotropin receptor (LH/CG-R) has been shown to decline during luteolysis (Spicer et al. 1981) and transcript abundance of LH/CG-R has been shown to decrease early during the process of luteolysis (Atli et al. 2012). Further, loss of luteal sensitivity to luteinizing hormone (LH) pulses has been postulated to mark the transition point from the pre-luteolytic phase, where progesterone rebounds can still occur, to the luteolytic phase with a continuous decline of progesterone in cattle (Ginther et al. 2011; Shrestha 2021). Downregulation of LH/CG-R has been suggested as a potential mechanism associated with transition from pre-luteolytic to luteolytic phase (Shrestha 2021). Thrombospondin-1 (THBS1) and pentraxin-3 (PTX3) were upregulated as early as 4 h after administration of PGF2α in responsive day 11 bovine corpora lutea (Zalman et al. 2012) and are, thus, also associated with early signs of initiated luteolysis. Therefore, we analyzed transcript abundance of LH/CG-R, THBS1 and PTX3 in the luteal tissue of heifers differing in AFC and pregnancy status to investigate if earlier endometrial OXTR upregulation coincides with early signs of luteolysis in the corpus luteum (CL) of Open Low AFC heifers but not in Open High AFC heifers.
It is well established that conceptus derived interferon tau (IFNτ) exerts effects on the endometrium, leading to inhibition of the secretion of luteolytic pulses of PGF2α from the endometrium (Hansen et al. 2017). Further, IFNτ stimulates the upregulation of classical interferon stimulated genes (ISGs; interferon stimulated gene 15 (ISG15) and MX dynamin like GTPase 1 (MX1), among others) in the endometrium, which are involved in embryo–maternal signaling and are critical for establishment of pregnancy (Hansen et al. 2017). Upregulation of ISGs was also described in extrauterine tissues such as peripheral blood leukocytes (PBL) and in the CL (Yankey et al. 2001; Han et al. 2006; Oliveira et al. 2008; Guzeloglu et al. 2024). Further, local effects of IFNτ in the CL of early pregnancy have been reported via upregulation of classical ISGs and the stimulation of antiluteolytic pathways, stabilization of steroidogenesis and promoting resistance to luteolytic pulses of PGF2α (Basavaraja et al. 2017; Sakumoto et al. 2020; Guzeloglu et al. 2024). We, therefore, also investigated luteal transcript abundance of ISGs (ISG15, MX1) to identify potential differences in their abundance in response to IFNτ in heifers with high or low AFC. Differences in the magnitude of luteal ISG response might be indicative of higher probability of luteal maintenance and could be linked to higher probability of early conception in High AFC heifers.
Peroxisome proliferator-activated receptor delta (PPARD) has been shown to be upregulated in the CL of early pregnancy and to be associated with improved steroidogenic capacity of luteal cells in vitro (Sakumoto et al. 2020). Further Galectin-9 (encoding gene: LGALS9) has been identified in an RNA sequencing study as a candidate gene associated with reduced sensitivity of the CL to PGF2α (Pate and Hughes 2023). We, therefore, analyzed transcription patterns of those two candidate genes to investigate potential differences associated with luteal sensitivity to PGF2α and luteal steroidogenic capacity, which are independent of interferon in heifers differing in pregnancy status and AFC.
The overall aim was to investigate the abundance of selected genes involved in regulation of luteolysis and luteal maintenance around the window of maternal recognition in heifers differing in pregnancy status and AFC. We hypothesized that (1) transcript abundance of LH/CG-R will be decreased while THBS1 and PTX3 abundance will be increased in Open Low AFC heifers, where the prerequisites for the initiation of luteolysis are given; (2) abundance of ISG15 and MX1 in luteal tissue and PBL will be greater in pregnant heifers in consequence of the presence of IFNτ, with a more pronounced increase in High versus Low AFC heifers; and (3) abundance of LGALS9 and PPARD will be greater in High AFC heifers than Low AFC heifers.
Materials and methods
This manuscript contains results and information generated from additional analyzes of the same data set presented previously (Kaps et al. 2024).
Heifer management
All animal procedures were approved by the U.S. Meat Animal Research Center (USMARC) Institutional Animal Care and Use Committee (Experimental Outline # 94.1). All animal procedures were described in detail previously (Kaps et al. 2024). Briefly, for a period of 4 years, 120 heifers per year underwent transrectal ultrasonographic examination for determination of AFC. From those 120 heifers each year, the 10 cyclic heifers with the greatest AFC (High AFC, n = 10/year, total n = 40) and the 10 cyclic heifers with the lowest AFC (Low AFC, n = 10/year, total n = 40) were selected to be estrous cycle synchronized and artificially inseminated each year. After the 4 years, a total of 80 heifers (n = 40 High AFC and n = 40 Low AFC) had undergone the study protocol. Each year, the selected heifers were synchronized with a 7-day CO-Synch protocol and artificially inseminated 48 h after administration of a PGF2α analog (25 mg, Lutalyse®, Zoetis, Florham Park, NJ, USA) with a standard artificial insemination (AI) dose from a single bull of proven fertility. On day 15 or 16 after AI, selected heifers were sent to the USMARC abattoir, blood samples were collected at exsanguination and reproductive tracts were collected and returned to the laboratory within 15 min. Heifers harvested on day 15 and 16 after AI were equally distributed between High and Low AFC group.
Reproductive assessment and sample collection
Upon arrival of the reproductive tract at the laboratory, the uterine lumen was flushed as previously described with slight modifications (Northrop-Albrecht et al. 2022), for recovery of a conceptus and determination of pregnancy status. To further support the determination of pregnancy status, ISG15 transcript abundance in endometrial tissue was analyzed (data not shown). The number of CL on the ovaries of an individual animal was assessed. Each CL was dissected, weighed and tissue was snap frozen individually and stored at −80°C until further analysis. The number of follicles on the surface of the two ovaries of an individual animals was counted and a cross-section of the ovary that did not contain the active CL was fixed in at least a 10-fold volume of neutral buffered formalin (10%) for subsequent histological evaluation of pre-antral follicle numbers. For a subset of heifers (n = 48, from the second year of the study onwards), blood samples were collected at exsanguination for RNA preservation (Tempus™ Blood RNA Tube, Thermo Fisher Scientific, Waltham, MA) and stored at −20°C until extraction and further analysis.
RNA extraction
Total cellular RNA was extracted from 15 mg of luteal tissue using the Qiagen RNAeasy mini kit (Qiagen Inc., Valencia, CA, USA), with on-column DNAse treatment following the manufacturer’s protocol. Total cellular RNA was purified from Tempus stabilized blood samples using the Tempus Spin RNA isolation kit according to the manufacturer’s protocol. Quantity of RNA was assessed by Nanodrop 8000 spectrophotometer (Thermo Fisher Scientific, Thermo Fisher Scientific, Waltham, MA). RNA integrity was assessed using the Agilent 2200 screentape assay (Applied Biosystems, Carlsbad, CA, USA). Mean RNA integrity number was 9.3 ± 0.5 s.d. for all luteal and 6.8 ± 2.2 s.d. for all blood samples. Following extractions, a total of 1 μg RNA per sample was reverse transcribed to cDNA using the iScript™ Reverse Transcription kit (Bio-Rad Laboratories, Hercules, CA, USA) and cDNA was diluted to a final working concentration of 2.5 ng/μL.
Quantitative real-time PCR (qPCR)
Quantitative real-time PCR assays were performed in duplicates, using previously validated primer sets for ISG15, MX1, LH/CG-R, THBS1, PTX3, PPARD and LGALS9, listed in Table 1. Two microliters of RNA-equivalent cDNA (5 ng total) were added to a 20 μL reaction containing 10 μL of iTaq™ Universal SYBR Green Supermix (BioRad Laboratories, Hercules, CA, USA), 6 μL of nuclease-free water and 1 μL each of the appropriate forward and reverse primers (10 μM). Analysis was performed on a CFX96 Realtime System (BioRad Laboratories, Hercules, CA, USA). Conditions for PCR included 5 min of denaturation at 95°C followed by amplification 95°C for 15 s, annealing for 15 s for 40 cycles using the annealing temperatures provided in Table 1. A single melting curve peak was confirmed for all primer pairs. The transcript abundance of potential reference genes GAPDH, β-Actin, YWHAZ and SDHA was analyzed. Gene stability was calculated via BestKeeper© Software and was used in conjunction with the stability in the statistical model (see Statistical analysis) as criterion for the selection of the reference gene, confirming the validity of SDHA as an endogenous reference gene for this study. Nuclease-free water was used as a negative control to exclude background contamination. Intra-assay coefficients of variation for SDHA and the target genes were ≤20%. Relative transcript abundance was determined following the 2−ΔΔCT method (Livak and Schmittgen 2001). The sample with the lowest normalized amount of expression was used as the calibrator (set to 1) and remaining samples were displayed as fold changes to the calibrator.
| Gene | Primer | Sequence | Annealing temperature | Reference | Gene accession number | |
|---|---|---|---|---|---|---|
| ISG15 | Forward | 5′-GGTATCCGAGCTGAAGCAGTT-3′ | 62°C | Han et al. (2006) | NM_174366.1 | |
| Reverse | 5′-ACCTCCCTGCTGTCAAGGT-3′ | |||||
| MX1 | Forward | 5′-GAGGTGGACCCCCAAGGA-3′ | 60°C | Sakumoto et al. (2020) | NM_173940 | |
| Reverse | 5′-CCACCAGATCGGGCTTTGT-3′ | |||||
| LH/CG-R | Forward | 5′-TGACCATGGCCCGTCTAAAA-3′ | 60°C | Jimenez-Krassel et al. (2009) | NM_174381 | |
| Reverse | 5′-TACTACCCAAAGCAATTTATAGATTCAATG-3′ | |||||
| PTX3 | Forward | 5′-TCTTTATTTATCTTGGCAAAATACTGAGTAA-3′ | 60°C | Chitko-McKown et al. (2004) | NM_002852 | |
| Reverse | 5′-AAGCACCATGGCATAAAATCT-AGTAA-3′ | |||||
| THBS1 | Forward | 5′-ATCATGGCTGACTCAGGAC-3′ | 60°C | Farberov and Meidan (2016) | NM_174196 | |
| Reverse | 5′-TAAGCCCATGGTTCCAGAA-3′ | |||||
| LGALS9 | Forward | 5′-TCAGCTTCCAGCCTCCAGGG-3′ | 60°C | Chaney et al. (2020) | NM_001015570.3 | |
| Reverse | 5′-TCCAGGGGCGCTGTGTATGGT-3′ | |||||
| PPARD | Forward | 5′-ACTCACTTCCTTCCAGCAGC-3′ | 60°C | Sakumoto et al. (2020) | NM_001083636 | |
| Reverse | 5′-TATTGAGGCTGCCACACGAG-3′ | |||||
| SDHA | Forward | 5′-ACCTGATGCTTTGTGCTCTG-3′ | 56°C | Hwang et al. (2021) | NM_174178.2 | |
| Reverse | 5′-TCGTACTCGTCAACCCTCTC-3′ |
Progesterone analysis
Blood samples were collected at exsanguination into clot-activator containing tubes. After clotting, tubes were centrifuged at 1000g for 30 min and serum was stored at −20°C until further analysis. For analysis of progesterone concentrations, a previously described and validated radioimmunoassay (Engel et al. 2008) was used. Intra- and inter-assay CVs for progesterone were 4.0% and 4.4%, respectively, and assay sensitivity was 0.4 pg/mL.
Statistical analysis
Data for ultrasonographic follicle count determination were analyzed using the MIXED procedure for ANOVA in SAS (SAS Inst. Inc., Cary, NC, USA) with AFC group as a fixed effect. Data of CL weight and abundance of the target genes (LH/CG-R, THBS1, PTX3, ISG15, MX1, PPARD and LGALS9) were analyzed using the MIXED procedure for ANOVA in SAS with AFC group (Low or High), pregnancy status (Open or Pregnant), and their interaction as fixed effects. The linear effect of day (15 or 16) was included as a co-variate. Where applicable, Tukey’s test was applied for post-hoc analysis. The number of animals with serum progesterone less than 3 ng/mL and pregnancy rate were analyzed by Fisher’s exact test. Correlations were analyzed using the CORR procedure in SAS. Data are presented as least squares mean ± s.e.m. A P-value ≤0.05 was considered significant. Animals with two CL present on the excised ovaries were classified as double ovulators (n = 4) and were excluded. Heifers without any CL present on the excised ovary were considered to have failed to ovulate in response to the synchronization protocol (n = 9) and were also excluded. The CL tissue sample of one animal was not available for RNA extraction and analysis, this heifer was excluded from all analysis. Pregnancy was defined by presence of a conceptus recovered by uterine flushing. In one animal, no conceptus was detected in the uterine flush, whereas relative ISG15 transcript abundance was increased more than 300-fold. It cannot be completely excluded that a conceptus was present but not detected. As determination of pregnancy status for this animal was not possible, it was excluded from all analyzes. A second animal had a conceptus-like structure that best resembled a degenerated, non-viable conceptus from which no RNA could be extracted and that additionally lacked increased endometrial abundance of ISG15. This heifer was defined as non-pregnant. The final analysis included a total of 65 of the initial 80 heifers.
Results
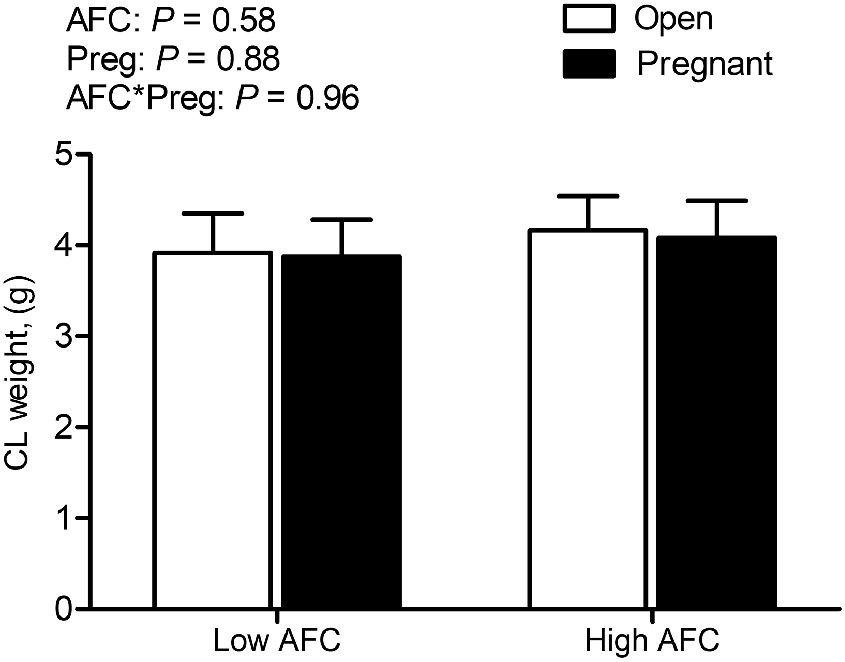
Among the 65 included heifers, 30 heifers were in the Low AFC group and 35 heifers were in the High AFC group, according to the ultrasonographic determination of AFC. The average number of antral follicles detectable by ultrasonography was 14.4 ± 1.1 follicles in Low AFC heifers and 33.5 5 ± 1.2 follicles in High AFC heifers (P < 0.0001). In accordance with ultrasonography, the number of follicles present on the ovarian surface after slaughter and the number of primordial follicles per histological section differed due to AFC group (data previously presented, Kaps et al. 2024). Pregnancy rate did not differ between AFC groups (Table 2). The overall number of heifers with serum progesterone concentration less than 3 ng/mL was 8 out of 65 (12.3%), with five (16.6%) heifers among the 30 Low AFC heifers and three (8.6%) among the 35 High AFC heifers (Table 2). The interaction of AFC group and pregnancy status was not significant for total weight of the CL and there was no difference between AFC groups or heifers differing in pregnancy status (Fig. 1).
| AFC group | Progesterone <3 ng/mL | ||||||
|---|---|---|---|---|---|---|---|
| Low AFC | High AFC | Total | Low AFC | High AFC | Total | ||
| Open | 46.7% 14/30 | 54.3% 19/35 | 50.8% 33/65 | 28.6% 4/14 | 15.8% 3/19 | 21.2% 7/33 | |
| Pregnant | 53.3% 16/30 | 45.7% 16/35 | 49.2% 32/65 | 6.3% 1/16 | 0% 0/16 | 3.1% 1/32 | |
| n = 30 | n = 35 | n.s. | 16.6% 5/30 | 8.6% 3/35 | n.s. | ||
Distribution did not differ between groups for both variables (Fisher’s exact test).
Least square means for weight of the CL for Open and Pregnant heifers in High and Low AFC groups, respectively. The P-values for the main effect of group, pregnancy status and their interaction are provided in the figure.
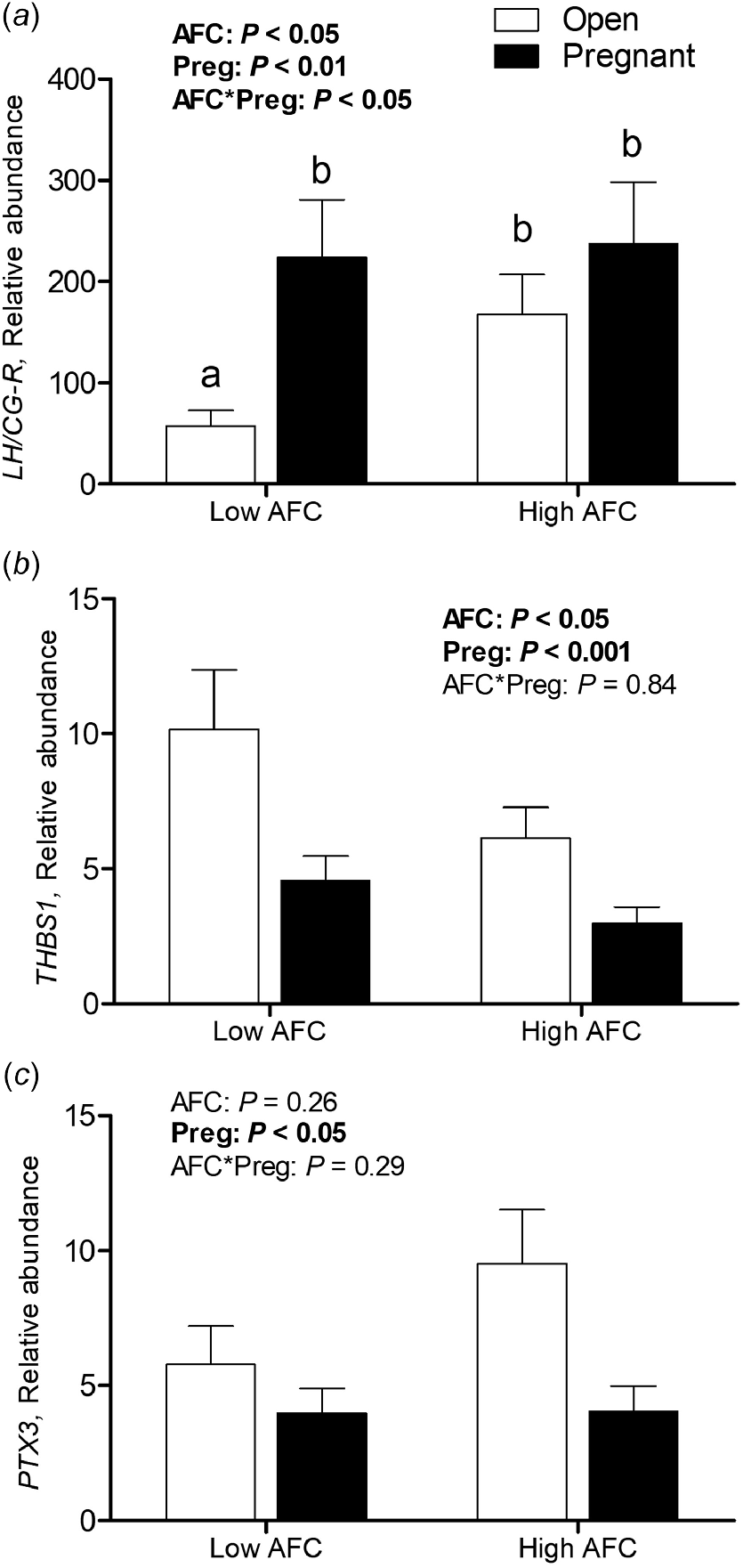
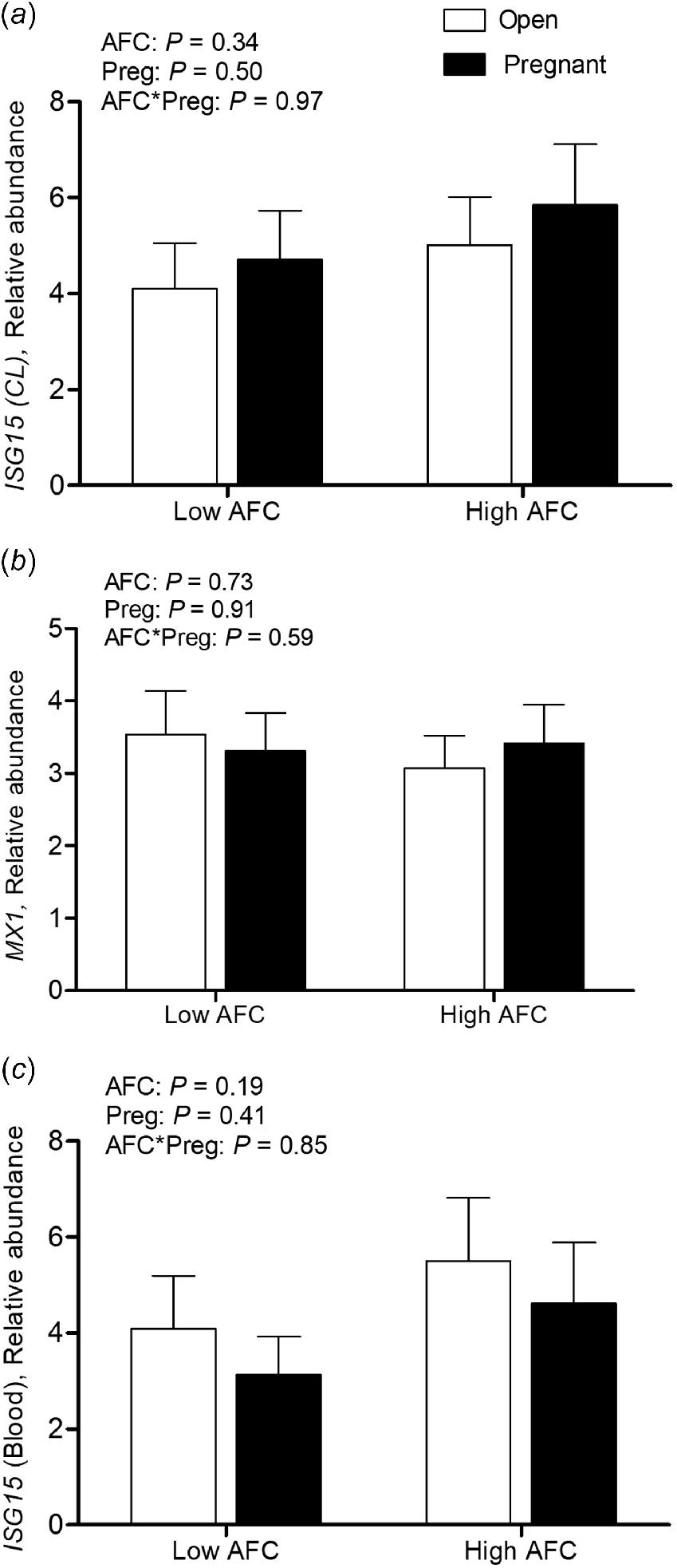
For luteal LH/CG-R abundance, an interaction of AFC group and pregnancy status was observed (P < 0.05), with decreased transcript abundance only present in Open heifers from the Low AFC group (P < 0.05, Fig. 2a). For luteal THBS1 transcript abundance, the interaction of AFC group and pregnancy status was not significant, but abundance was greater in Low than High AFC heifers (P < 0.05) and greater in Open than Pregnant heifers (P < 0.001, Fig. 2b). No interaction of AFC group and pregnancy status was observed for PTX3 transcript abundance, but its abundance was greater among Open compared to Pregnant heifers (P < 0.05, Fig. 2c). For luteal ISG15 and MX1, as well as for ISG15 transcript abundance in PBL, no interaction of AFC group and pregnancy status was observed. No difference in luteal and PBL transcript abundance of MX1 and ISG15 was observed between AFC groups or between heifers of differing pregnancy status (Fig. 3a–c).
Least square means for (a) LH/CG-R, (b) THBS1 and (c) PTX3 transcript abundance in luteal tissue for Open and Pregnant heifers in High and Low AFC groups, respectively. The P-values for the main effect of group, pregnancy status and their interaction are provided in the figure.
Least square means for luteal transcript abundance of (a) ISG15 and (b) MX1 and transcript abundance for (c) ISG15 in blood leukocytes (n = 48) for Open and Pregnant heifers in High and Low AFC groups, respectively. The P-values for the main effect of group, pregnancy status and their interaction are provided in the figure.
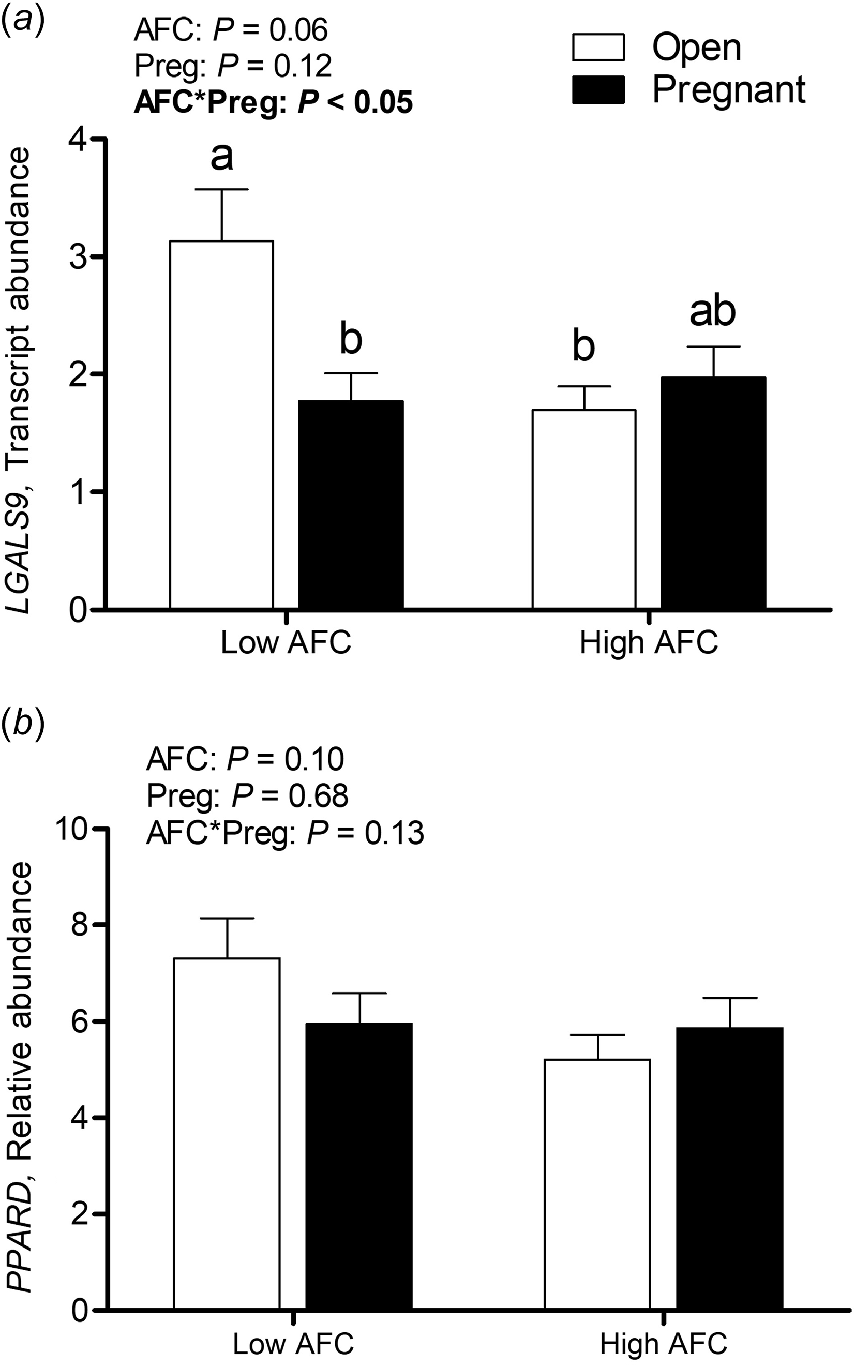
A significant interaction between AFC group and pregnancy status was observed for transcript abundance of LGALS9 (P < 0.05), with greatest expression in Open Low AFC heifers, intermediate expression in Pregnant High AFC heifers and lowest relative abundance in Pregnant Low and Open High AFC heifers (Fig. 4a). The interaction of AFC group and pregnancy status was not significant for transcript abundance of PPARD, and no differences were observed for AFC group or pregnancy status (Fig. 4b).
Least square means for luteal transcript abundance of (a) LGALS9 and (b) PPARD for Open and Pregnant heifers in High and Low AFC groups, respectively. The P-values for the main effect of group, pregnancy status and their interaction are provided in the figure.
Results of correlation analyzes of CL associated variables are given in Table 3. A weak negative correlation of THBS1 and PPARD with serum progesterone was observed, whereas LH/CG-R abundance was correlated negatively to THBS1, LGALS9 and PTX3, and positively to CL weight. The interferon stimulated genes ISG15 in CL tissue and blood and MX1 in CL tissue showed a strong positive correlation. Endometrial OXTR transcript abundance was negatively correlated to serum progesterone and LH/CG-R abundance, and positively correlated to abundance of THBS1, LGALS9 and PPARD in luteal tissue (Table 4).
| Weight corpus luteum (g) | Relative transcript abundance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LH/CG-R | THBS1 | PTX3 | ISG15 (CL) | MX1 | ISG15 (blood, n = 48) | LGALS9 | PPARD | |||
| Serum progesterone (ng/mL) | 0.14 (0.26) | 0.07 0.57 | −0.27 (<0.05) | −0.18 0.16 | −0.03 0.82 | −0.07 0.60 | 0.17 0.26 | −0.24 (0.05) | −0.32 (<0.01) | |
| Corpus luteum weight (g) | 0.39 (<0.01) | 0.18 (0.15) | −0.36 (<0.05) | −0.03 (0.83) | 0.02 (0.85) | 0.08 (0.60) | –0.30 (<0.05) | −0.12 (0.34) | ||
| Relative transcript abundance | ||||||||||
| LH/CG-R | −0.32 (<0.01) | −0.31 (<0.05) | −0.05 (0.69) | 0.04 (0.77) | −0.08 (0.57) | −0.32 (<0.01) | 0.05 (0.71) | |||
| THBS1 | 0.19 (0.13) | −0.09 (0.49) | 0.05 (0.68) | −0.11 (0.46) | 0.35 (<0.05) | 0.45 (<0.001) | ||||
| PTX3 | 0.20 (0.11) | 0.11 (0.37) | −0.08 (0.57) | −0.08 (0.51) | 0.02 (0.89) | |||||
| ISG15 (CL) | 0.70 (<0.0001) | 0.75 (<0.0001) | −0.01 (0.97) | −0.10 (0.41) | ||||||
| MX1 | 0.78 (<0.0001) | 0.03 (0.80) | 0.17 (0.18) | |||||||
| ISG15 (blood, n = 48) | −0.03 (0.84) | −0.09 (0.56) | ||||||||
| LGALS9 | 0.35 (<0.01) | |||||||||
Pearson correlation coefficient and level of significance (P-value) are given in the table; significant correlations are bolded.
| Endometrial OXTR abundance (r) | P-value | ||
|---|---|---|---|
| Corpus luteum weight | −0.06 | 0.63 | |
| Serum progesterone | −0.29 | <0.05 | |
| LH/CG-R | −0.51 | <0.001 | |
| THBS1 | 0.85 | <0.001 | |
| PTX3 | 0.13 | 0.29 | |
| LGALS9 | 0.56 | <0.001 | |
| PPARD | 0.44 | <0.001 |
Pearson correlation coefficient and level of significance (P-value) are given in the table; significant correlations are bolded.
Discussion
In the present study, we demonstrate that LH/CG-R is in fact downregulated by day 15/16 in luteal tissue of Open Low AFC heifers but not in Open High AFC or Pregnant heifers. This indicates that the transition from the pre-luteolytic to the luteolytic stage is already ongoing in Open Low AFC heifers only. The strong negative correlation of LH/CG-R abundance with endometrial OXTR expression indicates that prerequisites for the onset of luteolysis are not only given in the endometrium, but the consequent effects are already apparent in the associated corpus luteum. There is thus increasing evidence that timing of the onset of luteolysis differs among heifers with differing AFC.
The fact that CL weight did not differ among heifers of differing AFC group and pregnancy status suggests that the stage of structural luteolysis had not yet been reached in any group in this study. The differences found in transcriptional patterns in luteal tissue are, thus, indicative of the early stage of functional luteolysis. This is further supported by the lack of correlation of serum progesterone and luteal weight observed in this study, which has also been described during mid-luteal phase in previous studies (Jimenez-Krassel et al. 2009; Mann 2009).
As described in our previous publication, serum progesterone was greater in Pregnant than Open heifers (Kaps et al. 2024). In a study by Kenyon et al. (2013), no pregnancies were achieved after transfer of an embryo to cows with artificially reduced progesterone concentrations during early luteal phase (mean 1.5 ng/mL). In the same study, non-pregnant control heifers had a mean progesterone concentration of 2.7 ng/mL (Kenyon et al. 2013). In a study by Han et al. (2006), day 18 progesterone concentrations were above 4 ng/mL in pregnant cows, whereas in non-pregnant cows progesterone concentrations were below 3 ng/mL. Consequently, progesterone concentration below 3 ng/mL seems to be associated with negative pregnancy outcome. We, therefore, analyzed the number of heifers with progesterone concentration less than 3 ng/mL in the four subgroups of this study. The number of heifers with progesterone concentration less than 3 ng/mL was relatively low and there was no significant difference among the four subgroups. Nevertheless, the proportion of heifers with progesterone concentration less than 3 ng/mL was almost double in the Low AFC group compared to the High AFC group (16.6% Low AFC vs 8.6% High AFC), with the highest proportion among Open Low AFC heifers (28.6%). This corresponds to the increased endometrial OXTR and decreased luteal LH/CG-R transcript abundance observed in Open Low AFC heifers and matches the concept of ongoing luteolysis.
Loss of luteal sensitivity to LH has recently been postulated to compromise luteal survival mechanisms and consequently sets the necessary conditions for local luteolytic factors to cause further luteolysis (Shrestha 2021). The underlying mechanisms leading to the loss of luteal sensitivity to LH-pulses are, yet, not completely understood. Potential mechanisms are desensitization of LH/CG-R by uncoupling from the adenylate cyclase system and downregulation of LH/CG-R (Segaloff and Ascoli 1993; Shrestha 2021). During spontaneous luteolysis and after PGF2α exposure in vitro, abundance of LH/CG-R indeed decreases in luteal cells (Garverick et al. 1985; Mamluk et al. 1998; Weems et al. 2011). Consequently, the decrease in LH/CG-R abundance in Open Low AFC heifers in our study can be interpreted as an early sign of initiation of luteolysis. The strong correlation of LH/CG-R abundance in the CL with OXTR abundance in the endometrium further supports this concept. In addition, uncoupling of LH/CG-R from adenylate cyclase, leading to LH-desensitization, is driven by the receptor agonist LH (Segaloff and Ascoli 1993). Chronically increased LH plasma concentrations have been described in young beef cattle with diminished ovarian reserve (Jimenez-Krassel et al. 2009). The earlier loss of luteal sensitivity to LH that is resulting from chronically increased LH plasma concentrations might consequently also be involved in earlier onset of luteolysis in heifers with diminished ovarian reserve, additionally to the temporal difference in the onset of OXTR expression in the endometrium.
The pattern of THBS1 transcript abundance, despite being increased instead of decreased, followed a similar pattern among groups as observed for LH/CG-R. However, no interaction of AFC group and pregnancy status was determined. Abundance was numerically greatest in Open Low AFC heifers, with no statistical difference to Open High AFC heifers. This might reflect a slight time-lag between the onset of functional luteolysis as indicated by LH/CG-R downregulation and the transition towards structural luteolysis indicated by THBS1 upregulation in response to endogenous PGF2α. The strong positive correlation with endometrial OXTR expression and the weak negative correlation with serum progesterone and LH/CG-R abundance in the present study further emphasizes the importance of THBS1 upregulation in the context of luteolysis, as described previously (Farberov and Meidan 2016). Thrombospondin-1 determines the fate of the CL via the promotion of vascular instability, induction of apoptosis, and matrix remodeling during structural luteolysis (Farberov et al. 2019). In the study by Zalman et al. (2012), THBS1 was already upregulated in CL recovered 4 h after the administration of a PGF2α analogue to the heifer. In addition to the mentioned time-lag, there might also be a difference in the transcriptional response of the CL to endogenous PGF2α pulses as compared to exogenous analogues.
In the present study, PTX3 was more abundant in Open than Pregnant heifers, with no difference between AFC groups. Despite its antiangiogenic properties and upregulation in response to exogenous PGF2α (Zalman et al. 2012), the higher abundance of PTX3 in Open heifers of this study is probably not related to PGF2α pulses derived from the endometrium, as this contradicts with the lack of endometrial OXTR expression in Open High AFC heifers. This is further supported by the lack of correlation of PTX3 transcript abundance with endometrial OXTR transcript abundance. In contrast to the results of Zalman et al. (2012), pro-proliferative properties of PTX3 have been the focus of investigation in CL tissue exposed to IFNτ. Interferon tau robustly induces expression of PTX3 in luteal tissue and promotes its pro-proliferative properties, which have recently been determined in vitro (Basavaraja et al. 2020). As there was not yet any local effect of IFNτ on the CL of Pregnant heifers in this study (discussed below) and PTX3 was more greatly abundant in Open than Pregnant heifers, the role of PTX3 and its regulation in the CL of mid-luteal phase and the CL of early pregnancy prior to IFNτ stimulation remains unclear and requires further characterization.
The supportive actions of IFNτ on the CL have recently been proven to occur independently from endometrial expression of ISG15 and are derived by direct effects of conceptus-derived IFNτ action on the CL (Guzeloglu et al. 2024). The earliest upregulation of ISG15 and MX1 in the bovine CL has been described on day 18 of early pregnancy in cattle (Yang et al. 2010; Sakumoto et al. 2020). In PBL, an increase in ISG15 abundance has been described earliest on day 16 of pregnancy with a peak on day 20 (Han et al. 2006), whereas Gifford et al. (2007) observed an increase in ISG15 abundance in PBL on day 18. In the present study, we did not find any difference in luteal ISG15 and MX1 transcript abundance between Open and Pregnant heifers. Further, no differences for ISG15 transcript abundance in PBL were observed between Open and Pregnant heifers. This indicates that extrauterine tissues (neither the CL nor PBL) were not yet affected by the presence of a conceptus and conceptus-derived IFNτ. Most probably, the timepoint of sample collection was too early to determine an effect of IFNτ on the luteal and PBL transcription pattern of classical ISGs. Nevertheless, there was a strong positive correlation of transcript abundance of the analyzed ISGs in luteal tissue and PBL, indicating a similar transcription pattern of ISGs irrespective of the type of extrauterine tissue. The positive aspect of the lacking IFNτ action on the CL is that differences in THBS1 expression between Open and Pregnant heifers are consequently not related to the inhibition of THBS1 transcription by IFNτ (Basavaraja et al. 2017).
Peroxisome proliferator-activated receptor delta (PPARD) transcript abundance did not differ between heifers with differing pregnancy status or AFC group. An increase in PPARD abundance in luteal tissue of early pregnancy has previously been described (Sakumoto et al. 2020). In a more recent study, PPARD transcript abundance and protein expression was evaluated in different cycle stages of the CL. No difference in PPARD transcript abundance was found in any stage, whereas protein abundance was greater during days 19–21 of the estrous cycle (Socha et al. 2022). Those observations suggest that PPARD might be involved in the late luteolytic stage of the CL. The weak negative correlation with serum progesterone and the weak positive correlation with THBS1 support that, whereas involvement in a later phase of luteolysis might also be causative for the lack of differences among groups in this study.
Galectin-9 is involved in adhesion and aggregation processes. A potential role in the regulation of maternal–conceptus immune tolerance, together with other galectins, as in humans and rodents, has recently been reported (Chaney et al. 2022). Indeed greater transcript abundance of its encoding gene LGALS9 has been described in endometrial tissue of pregnant heifers than in non-pregnant heifers on day 16 of the estrous cycle (Forde et al. 2011; Chaney et al. 2022). In addition, LGALS9 has previously been identified as a potential marker for increased luteal resistance to PGF2α pulses in an RNA sequencing study of bovine luteal tissue. Its transcript abundance was greater in PGF2α-unresponsive luteal tissue of day 4 as compared to PGF2α-responsive luteal tissue of day 6 (Pate and Hughes 2023). To the best of our knowledge, there are yet no reports of LGALS9 abundance in luteal tissue around the window of maternal recognition of pregnancy. In contrast to our initial hypothesis, transcript abundance of LGALS9 was greater in Open Low AFC heifers as compared to Pregnant Low AFC or Open High AFC heifers. Despite no statistical difference between Open Low AFC heifers and Pregnant High AFC heifers being determined, the weak positive correlation with THBS1 and weak negative correlation with LH/CG-R abundance and luteal weight are more supportive of an involvement of LGALS9 in luteal regression than decreasing its sensitivity to PGF2α.
Conclusion
In conclusion, the transcript abundance patterns of LH/CG-R and THBS1 as early transcriptional indicators for onset of luteolysis, as well as their positive and negative correlation to endometrial OXTR abundance, support the concept that luteolysis is initiated earlier in heifers with reduced AFC. Nevertheless, further investigations, including repeated blood sample collections for progesterone measurements, are required to further confirm that the prerequisites for the initiation of luteolysis are given earlier in Low AFC heifers and that luteolysis can be induced earlier in those heifers. Further determination of the shape of the progesterone profile over the entire estrous cycle (Meier et al. 2009a, 2009b) might be helpful to determine how the earlier onset of luteolysis can be brought into context with identical length of the estrous cycle reported in heifers differing in AFC (Burns et al. 2005; Cushman et al. 2024). The observed transcriptional differences were not altered by the presence of a local action of IFNτ in the CL, as classical ISGs were not yet upregulated in luteal tissue or PBL. It still needs to be determined if differences can be found in the luteal response to IFNτ between heifers with High or Low AFC, which might contribute to differences in reproductive efficiency.
Data availability
The data that support this study can be shared upon reasonable request to the corresponding author.
Declaration of funding
This research was funded in part by ARS project number 3040-31000-096D and in part by the Austrian Science Fund (FWF) [J4690].
Author contributions
Martim Kaps: conceptualization, laboratory analysis, data analysis, writing: original draft. Lacey Quail: sample collection, data collection, data management, writing. Alexandria Snider: conceptualization, sample collection, laboratory analysis, data collection, writing. Jeremy Miles: conceptualization, laboratory analysis, data collection, data analysis, writing. George Perry: sample collection, laboratory analysis, data collection, writing. Robert Cushman: conceptualization, sample collection, data collection, laboratory analysis, data analysis, writing.
Acknowledgements
The authors thank USMARC Cattle Operations for care of the animals and assistance with collection of the ultrasonographic data. The authors thank Kaleb Corbett, Haley Sandoe and David Sypherd for expert laboratory assistance. Gratitude is extended to Lisa Stone for assistance with preparation of the manuscript for submission.
References
Atli MO, Bender RW, Mehta V, Bastos MR, Luo W, Vezina CM, Wiltbank MC (2012) Patterns of gene expression in the bovine corpus luteum following repeated intrauterine infusions of low doses of prostaglandin F2alpha. Biology of Reproduction 86(4), 130.
| Crossref | Google Scholar | PubMed |
Basavaraja R, Przygrodzka E, Pawlinski B, Gajewski Z, Kaczmarek MM, Meidan R (2017) Interferon-tau promotes luteal endothelial cell survival and inhibits specific luteolytic genes in bovine corpus luteum. Reproduction 154(5), 559-568.
| Crossref | Google Scholar | PubMed |
Basavaraja R, Madusanka ST, Shrestha K, Przygrodzka E, Kaczmarek MM, Meidan R (2020) Pentraxin-3 mediates prosurvival actions of interferon tau in bovine luteinized granulosa cells. Reproduction 160(4), 603-612.
| Crossref | Google Scholar |
Burns DS, Jimenez-Krassel F, Ireland JLH, Knight PG, Ireland JJ (2005) Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biology of Reproduction 73(1), 54-62.
| Crossref | Google Scholar | PubMed |
Chaney HL, Grose LF, LaBarbara JM, Sirk AW, Blancke AM, Sánchez JM, Passaro C, Lonergan P, Mathew DJ (2022) Galectin-1 induces gene and protein expression related to maternal-conceptus immune tolerance in bovine endometrium. Biology of Reproduction 106(3), 487-502.
| Crossref | Google Scholar | PubMed |
Chitko-McKown CG, Fox JM, Miller LC, Heaton MP, Bono JL, Keen JE, Grosse WM, Laegreid WW (2004) Gene expression profiling of bovine macrophages in response to Escherichia coli O157:H7 lipopolysaccharide. Developmental and Comparative Immunology 28(6), 635-645.
| Crossref | Google Scholar | PubMed |
Cushman RA, DeSouza JC, Hedgpeth VS, Britt JH (1999) Superovulatory response of one ovary is related to the micro- and macroscopic population of follicles in the contralateral ovary of the cow. Biology of Reproduction 60(2), 349-354.
| Crossref | Google Scholar |
Cushman RA, Hedgpeth VS, Echternkamp SE, Britt JH (2000) Evaluation of numbers of microscopic and macroscopic follicles in cattle selected for twinning. Journal of Animal Science 78(6), 1564-1567.
| Crossref | Google Scholar | PubMed |
Cushman RA, Kill LK, Funston RN, Mousel EM, Perry GA (2013) Heifer calving date positively influences calf weaning weights through six parturitions. Journal of Animal Science 91(9), 4486-4491.
| Crossref | Google Scholar | PubMed |
Cushman RA, McNeel AK, Freetly HC (2014) The impact of cow nutrient status during the second and third trimesters on age at puberty, antral follicle count, and fertility of daughters. Livestock Science 162, 252-258.
| Crossref | Google Scholar |
Cushman RA, Yake HK, Snider AP, Lents CA, Murphy TW, Freking BA (2023) An extreme model of fertility in sheep demonstrates the basis of controversies surrounding antral follicle count and circulating concentrations of anti-Müllerian hormone as predictors of fertility in ruminants. Animal Reproduction Science 259, 107364.
| Crossref | Google Scholar | PubMed |
Cushman RA, Kaps M, Snider AP, Crouse MS, Woodbury BL, Keel BN, McCarthy KL (2024) Relationship of length of the estrous cycle to antral follicle number in crossbred beef heifers. Translational Animal Science 8, txae074.
| Crossref | Google Scholar |
Engel CL, Patterson HH, Perry GA (2008) Effect of dried corn distillers grains plus solubles compared with soybean hulls, in late gestation heifer diets, on animal and reproductive performance. Journal of Animal Science 86(7), 1697-1708.
| Crossref | Google Scholar | PubMed |
Farberov S, Meidan R (2016) Thrombospondin-1 affects bovine luteal function via transforming growth factor-beta1-dependent and independent actions. Biology of Reproduction 94(1), 25.
| Crossref | Google Scholar | PubMed |
Farberov S, Basavaraja R, Meidan R (2019) Thrombospondin-1 at the crossroads of corpus luteum fate decisions. Reproduction 157(3), R73-R83.
| Crossref | Google Scholar | PubMed |
Flint AP, Lamming GE, Stewart HJ, Abayasekara DR (1994) The role of the endometrial oxytocin receptor in determining the length of the sterile oestrous cycle and ensuring maintenance of luteal function in early pregnancy in ruminants. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences 344(1309), 291-304.
| Crossref | Google Scholar | PubMed |
Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, Okumu LA, McGettigan PA, Mehta JP, McBride R, O’Gaora P, Roche JF, Lonergan P (2011) Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biology of Reproduction 85(1), 144-156.
| Crossref | Google Scholar | PubMed |
Garverick HA, Smith MF, Elmore RG, Morehouse GL, Agudo LS, Zahler WL (1985) Changes and interrelationships among luteal LH receptors, adenylate cyclase activity and phosphodiesterase activity during the bovine estrous cycle. Journal of Animal Science 61(1), 216-223.
| Crossref | Google Scholar | PubMed |
Gifford CA, Racicot K, Clark DS, Austin KJ, Hansen TR, Lucy MC, Davies CJ, Ott TL (2007) Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. Journal of Dairy Science 90(1), 274-280.
| Crossref | Google Scholar |
Ginther OJ, Beg MA (2012) The hour of transition into luteolysis in horses and cattle: a species comparison. Theriogenology 77(9), 1731-1740.
| Crossref | Google Scholar | PubMed |
Ginther OJ, Fuenzalida MJ, Shrestha HK, Beg MA (2011) The transition between preluteolysis and luteolysis in cattle. Theriogenology 75(1), 164-171.
| Crossref | Google Scholar |
Guzeloglu A, Bishop JV, van Campen H, Plewes MR, Gonzalez-Berrios CL, Kincade JN, Davis JS, Hansen TR (2024) Interferon-tau infusion into the ovine corpus luteum delays luteolysis. Biology of Reproduction 111, 667-677.
| Crossref | Google Scholar |
Han H, Austin KJ, Rempel LA, Hansen TR (2006) Low blood ISG15 mRNA and progesterone levels are predictive of non-pregnant dairy cows. Journal of Endocrinology 191(2), 505-512.
| Crossref | Google Scholar | PubMed |
Hansen TR, Sinedino LDP, Spencer TE (2017) Paracrine and endocrine actions of interferon tau (IFNT). Reproduction 154(5), F45-F59.
| Crossref | Google Scholar | PubMed |
Hwang JH, Spurlock ME, Kube JC, Li XZ, Smith SB (2021) Characterization of β-adrenergic receptors in bovine intramuscular and subcutaneous adipose tissue: comparison of lubabegron fumarate with β-adrenergic receptor agonists and antagonists. Journal of Animal Science 99(8), skab116.
| Crossref | Google Scholar |
Ireland JLH, Scheetz D, Jimenez-Krassel F, Themmen APN, Ward F, Lonergan P, Smith GW, Perez GI, Evans ACO, Ireland JJ (2008) Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biology of Reproduction 79(6), 1219-1225.
| Crossref | Google Scholar | PubMed |
Ireland JJ, Smith GW, Scheetz D, Jimenez-Krassel F, Folger JK, Ireland JLH, Mossa F, Lonergan P, Evans ACO (2011) Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reproduction, Fertility, and Development 23(1), 1-14.
| Crossref | Google Scholar | PubMed |
Jimenez-Krassel F, Folger JK, Ireland JLH, Smith GW, Hou X, Davis JS, Lonergan P, Evans ACO, Ireland JJ (2009) Evidence that high variation in ovarian reserves of healthy young adults has a negative impact on the corpus luteum and endometrium during estrous cycles in cattle. Biology of Reproduction 80(6), 1272-1281.
| Crossref | Google Scholar | PubMed |
Juengel JL, Cushman RA, Dupont J, Fabre S, Lea RG, Martin GB, Mossa F, Pitman JL, Price CA, Smith P (2021) The ovarian follicle of ruminants: the path from conceptus to adult. Reproduction, Fertility, and Development 33, 621-642.
| Crossref | Google Scholar | PubMed |
Kaps M, Quail LK, Rosasco SL, Snider AP, Zoca SM, Epperson KM, Rich JJ, Miles JR, Crouse MS, Keel BN, Summers AF, Perry GA, Lents CA, Cushman RA (2024) Delayed endometrial preparation for the induction of luteolysis as a potential factor for improved reproductive performance in Angus beef heifers with high antral follicle counts. Biology of Reproduction ioae146.
| Crossref | Google Scholar |
Kenyon AG, Mendonça LGD, Lopes G, Jr., Lima JR, Santos JEP, Chebel RC (2013) Minimal progesterone concentration required for embryo survival after embryo transfer in lactating Holstein cows. Animal Reproduction Science 136(4), 223-230.
| Crossref | Google Scholar | PubMed |
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4), 402-408.
| Crossref | Google Scholar | PubMed |
Mamluk R, Chen D, Greber Y, Davis JS, Meidan R (1998) Characterization of messenger ribonucleic acid expression for prostaglandin F2 alpha and luteinizing hormone receptors in various bovine luteal cell types. Biology of Reproduction 58(3), 849-856.
| Crossref | Google Scholar |
Mann GE (2009) Corpus luteum size and plasma progesterone concentration in cows. Animal Reproduction Science 115(1–4), 296-299.
| Crossref | Google Scholar | PubMed |
Mann GE, Lamming GE (1994) Use of repeated biopsies to monitor endometrial oxytocin receptors in the cow. The Veterinary Record 135(17), 403-405.
| Crossref | Google Scholar | PubMed |
Mann GE, Lamming GE (2006) Timing of prostaglandin F2α release episodes and oxytocin receptor development during luteolysis in the cow. Animal Reproduction Science 93(3–4), 328-336.
| Crossref | Google Scholar | PubMed |
Martinez MF, Sanderson N, Quirke LD, Lawrence SB, Juengel JL (2016) Association between antral follicle count and reproductive measures in New Zealand lactating dairy cows maintained in a pasture-based production system. Theriogenology 85(3), 466-475.
| Crossref | Google Scholar | PubMed |
McCracken JA, Schramm W, Barcikowski B, Wilson L (1981) The identification of prostaglandin F2 alpha as a uterine luteolytic hormone and the hormonal control of its synthesis. Acta Veterinaria Scandinavica. Supplement 77, 71-88.
| Google Scholar |
McNeel AK, Cushman RA (2015) Influence of puberty and antral follicle count on calving day in crossbred beef heifers. Theriogenology 84(7), 1061-1066.
| Crossref | Google Scholar | PubMed |
McNeel AK, Soares ÉM, Patterson AL, Vallet JL, Wright EC, Larimore EL, Amundson OL, Miles JR, Chase CC, Jr., Lents CA, Wood JR, Cupp AS, Perry GA, Cushman RA (2017) Beef heifers with diminished numbers of antral follicles have decreased uterine protein concentrations. Animal Reproduction Science 179, 1-9.
| Crossref | Google Scholar | PubMed |
Meier S, Roche JR, Kolver ES, Boston RC (2009a) A compartmental model describing changes in progesterone concentrations during the oestrous cycle. Journal of Dairy Research 76(2), 249-256.
| Crossref | Google Scholar | PubMed |
Meier S, Roche JR, Kolver ES, Verkerk GA, Boston RC (2009b) Comparing subpopulations of plasma progesterone using cluster analyses. Journal of Dairy Science 92(4), 1460-1468.
| Crossref | Google Scholar | PubMed |
Mossa F, Evans ACO (2023) Review: The ovarian follicular reserve – implications for fertility in ruminants. Animal 17(Suppl 1), 100744.
| Crossref | Google Scholar | PubMed |
Northrop-Albrecht EJ, Rich JJJ, Cushman RA, Yao R, Ge X, Perry GA (2022) Influence of conceptus presence and preovulatory estradiol exposure on uterine gene transcripts and proteins around maternal recognition of pregnancy in beef cattle. Molecular and Cellular Endocrinology 540, 111508.
| Crossref | Google Scholar | PubMed |
Oliveira JF, Henkes LE, Ashley RL, Purcell SH, Smirnova NP, Veeramachaneni DNR, Anthony RV, Hansen TR (2008) Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein. Endocrinology 149(3), 1252-1259.
| Crossref | Google Scholar | PubMed |
Pate JL (1994) Cellular components involved in luteolysis. Journal of Animal Science 72(7), 1884-1890.
| Crossref | Google Scholar |
Pate JL, Hughes CHK (2023) Review: Luteal prostaglandins: mechanisms regulating luteal survival and demise in ruminants. Animal 17(Suppl 1), 100739.
| Crossref | Google Scholar | PubMed |
Robinson RS, Mann GE, Lamming GE, Wathes DC (2001) Expression of oxytocin, oestrogen and progesterone receptors in uterine biopsy samples throughout the oestrous cycle and early pregnancy in cows. Reproduction 122(6), 965-979.
| Crossref | Google Scholar | PubMed |
Sakumoto R, Hayashi KG, Hosoe M, Iga K, Kizaki K (2020) Pregnancy-associated changes of peroxisome proliferator-activated receptor delta (PPARD) and cytochrome P450 family 21 subfamily A member 2 (CYP21A2) expression in the bovine corpus luteum. Journal of Reproduction and Development 66(3), 205-213.
| Crossref | Google Scholar | PubMed |
Santa Cruz R, Cushman RA, Viñoles C (2018) Antral follicular count is a tool that may allow the selection of more precocious Bradford heifers at weaning. Theriogenology 119, 35-42.
| Crossref | Google Scholar | PubMed |
Segaloff DL, Ascoli M (1993) The lutropin/choriogonadotropin receptor … 4 years later. Endocrine Reviews 14(3), 324-347.
| Crossref | Google Scholar | PubMed |
Shrestha HK (2021) Loss of luteal sensitivity to luteinizing hormone underlies luteolysis in cattle: a hypothesis. Reproductive Biology 21(4), 100570.
| Crossref | Google Scholar | PubMed |
Singh J, Domínguez M, Jaiswal R, Adams GP (2004) A simple ultrasound test to predict the superstimulatory response in cattle. Theriogenology 62(1–2), 227-243.
| Crossref | Google Scholar | PubMed |
Snider AP, Crouse MS, Rosasco SL, Epperson KM, Northrop-Albrecht EJ, Rich JJJ, Chase CC, Jr., Miles JR, Perry GA, Summers AF, Cushman RA (2022) Greater numbers of antral follicles in the ovary are associated with increased concentrations of glucose in uterine luminal fluid of beef heifers. Animal Reproduction Science 239, 106968.
| Crossref | Google Scholar | PubMed |
Socha BM, Łada P, Jończyk AW, Korzekwa AJ, Skarżyński DJ (2022) The role of peroxisome proliferator-activated receptors in PGF2α-induced luteolysis in the bovine corpus luteum. Animals 12(12), 1542.
| Crossref | Google Scholar |
Spicer LJ, Ireland JJ, Roche JF (1981) Changes in serum LH, progesterone, and specific binding of 125I-hCG to luteal cells during regression and development of bovifle corpora lutea. Biology of Reproduction 25(4), 832-841.
| Crossref | Google Scholar | PubMed |
Weems YS, Arreguin-Arevalo JA, Nett TM, Vann RC, Ford SP, Bridges PJ, Welsh TH, Jr., Lewis AW, Neuendorff DA, Randel RD, Weems CW (2011) In vivo intra-luteal implants of prostaglandin (PG) E(1) or E(2) (PGE(1), PGE(2)) prevent luteolysis in cows. I. Luteal weight, circulating progesterone, mRNA for luteal luteinizing hormone (LH) receptor, and occupied and unoccupied luteal receptors for LH. Prostaglandins & Other Lipid Mediators 95(1–4), 35-44.
| Crossref | Google Scholar | PubMed |
Yang L, Wang XL, Wan PC, Zhang LY, Wu Y, Tang DW, Zeng SM (2010) Up-regulation of expression of interferon-stimulated gene 15 in the bovine corpus luteum during early pregnancy. Journal of Dairy Science 93(3), 1000-1011.
| Crossref | Google Scholar | PubMed |
Yankey SJ, Hicks BA, Carnahan KG, Assiri AM, Sinor SJ, Kodali K, Stellflug JN, Ott TL (2001) Expression of the antiviral protein Mx in peripheral blood mononuclear cells of pregnant and bred, non-pregnant ewes. Journal of Endocrinology 170(2), R7-R11.
| Crossref | Google Scholar |
Zalman Y, Klipper E, Farberov S, Mondal M, Wee G, Folger JK, Smith GW, Meidan R (2012) Regulation of angiogenesis-related prostaglandin f2alpha-induced genes in the bovine corpus luteum. Biology of Reproduction 86(3), 92.
| Crossref | Google Scholar | PubMed |
Footnotes
§ Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program (Not all prohibited bases apply to all programs.). Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.


