Asian elephant (Elephas maximus) seminal plasma: establishing the proteome and effect on spermatozoa when added to cryomedium
Cameron Negus A * , Anuchai Pinyopummin
A * , Anuchai Pinyopummin  B , Sittidet Mahasawangkul
B , Sittidet Mahasawangkul  C , Rebecca Hobbs
C , Rebecca Hobbs  D and Roslyn Bathgate
D and Roslyn Bathgate  A
A
A
B
C
D
Abstract
The removal or supplementation of ejaculates with seminal plasma (SP) can affect cryotolerance and post-thaw survival of spermatozoa in many species. In the Asian elephant (Elephas maximus), elucidation of the SP proteome and investigation of how it affects spermatozoa may enable improvement of cryopreservation protocols.
Herein, we characterise the Asian elephant SP proteome and investigate the impacts of SP on sperm cryotolerance in the presence of conspecific or heterospecific SP.
Proteomic analysis of Asian elephant SP was performed using mass spectrometry on nine samples from three individuals. In a separate study, SP was removed from six ejaculates and spermatozoa were resuspended in Tris extender supplemented with: no seminal plasma (NOSP), conspecific SP from ejaculates exhibiting ‘good’ (GSP, >60%) or mixed sperm total motility (MSP), or horse SP (HSP). Samples underwent cryopreservation, and sperm parameters were compared prior to cryopreservation and after thawing (0 and 2 h).
Mass spectrometry identified 155 proteins from an array of families. Significant differences were observed in post-thaw sperm quality between SP treatments: high concentrations of MSP (25%, v/v) displayed greater average path and straight-line velocity immediately after thawing (P < 0.05) and greater sperm motility index and beat cross frequency than NOSP after 2 h post-thaw incubation (P < 0.05). The addition of HSP improved sperm kinematic parameters compared to NOSP and GSP treatments (P < 0.05).
These preliminary findings suggest the potential of SP to enhance the cryosurvival of Asian elephant spermatozoa, with HSP showing particularly promising results compared to conspecific SP (GSP). Further research into the specific effects of Asian elephant SP proteins is warranted.
Keywords: Asian elephant, cryopreservation, Elephas maximus, mass spectrometry, preservation, proteins, semen, seminal plasma.
Introduction
The Asian elephant (Elephas maximus) is categorised by the IUCN (Williams et al. 2020) as endangered with declining numbers, and captive breeding is a vital component of preserving this species. The management of captive Asian elephants incorporates assisted reproductive techniques such as artificial insemination (AI) with fresh and chilled preserved semen to supplement natural breeding. This is in an effort to improve reproductive output, enable outbreeding to enhance genetic diversity within isolated populations, and thus ensure the sustainability of captive populations (Hildebrandt et al. 2006). However, the use of cryopreserved Asian elephant semen has been unsuccessful, attributed to inconsistent quality of collected semen (Hildebrandt et al. 2000) and poor post-thaw sperm survival. Despite multiple attempts using semen samples displaying ≥40% post-thaw motility, no live Asian elephant calves have been born from frozen-thawed artificially inseminated semen (Thongtip et al. 2009). Although research suggests methods for cryo-sensitive spermatozoa (O’Brien et al. 2013), cryopreservation of Asian elephant semen still requires fundamental improvements in processing and freezing techniques to achieve success after AI.
An ejaculate is composed of spermatozoa and the acellular seminal plasma (SP), primarily secreted from the accessory sex glands (Flint et al. 2015). Seminal plasma is a complex medium of inorganic ions, sugars, organic salts, enzymes, proteins and various other factors. It is involved in a multitude of sperm functions and processes preceding fertilisation, as reviewed by Juyena and Stelletta (2012), though the constituents are highly species-dependent. Within the elephant species, the seminal vesicles are the largest accessory sex glands and believed to contribute the greatest proportion of volume to the SP in the ejaculate, as indicated by a large reduction in internal fluid volume of the seminal vesicles observed via ultrasonography after ejaculation (Hildebrandt et al. 2000). Similarly, in stallions, seminal vesicles also significantly decrease in size after ejaculation (Weber et al. 1990). Although similar effects have been recorded in the stallion ampulla, these accessory sex glands are shown to vary in size between breeds and seasons (Pozor and McDonnell 2002).
The protein component of SP has recently become a focal point of reproductive research in domestic animals (Rickard et al. 2015; Perez-Patiño et al. 2016; Aquino-Cortez et al. 2017), as SP proteins are known to contribute to the fertilisation process by remodelling the sperm surface (Mogielnicka-Brzozowska and Kordan 2011; Rodríguez-Martínez et al. 2011). Several studies have demonstrated that specific SP proteins influence the function and fertilising capacity of spermatozoa (Moura et al. 2006; Vilagran et al. 2015) and, in some species, are utilised as markers of sperm freezability (Jobim et al. 2011; Vilagran et al. 2015). Minimal research has been done on the proteomics of Asian elephant SP. One- and two-dimensional gel electrophoresis have revealed a large protein component of Asian elephant SP collected via manual rectal massage technique, but gel spots varied greatly in size between ejaculates (Kiso et al. 2013). A recent non-targeted proteomic analysis using tandem-mass spectrometry revealed 1183 proteins in Asian elephant SP, of which 597 proteins were mapped to identified proteins from 58 species, with only a small proportion (29 proteins) recognised to be related to reproductive processes (Wattananit et al. 2023). Continuing to establish an Asian elephant SP proteome will likely make a significant contribution to the development of sperm cryo-protocols and guide selection of males and semen samples for AI and breeding programs.
The role and benefits of SP when applying sperm preservation techniques, such as cryopreservation, is still under debate, often with varying results observed across species. For example, horse spermatozoa are able to maintain greater motility during cooled storage and cryopreservation when SP is removed prior (Jasko et al. 1991; Moore et al. 2005). However, for other species, such as humans (Ben et al. 1997), goats (Azerêdo et al. 2001), and red deer (Martínez-Pastor et al. 2006), SP inclusion has proven to improve sperm cryosurvival. Even within species, the reported effects of SP during cryopreservation are conflicting. While the beneficial effects of SP removal prior to freezing have already been shown with stallions and boars (Kawano et al. 2004; Moore et al. 2005), other studies in the same species have demonstrated that SP obtained from certain males (classified as ‘good freezers’; ones demonstrating high post-thaw quality) may improve the cryosurvival of spermatozoa from other males (Aurich et al. 1996; Hernández et al. 2007). Similarly, the addition of SP from infertile bulls can reduce the fertilising ability of spermatozoa from bulls with high fertility (Henault and Killian 1996), which is hypothesised to be due to differences in protein composition of the SP (Zahn et al. 2005; Vilagran et al. 2015). Because of the high variability of sperm quality seen in fresh Asian elephant ejaculates (Hildebrandt et al. 2000), it is possible that the SP composition, including proteins, may be highly variable and strongly influence the observed sperm quality (Kiso et al. 2013).
Previous studies have advocated for removal of Asian elephant SP prior to cryopreservation (Saragusty et al. 2009). However, others have retained the SP during freezing with similar success (Thongtip et al. 2004, 2009). The beneficial effects of Asian elephant SP have been more clearly demonstrated during chilled liquid storage where sperm motility and acrosome integrity were better maintained in the presence of SP compared to its absence (Pinyopummin et al. 2017). Further studies directly comparing the effects of SP, and source of ejaculate quality, on Asian elephant spermatozoa during cryopreservation are required to understand the role of SP during and after the freezing process.
Studies on cryopreservation of spermatozoa using heterospecific SP have demonstrated beneficial in vitro effects. For example, rainbow trout (Ustuner et al. 2016) and dog (Mataveia et al. 2010) SP have been shown to improve ram spermatozoa cryosurvival. Horse SP (HSP) has previously been added to Asian elephant spermatozoa during chilled storage and has demonstrated greater preservation of sperm motility and velocity compared to conspecific SP (Pinyopummin et al. 2017). Furthermore, HSP was found to reverse the detrimental effects of high dilution rates on Asian elephant sperm motility during chilled storage (Pinyopummin et al. 2018). Despite this, the cryoprotective effects of HSP on Asian elephant spermatozoa have not previously been investigated.
The aims of this study were to undertake two preliminary investigations to improve the cryosurvival of Asian elephant spermatozoa by addition of SP proteins. Working within the constraints of limited samples from this species, the first investigation aimed to characterise the Asian elephant SP proteome using mass spectrometry. The second part aimed to investigate the effects of the presence and absence of both con- and heterospecific SP on Asian elephant spermatozoa during cryopreservation. This study serves as a first step towards more thorough investigation of the effect of specific proteins on Asian elephant spermatozoa during cryopreservation, contributing to the development of more effective assisted reproductive technologies for this endangered species.
Materials and methods
Experimental design
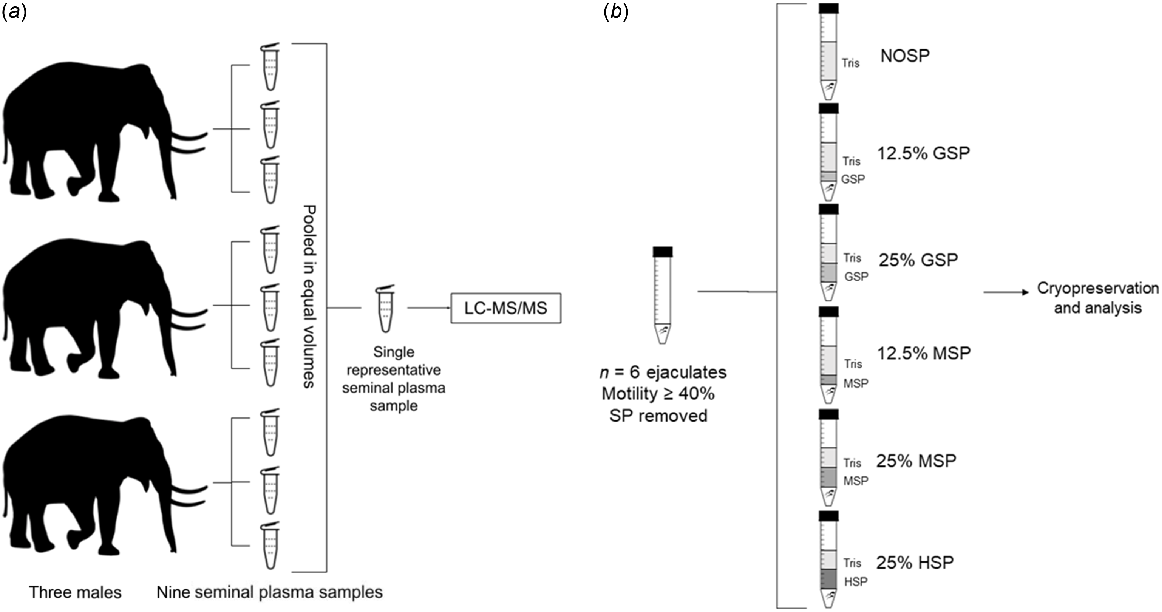
This study is presented as two experimental designs: (1) proteomic analysis of Asian elephant seminal plasma (Fig. 1a), and (2) cryopreservation of semen samples with and without con- and heterospecific SP (Fig. 1b).
Experimental designs for the study. (a) Proteomic analysis of Asian elephant seminal plasma. Seminal plasma samples were pooled from three males to analyse a simple representative sample of the species via liquid chromatography tandem mass spectrometry (LC-MS/MS). (b) Cryopreservation study with and without heterospecific and conspecific seminal plasma (SP). Semen samples removed of initial seminal plasma (n = 6 replicates) and diluted with Tris extender supplemented with varying final concentrations (v/v) of good quality seminal plasma (GSP), mixed seminal plasma (MSP), and horse seminal plasma (HSP). NOSP, no seminal plasma. The Figure was created using Google Drawings.
Animals
All experimental procedures were carried out with the approval of the Animal Ethics Committee of the Taronga Conservation Society Australia (4a/04/114), the Animal Usage and Ethics Committee of Kasetsart University (ACKU 01858), and endorsed by the University of Sydney Animal Ethics Committee (2016/1010).
For the SP proteomic study, three sexually mature Asian elephant bulls (14–40 years of age) housed at different zoological institutions across Australia were used for semen collection. Animals were given water ad libitum and fed a varied diet as managed by each institution, including mostly hay and lucerne, along with treats of fruit, vegetables, bread, sugar cane, bamboo, and leafy branches. Elephants used in this study had proven fertility. Ejaculate samples were collected from these males between May 2013 and October 2015.
For the cryopreservation study, nine sexually mature Asian elephant bulls (20–35 years of age) housed at the National Elephant Institute of the Forest Industry Organization, Lampang, Thailand (latitude 18°21.60′N and longitude 99°14.92′E) were used for semen collection. The animals were fed a mixture of pangola grass, sugarcane, banana, and corn, and supplemented with concentrated feed (8% protein, 2% fat, and 20% fibre). Elephants used in this study varied in terms of proven fertility. Ejaculate samples were collected from these males and analysed between August and September 2018.
Semen collection and processing
Semen samples were collected by manual rectal massage method (Schmitt and Hildebrandt 1998). To avoid urine contamination, semen collecting tubes were changed frequently during the collection process, and samples were collected in multiple fractions. Fractions with urine contamination as determined by colour and odour were discarded. Non-contaminated fractions were pooled as a single sample for further processing. Semen volume and pH (pH indicator strips; Universal indicator, Merck, Germany), sperm concentration (Neubauer hemocytometer; Evans et al. 1987), and the percentage of spermatozoa with normal viability, functional plasma membranes, and normal acrosome integrity were determined with fresh semen samples as described below. Samples were held at room temperature (22–26°C) during processing, dilution, and evaluation. Semen samples were collected and processed similarly for both the proteomic analysis and cryopreservation study.
Proteomic analysis of Asian elephant seminal plasma
Three ejaculates each from three mature Asian elephant bulls were selected for this study. To detect the maximum range of proteins, ejaculate samples of varying initial seminal quality (0–90% fresh total motility) were chosen. Seminal plasma was separated from spermatozoa by centrifuging at 10,000g for 20 min, then aspirating the supernatant void of spermatozoa, and stored frozen (−80°C) until further analysis. On the day of proteomic analysis, SP samples were thawed on ice and centrifuged (10,000g for 5 min at 4°C) to isolate the supernatant, free of any potential spermatozoa or debris. Before proteomic analysis, total protein concentrations (mg/mL) of each SP sample were determined by bicinchoninic acid (BCA) protein quantification assay (Pierce, Rockford, IL, USA) as per the manufacturer’s instructions. Bovine serum albumin was used as the protein standard. Equal volumes of each SP sample were then pooled together to create a single representative sample of the species for proteomic analysis. Equal volumes of each SP sample were used to ensure equal representation of each male and ejaculate. The pooled SP sample was kept on ice until proteomic analysis.
To identify proteins in Asian elephant SP, the pooled sample was analysed by liquid chromatography tandem mass spectrometry (LC-MS/MS) at the Bioanalytical Mass Spectrometry Facility, University of New South Wales, Australia. The liquid sample was first reduced, alkylated, and digested overnight with trypsin at 37°C. Digest peptides were separated by nano-LC using an Ultimate 3000 high performance liquid chromatography and autosampler system (Dionex, Amsterdam, Netherlands). Samples (2.5 μL) were concentrated and desalted onto a micro C18 precolumn (300 μm × 5 mm, Dionex) with H2O:CH3CN (98:2, 0.05% trifluoroacetic acid) at 15 μL/min. After a 4-min wash, the pre-column was switched (Valco 10 port valve, Dionex) into line with a fritless nano column (75 μm × ~10 cm) containing C18 media (1.9 μ, 120 Å, Dr Maisch, Ammerbuch-Entringen, Germany) manufactured according to Gatlin et al. (1998). Peptides were eluted using a linear gradient of H2O:CH3CN (98:2, 0.1% formic acid) to H2O:CH3CN (64:36, 0.1% formic acid) at 200 nL/min over 30 min. High voltage (2000 V) was applied to a low volume tee (Upchurch Scientific), and the column tip was positioned ~0.5 cm from the heated capillary (T = 275°C) of an Orbitrap Velos (Thermo Electron, Bremen, Germany) mass spectrometer. Positive ions were generated by electrospray, and the Orbitrap operated in data dependent acquisition mode (DDA).
A survey scan m/z 350–1750 was acquired in the Orbitrap (Resolution = 30,000 at m/z 400, with an accumulation target value of 1,000,000 ions) with lockmass enabled. Up to the 10 most abundant ions (>4000 counts) with charge states > +2 were sequentially isolated and fragmented within the linear ion trap using collisionally induced dissociation with an activation q = 0.25 and activation time of 30 ms at a target value of 30,000 ions. M/z ratios selected for MS/MS were dynamically excluded for 30 s.
All MS/MS spectra were searched against Uniprot and a customised database using MASCOT (ver. 2.4, Matrix Science, London, UK) with the following search criteria: enzyme specificity was trypsin; precursor and product ion tolerances were at 4 ppm and ±0.4 Da, respectively; variable modification of methionine oxidation; and one missed cleavage was allowed. The ions score significance threshold was set to 0.5, and each protein was provided with a probability-based MOWSE (Molecular Weight Search) score (Pappin et al. 1993). Ions scores were determined by −10 × log(P), where P is the probability that the observed match is a random event. Individual ion scores >20 indicated identity or extensive homology (P < 0.05). Protein scores were derived from ions scores as a non-probabilistic basis for ranking protein hits. A higher protein score indicated a higher probability of a non-spurious match.
For identifying proteins, peptides were first searched against the completed African elephant (Loxodonta africana) genome (tax ID: 9784). If no match was found, then a non-restricted search was performed. To predict uncharacterised proteins in L. africana, FASTA codes were entered into a BLAST search restricted to the Mammalia class (tax ID: 40674).
The identified proteins were further characterised for molecular functions, biological processes, cellular components, and protein classes using the PANTHER classification system (ver. 13.1; www.pantherdb.org). Gene symbols were used as input for Gene Ontology (GO) annotations for functional categorisation. To maximise the number of matched gene names and classifications of proteins, Homo sapiens was used as the reference species.
Evaluation of spermatozoa
Sperm total motility was subjectively estimated under phase contrast microscopy at ×400 magnification as described by Evans et al. (1987). Spermatozoa were considered non-motile if there was no flagellar movement. Simultaneously, the samples were given a kinematic rating on a scale 0–5, where 0 represented no flagellar movement and 5 represented rapid forward progressive movement (>1 sperm length/s). For an overall sperm motility rating with equal emphasis on total motility and forward progressive motion, a sperm motility index (SMI), as described by Howard and Wildt (1990), was calculated as follows:
Further motility information and sperm kinematic properties of post-thawed samples were evaluated in the laboratory using computer-assisted semen analysis (CASA; IVOS model 12.0, Hamilton–Thorne Biosciences, Beverly, MA, USA). The CASA settings and procedures have been previously described for Asian elephant spermatozoa (Thongtip et al. 2008). The following kinematic parameters were recorded: percentage of motile spermatozoa (MOT, %), percentage of progressively motile spermatozoa (pMOT; %), average velocity path (VAP, μm/s), straight-line velocity (VSL, μm/s), curvilinear velocity (VCL, μm/s), amplitude of lateral head displacement (ALH; mm), beat-cross frequency (BCF; Hz), linearity (LIN; %), and straightness (STR; %). A minimum of 300 spermatozoa over four fields of view were recorded. The CASA system could not be used for fresh and pre-freeze assessments because of the field location where semen collection and processing occurred. However, it was deemed appropriate to enhance comparison of post-thaw parameters between treatment groups.
For assessment of viability, eosin-nigrosin stain was used as previously described (Björndahl et al. 2003). A minimum of 200 spermatozoa per sample were classified as viable (no stain uptake) or non-viable (partial or complete stain uptake). Viability assessments were performed within 8 h of staining and smearing. Sperm morphology was assessed on eosin-nigrosin stains by evaluating a minimum of 200 spermatozoa for abnormalities, as described by Kiso et al. (2013).
The functional integrity of the sperm plasma membrane was evaluated by means of the hypo-osmotic swelling test (HOST) using a modified protocol (Matson et al. 2009) for Asian elephant spermatozoa. A minimum of 200 spermatozoa were assessed under phase contrast microscopy (×400) for morphological changes. A positive response to hypo-osmotic stress (HOST+) resulted in spermatozoa exhibiting signs of either tail coiling or swelling to various degrees (Jeyendran et al. 1984), indicating normal plasma membrane integrity and function.
The acrosome integrity of the spermatozoa was assessed by means of Coomassie blue staining technique (Larson and Miller 1999). Briefly, 10–20 μL of sample was fixed in 250 μL of 4% paraformaldehyde. A minimum of 200 stained spermatozoa per sample were evaluated for acrosomal integrity using bright-field microscopy (×1000) under oil immersion. Spermatozoa exhibiting uniform staining over the acrosomal region were categorised as intact acrosomes, whereas those that showed non-uniform staining, abnormal shape, or lack of staining altogether in the acrosomal region were categorised as non-intact acrosomes.
The integrity of sperm chromatin DNA was assessed by acridine orange (AO) fluorescence (Tejada et al. 1984). A minimum of 200 spermatozoa were counted on each slide by the same examiner, and the duration of evaluation per field of view did not exceed 40 s to minimise photo-bleaching effects. Spermatozoa displaying green fluorescence were considered to contain normal, intact DNA, whereas spermatozoa displaying a spectrum of yellow–orange to red fluorescence were considered to contain damaged non-intact DNA.
Cryopreservation study
Asian elephant SP was isolated from non-urine contaminated semen samples. Samples were centrifuged at 10,000g for 20 min, and then the supernatant void of spermatozoa was aspirated and stored frozen (−20°C) until used. Because of the limitations in sample availability, the SP from multiple ejaculates of Asian elephant bulls were pooled to form two SP quality groups. Ejaculates (n = 2) displaying fresh total motility ≥60% were pooled in equal volumes to form the ‘good’ seminal plasma (GSP) treatment. Ejaculates (n = 9) with varying fresh motility (range 0–65%) were pooled in equal volumes to form the mixed seminal plasma (MSP) treatment. A range of fresh ejaculate motility was chosen for the MSP treatment to encompass all types of ejaculate quality and better represent the ‘average’ Asian elephant ejaculate. Horse seminal plasma (HSP) was obtained from stallion ejaculates (n = 2) exhibiting greater than 60% fresh sperm motility. Two stallions (from Pinyopummin et al. 2017, 2018) were used for semen collection via artificial vagina (Davies Morel 1999). Upon pooling, semen samples were centrifuged (12,000g for 5 min) and seminal plasma aspirated. Spermatozoa from ejaculates pooled for the SP treatments (Table 1) were discarded, and new semen samples were collected for cryopreservation experiments.
| Male | Total motility (%) | ||
|---|---|---|---|
| Good quality seminal plasma (GSP) | Bull 1 | 60 | |
| Bull 2 | 65 | ||
| Mixed quality seminal plasma (MSP) | Bull 2 | 65 | |
| Bull 3 | 5 | ||
| 50 | |||
| Bull 4 | 0 | ||
| Bull 5 | 0 | ||
| Bull 6 | 40 | ||
| 20 | |||
| Bull 7 | 0 | ||
| Bull 8 | 5 | ||
| Horse seminal plasma (HSP) | Stallion 1 | 65 | |
| Stallion 2 | 80 |
Total sperm motility of original ejaculate samples selected for seminal plasma extraction to form the seminal plasma quality groups for the cryopreservation study. Selection criteria based on total sperm motilities of fresh ejaculates or species. All samples in the table were used only for seminal plasma extraction (spermatozoa fractions were discarded).
Only ejaculates with an initial motility ≥40% were processed for cryopreservation. A lower than usual fresh motility criterion for Asian elephant semen cryopreservation (motility ≥60%; Thongtip et al. 2009; Kiso et al. 2012) was used to allow inclusion of a greater number of replicates for the purpose of statistical validity.
Fresh Asian elephant semen samples meeting this criterion (n = 6) were each diluted (1:1) with Tris-based extender containing 198.1 mM Tris-(hydroxymethyl)-aminomethane, 66.6 mM citric acid monohydrate, 44.4 mM glucose (Merck Millipore, USA), and 20% (v/v) egg yolk with penicillin G potassium salt (0.6 mg/mL) and streptomycin sulfate salt (1 mg/mL). This extender was adapted from use with dog spermatozoa (Peña and Linde-Forsberg 2000), and has previously shown success in preserving Asian elephant spermatozoa (Pinyopummin et al. 2017). All chemicals in this study were purchased from Sigma Chemical Company (St Louis, MO, USA) unless stated otherwise. The diluted samples were then centrifuged (125g for 10 min) for the removal of pre-existing SP. The sperm pellet was re-diluted with a Tris-SP mixture (from categories of SP described in Table 1) to divide each ejaculate across six treatments with final concentration of prepared SP (v/v) before freezing of: 0% SP (no seminal plasma, NOSP; as a comparative control), 12.5% GSP (GSP-low), 25% GSP (GSP-high), 12.5% MSP (MSP-low), 25% MSP (MSP-high) and 25% HSP (HSP). These concentrations were based on those used by Pinyopummin et al. (2017). Diluted spermatozoa were then placed into a waterbath consisting of 100 mL of water at room temperature, fully submerged, and chilled to 4°C, over 90–105 min. After chilling, samples were gradually diluted (quarter of the final volume every 15 min) with chilled Tris extender supplemented with glycerol (Bio Basic, Canada) and STM Equex paste (Nova Chemical Sales, USA) to achieve final concentrations of 5% and 0.5% (v/v), respectively. Samples were allowed to equilibrate with cryoprotectants at 4°C for approximately 15 min. Thereafter, spermatozoa were loaded into 0.5 mL straws, sealed with sealing powder, and cryopreserved by resting on a stainless-steel rack 2.5 cm above liquid nitrogen for 10 min, before being plunged and stored in liquid nitrogen until thawing (Kiso et al. 2012). Pre-freeze assessments were conducted after the cryoprotectant was completely added and immediately before straw loading.
Semen straws containing cryopreserved spermatozoa were thawed by agitating in a 37°C waterbath for 30 s. Spermatozoa were expelled into glass tubes and slowly diluted (1:1) with the base Tris extender warmed to 37°C. An aliquot of spermatozoa was immediately assessed for post-thaw evaluation of sperm motility, kinematics, sperm viability, plasma membrane integrity, acrosome integrity, and DNA integrity as described above (0 h). The original tube was kept at 37°C for a further 2 h and then reassessed.
Statistical analyses
Data from the cryopreservation study were analysed using Linear Mixed Model in GenStat (ver. 16, VSN International Ltd, Hemel Hempstead, UK) to determine the effects of SP treatments, time of sperm assessment, and the interaction between both. All measured sperm parameters were statistically analysed. The effects of SP treatment (GSP-low, GSP-high, MSP-low, MSP-high, NOSP and HSP) and time point (pre-freeze where applicable, and 0 h and 2 h post-thaw incubation) were included in the fixed model with bull/ejaculate as the random term. If interactions were nonsignificant they were removed from the fixed model. Data were checked for normality and homogeneity of variances before analysis. Means were compared on the basis of least significant difference and all values are reported as means ± standard error of the mean (s.e.m.). The decision was made to present the data as means ± s.e.m. despite the small sample size to enable better comparison between this study and those previously published. For all analyses, statistical significance was defined as P < 0.05.
Results
Proteomic analysis of Asian elephant seminal plasma
The fresh seminal characteristics of ejaculates selected for SP pooling for proteomic analysis are displayed in Table 2. Total protein concentration of Asian elephant SP ranged from 3.3 to 11.9 mg/mL with an average of 8.0 ± 1.2 mg/mL.
| Ejaculate | Ejaculate fraction volume (mL) | Concentration (×106 sperm/mL) | pH | Total motility (%) | Viability (%) | Normal morphology (%) | Seminal plasma total protein (mg/mL) | ||
|---|---|---|---|---|---|---|---|---|---|
| Bull 1 | 1 | 12 | 1280 | 7 | 0 | 14.2 | 32.1 | 11.7 | |
| 2 | 5 | 1322.5 | 6 | 40 | 10.3 | 46.9 | 8.7 | ||
| 3 | 23 | 2415 | 6 | 20 | 8.8 | 66.3 | 11.6 | ||
| Bull 2 | 1 | 5 | 64 | 8.5 | 80 | 45.3 | n/a | 3.3 | |
| 2 | 3 | 815 | 8.5 | 50 | 37.0 | n/a | 8.0 | ||
| 3 | 4.5 | 715 | 9 | 0 | 62.7 | 57.9 | 9.0 | ||
| Bull 3 | 1 | 3 | 585 | 8.5 | 90 | 53.0 | 92.9 | 3.6 | |
| 2 | 15 | n/a | 8 | 70 | 78.5 | 81.5 | 4.2 | ||
| 3 | 7.5 | n/a | 7 | 50 | n/a | n/a | 11.9 | ||
| Mean | 8.7 | 1028.1 | 7.6 | 44.4 | 38.7 | 62.9 | 8.0 | ||
| s.e.m. | ±2.3 | ±282.1 | ±0.4 | ±10.9 | ±9.2 | ±9.1 | ±1.2 |
n/a, not applicable.
Mass spectrometry identified a total of 155 proteins (Supplementary Table S1) in the pooled sample of Asian elephant SP. The top 30 proteins with the highest protein scores are displayed in Table 3.
| UniprotKB accession | Protein name | Gene symbol | Protein mass (kDa) A | Protein score B | |
|---|---|---|---|---|---|
| G3SMX8 | Serum albumin | ALB | 68.8 | 575 | |
| G3UD48 | Epididymal-specific lipocalin-5 | LCN5 | 21.5 | 288 | |
| G3TBR7 | Low density lipoprotein receptor-related protein 2 | LRP2 | 518.1 | 264 | |
| G3T8L4 | Apolipoprotein D | APOD | 21.6 | 252 | |
| G3SS80 | Ribonuclease T2 | RNASET2 | 25.5 | 230 | |
| G3T752 | Zonadhesin | ZAN | 249.2 | 192 | |
| G3T0S5 | Lactotransferrin | LTF | 77.3 | 188 | |
| G3TUH4 | Chromosome 1 Open Reading Frame 56 | C1orf56 | 34.1 | 175 | |
| G3T643 | Superoxide dismutase [Cu-Zn] | SOD1 | 15.7 | 160 | |
| G3T3N6 | Carboxylesterase 5A | CES5A | 64.0 | 154 | |
| G3SZZ0 | Enolase 1, (alpha) | ENO1 | 47.2 | 146 | |
| G3UCL2 | Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 | GPIHBP1 | 20.7 | 137 | |
| G3THY2 | Tetraspanin (Fragment)/CD81 Molecule | CD81 | 23.2 | 134 | |
| G3U1Z4 | Cathepsin D | CTSD | 41.1 | 132 | |
| G3U2L5 | Angiopoietin-Like Protein 5 | ANGPTL5 | 26.0 | 121 | |
| G3UBT6 | Heat shock protein 90 kDa alpha (cytosolic), class A member 1 | HSP90AA1 | 85.1 | 117 | |
| G3TZ57 | Disintegrin and metalloproteinase domain-containing protein 18 | ADAM18 | 60.9 | 117 | |
| G3T9G3 | Transferrin | TF | 108.2 | 111 | |
| G3SLB1 | Glucosylceramidase | GBA | 57.6 | 110 | |
| G3T8N4 | Protein deglycase DJ-1 | PARK7 | 20.0 | 107 | |
| G3U416 | Cystatin | CST6 | 16.3 | 104 | |
| G3TBY5 | Glucose-6-phosphate isomerase | GPI | 62.3 | 104 | |
| G3SMQ4 | Proteasome subunit alpha type | PSMA8 | 27.8 | 102 | |
| G3SRG6 | Acrosin | ACR | 40.0 | 102 | |
| G3SNZ3 | Sperm acrosome membrane-associated protein 1 | SPACA1 | 32.8 | 102 | |
| G3U7Z4 | A-kinase anchor protein 4 | AKAP4 | 89.3 | 99 | |
| G3T7P8 | Gamma-glutamyl hydrolase | GGH | 35.9 | 96 | |
| G3UDP9 | Disintegrin and metalloproteinase domain-containing protein 21 | ADAM21 | 79.5 | 95 | |
| G3UJ16 | Peroxiredoxin 6 | PRDX6 | 25.1 | 95 | |
| G3T7L7 | Leucine-rich repeat-containing protein 37A3-like | LRRC37A3 | 151.3 | 91 |
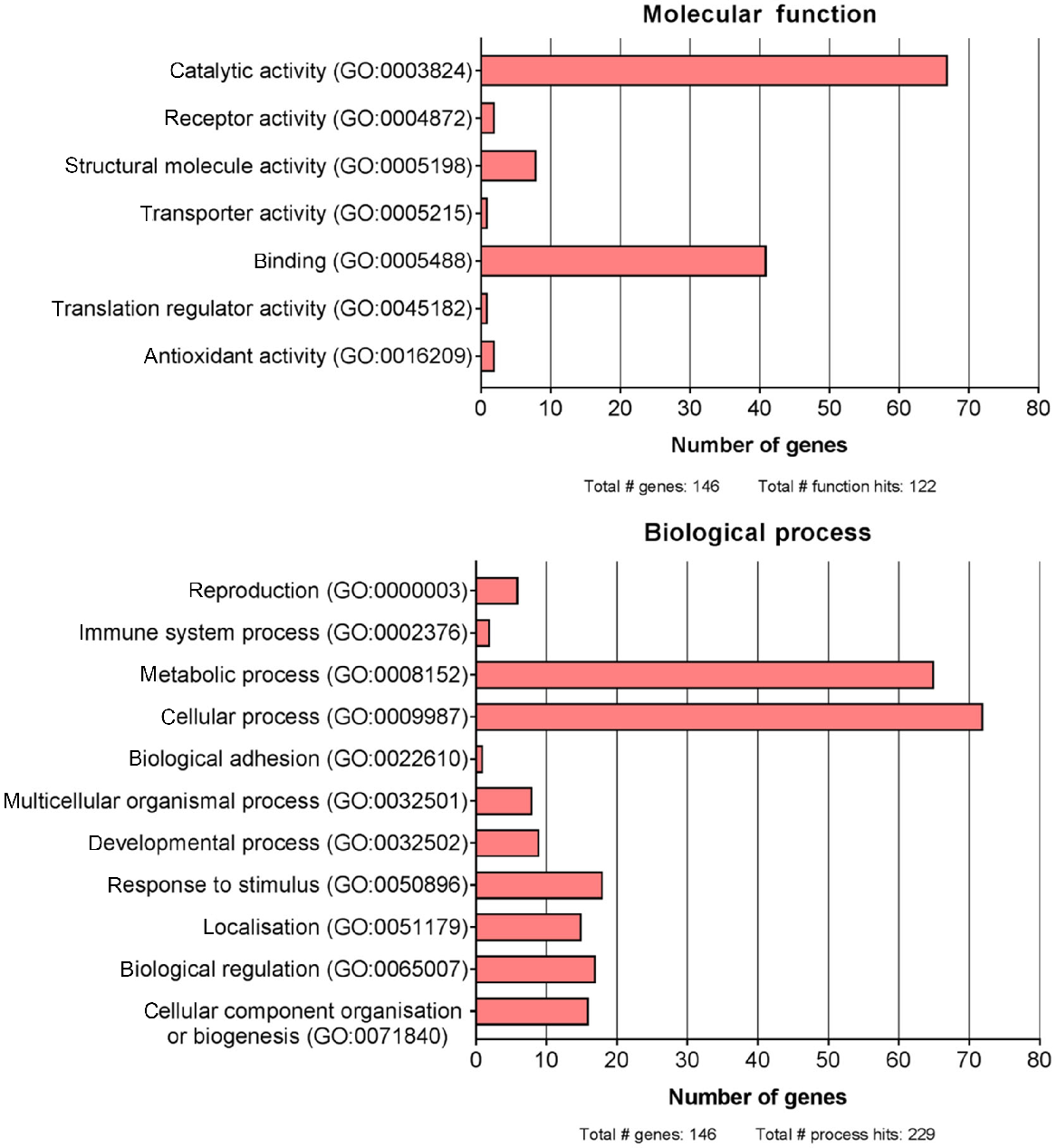
The proteins identified in Asian elephant SP are known to be involved in a wide range of molecular functions and biological processes, as per gene ontology assessment via PANTHER (Fig. 2). Of the total 155 Asian elephant SP proteins, nine gene symbols could not be identified within the gene ontology database. The most common molecular functions amongst the SP protein data set were catalytic activity (GO:0003824; 54.9% of total function hits) and binding (GO:0005488; 33.6% of total function hits). In terms of biological processes, cellular (GO:0009987; 31.4% of total process hits) and metabolic (GO:0008152; 28.4% of total process hits) processes were the most common amongst the identified Asian elephant SP proteins. Only six genes (4.1% of total gene hits) were categorised with ‘reproduction’ as a biological process. It should be noted that a protein may be listed under more than one category. Further categorising of Asian elephant SP proteins into cellular components and protein classes can be found in Fig. S1.
The molecular functions and biological processes of Asian elephant seminal plasma proteins. Proteins were categorised from the Gene Ontology database using PANTHER (ver. 13.1). The graph plots the number of proteins identified for each function and process (GO accession). Note: proteins can have multiple functions and processes.
Cryopreservation study
Out of 36 attempted collections from nine bulls, 26 collections (from seven bulls) resulted in semen samples. Among these, six samples (from five bulls) met the criteria for the cryopreservation study (fresh total motility ≥40%). The fresh semen parameters of these ejaculates are summarised in Table 4.
| Parameter | All ejaculates (seven bulls) | Ejaculates used for freezing (motility ≥ 40%; five bulls) | |||
|---|---|---|---|---|---|
| Mean ± s.e.m. | Range | Mean ± s.e.m. | Range | ||
| No. of ejaculates | 26 | 6 | |||
| Volume (mL) | 9.3 ± 1.5 | (0.5–35.0) | 5.1 ± 0.8 | (3.0–7.5) | |
| Sperm concentration (×106/mL) | 1375.1 ± 160.7 | (422.5–3642.5) | 1481.7 ± 337.1 | (680.0–2720.0) | |
| pH | 7.4 ± 0.2 | (6.0–8.5) | 7.7 ± 0.3 | (6.5–8.5) | |
| Normal morphology (%) | 43.2 ± 4.9 | (9.3–93.5) | 71.3 ± 8.7 | (35.0–93.5) | |
| Motility (%) | 24.8 ± 4.0 | (0.0–75.0) | 57.5 ± 4.6 | (40.0–75.0) | |
| Kinematic rating (0–5) | 2.8 ± 0.3 | (0.0–4.5) | 3.9 ± 0.3 | (2.5–4.5) | |
| Sperm motility index | 40.5 ± 4.3 | (0.0–82.5) | 67.9 ± 5.2 | (45.0–82.5) | |
| Viability (%) | 28.8 ± 4.6 | (1.0–86.5) | 56.8 ± 11.1 | (15.5–86.5) | |
| HOST positive (%)A | 17.2 ± 3.2 | (1.0–56.5) | 35.7 ± 7.7 | (5.5–56.5) | |
| Intact acrosomes (%) | 30.1 ± 4.7 | (4.0–89.0) | 59.0 ± 9.8 | (28.5–89.0) | |
| Intact DNA (%) | 45.3 ± 5.4 | (1.5–92.0) | 70.3 ± 8.7 | (35.0–92.0) | |
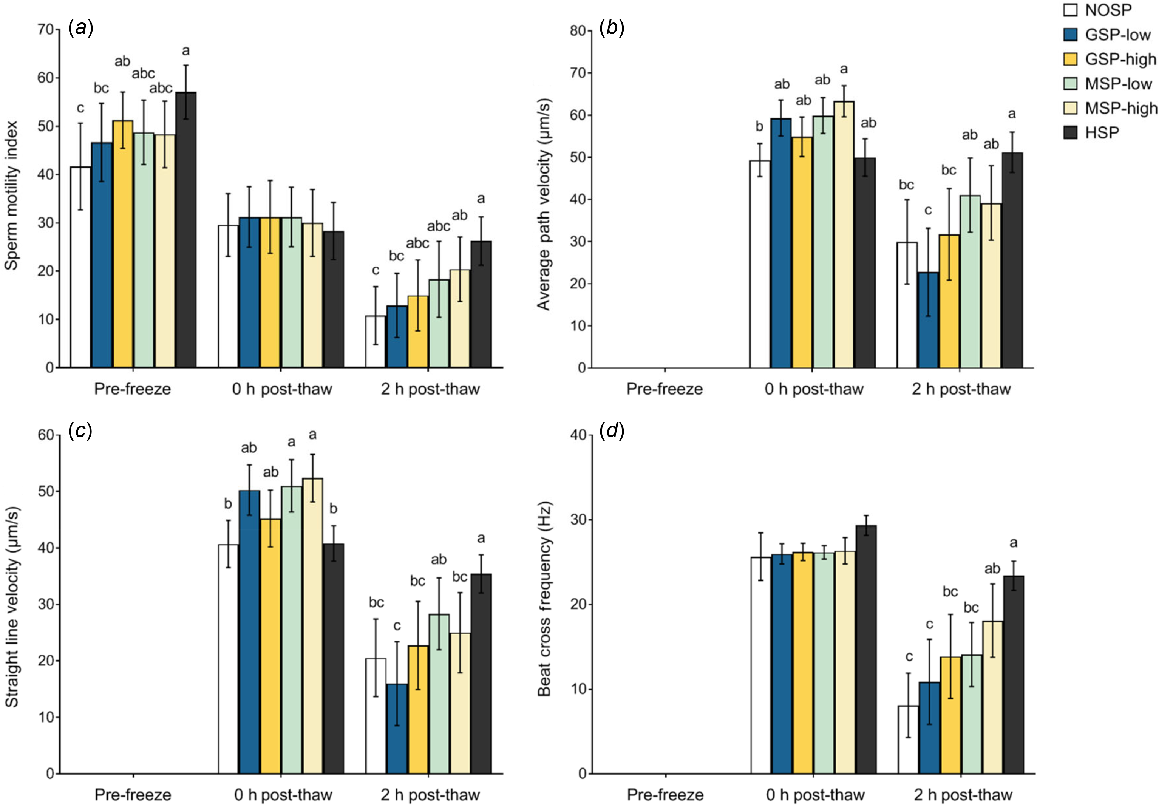
No significant effects of SP treatments were detected on total sperm motility, or on plasma membrane, acrosome and DNA integrity, both pre-freeze and post-thaw (P > 0.05; Table 5). The only significant effects of SP treatments on Asian elephant spermatozoa quality were detected with some sperm kinematic parameters. Sperm motility index (SMI) was higher in HSP-treated spermatozoa than in NOSP at both pre-freeze assessment and at 2 h post-thaw (P = 0.011; Fig. 3a). In elephant SP treatment groups, GSP-high exhibited significantly higher SMI than NOSP at pre-freeze, while MSP-high displayed significantly higher SMI than NOSP at 2 h post-thaw. Within the same SP quality groups (GSP and MSP) there were no differences in SMI between high and low concentrations over time. Sperm kinematic metrics were only evaluated by CASA post-thaw. At 0 h post-thaw, the VAP of MSP-high treated spermatozoa was greater than that of NOSP, but by 2 h post-thaw, only HSP displayed greater VAP than NOSP (P = 0.013; Fig. 3b). Immediately after thawing, the VSL of MSP-low and MSP-high were significantly higher than those of NOSP (P = 0.004). By 2 h post-thaw, HSP and MSP-low both displayed significantly higher VSL than NOSP (P = 0.004; Fig. 3c). Treatments HSP and MSP-high exhibited higher BCF than NOSP at 2 h post-thaw (P = 0.008; Fig. 3d). Within CASA sperm parameters, no significant differences were found between high and low SP concentration within the same SP quality group (GSP and MSP; P > 0.05; Fig. 3). No significant effects of SP treatments were found in the other sperm kinematic parameters as measured by CASA (P > 0.05; Table 6).
| Sperm parameters | ||||||
|---|---|---|---|---|---|---|
| Total motility (%)A | Viability (%) | HOST positive (%) | Intact acrosomes (%) | Intact DNA (%) | ||
| Pre-freeze | ||||||
| NOSP | 28.3 ± 6.9 | 47.3 ± 13.5 | 30.2 ± 7.6 | 52.3 ± 8.6 | 56.8 ± 9.2 | |
| GSP-low | 33.3 ± 6.9 | 49.0 ± 13.0 | 30.9 ± 7.3 | 51.7 ± 8.5 | 56.9 ± 10.7 | |
| GSP-high | 39.2 ± 5.7 | 49.8 ± 13.5 | 36.3 ± 8.1 | 51.7 ± 8.9 | 58.1 ± 10.2 | |
| MSP-low | 32.5 ± 7.3 | 52.1 ± 14.6 | 31.6 ± 7.0 | 50.3 ± 8.0 | 56.3 ± 11.1 | |
| MSP-high | 38.3 ± 5.6 | 50.0 ± 13.2 | 33.8 ± 7.5 | 51.6 ± 8.6 | 54.3 ± 8.8 | |
| HSP | 40.8 ± 6.4 | 50.0 ± 12.8 | 32.1 ± 6.8 | 52.8 ± 7.6 | 58.9 ± 9.9 | |
| 0 h post-thaw | ||||||
| NOSP | 15.8 ± 6.6 | 37.2 ± 8.0 | 14.2 ± 2.0 | 44.3 ± 8.2 | 57.9 ± 12.1 | |
| GSP-low | 15.8 ± 6.6 | 35.9 ± 7.9 | 12.0 ± 1.7 | 44.4 ± 7.9 | 47.3 ± 9.9 | |
| GSP-high | 19.2 ± 8.2 | 35.4 ± 7.6 | 13.0 ± 2.1 | 44.1 ± 8.1 | 47.9 ± 9.1 | |
| MSP-low | 17.5 ± 5.9 | 36.1 ± 8.0 | 12.4 ± 2.1 | 48.3 ± 8.5 | 51.5 ± 10.6 | |
| MSP-high | 16.7 ± 7.5 | 37.1 ± 7.8 | 14.8 ± 2.1 | 42.9 ± 8.8 | 48.6 ± 10.0 | |
| HSP | 13.3 ± 5.4 | 33.6 ± 8.5 | 13.3 ± 1.9 | 46.0 ± 9.4 | 54.7 ± 8.8 | |
| 2 h post-thaw | ||||||
| NOSP | 5.0 ± 3.2 | 32.1 ± 7.1 | 10.4 ± 1.4 | 41.4 ± 8.4 | 37.7 ± 8.0 | |
| GSP-low | 7.5 ± 4.2 | 34.9 ± 7.5 | 9.2 ± 1.2 | 45.2 ± 8.1 | 39.6 ± 8.4 | |
| GSP-high | 10.0 ± 6.5 | 37.7 ± 8.9 | 10.5 ± 1.9 | 40.8 ± 8.7 | 41.2 ± 9.6 | |
| MSP-low | 11.7 ± 6.1 | 35.0 ± 7.9 | 10.4 ± 1.3 | 41.2 ± 8.6 | 41.8 ± 8.7 | |
| MSP-high | 10.8 ± 5.4 | 31.8 ± 6.5 | 11.2 ± 0.7 | 44.4 ± 9.7 | 41.8 ± 8.1 | |
| HSP | 12.5 ± 4.4 | 32.9 ± 7.2 | 13.2 ± 1.5 | 47.7 ± 9.2 | 42.8 ± 8.7 | |
The effects of the addition of ‘good’ seminal plasma (GSP) and mixed seminal plasma (MSP), at high or low concentrations, absence of seminal plasma (NOSP), and horse seminal plasma (HSP) on Asian elephant sperm parameters before and after cryopreservation. Values are presented as mean ± s.e.m. Within the parameters presented, there were no significant differences between seminal plasma treatments (P > 0.05).
Sperm motility and kinematic parameters of frozen-thawed Asian elephant spermatozoa. The effects of the absence of seminal plasma (NOSP), ‘good’ conspecific seminal plasma (GSP), and mixed conspecific seminal plasma (MSP), at low (12.5%, v/v) or high (25%) concentration, and horse seminal plasma (HSP 25%) on Asian elephant spermatozoa before and after cryopreservation. Data are shown as mean ± s.e.m. of (a) sperm motility index, (b) average path velocity (VAP), (c) straight line velocity (VSL), and (d) sperm head beat cross frequency (BCF). Sperm kinematic parameters assessed by CASA at post-thaw only. Within time points on each graph, different lowercase letters between treatment groups represent significant differences (P < 0.05).
| Sperm kinematic parameters | |||||||
|---|---|---|---|---|---|---|---|
| Motility (%) | pMOT (%) | VCL (μm/s) | ALH (μm) | STR (%) | LIN (%) | ||
| 0 h post-thaw | |||||||
| NOSP | 11.5 ± 4.1 | 1.8 ± 0.9 | 78.5 ± 4.0 | 5.0 ± 1.2 | 79.5 ± 3.5 | 52.2 ± 4.7 | |
| GSP-low | 10.3 ± 3.0 | 2.5 ± 1.1 | 89.6 ± 3.1 | 5.2 ± 0.6 | 82.0 ± 1.9 | 56.3 ± 4.4 | |
| GSP-high | 14.5 ± 6.4 | 3.3 ± 1.9 | 84.3 ± 4.6 | 3.5 ± 0.8 | 76.0 ± 3.7 | 54.2 ± 4.1 | |
| MSP-low | 14.0 ± 4.0 | 2.0 ± 0.9 | 91.4 ± 3.8 | 5.3 ± 0.5 | 82.8 ± 2.3 | 57.2 ± 4.8 | |
| MSP-high | 12.8 ± 4.1 | 3.2 ± 1.4 | 100.6 ± 2.3 | 5.5 ± 0.4 | 78.8 ± 2.0 | 52.2 ± 3.8 | |
| HSP | 10.2 ± 3.3 | 1.3 ± 0.5 | 83.3 ± 9.1 | 6.0 ± 0.4 | 78.3 ± 3.5 | 51.7 ± 3.1 | |
| 2 h post-thaw | |||||||
| NOSP | 4.5 ± 2.6 | 0.2 ± 0.2 | 53.1 ± 18.5 | 1.8 ± 1.2 | 45.0 ± 14.5 | 26.5 ± 8.7 | |
| GSP-low | 6.3 ± 3.3 | 0.3 ± 0.2 | 42.9 ± 20.1 | 3.8 ± 1.7 | 33.7 ± 15.1 | 19.2 ± 8.6 | |
| GSP-high | 7.2 ± 4.1 | 0.7 ± 0.5 | 62.6 ± 20.9 | 3.8 ± 1.7 | 46.7 ± 14.8 | 24.5 ± 7.8 | |
| MSP-low | 7.8 ± 4.0 | 0.5 ± 0.3 | 74.1 ± 16.6 | 3.1 ± 1.4 | 56.7 ± 11.5 | 32.5 ± 6.6 | |
| MSP-high | 9.5 ± 4.8 | 0.8 ± 0.7 | 76.1 ± 16.7 | 3.7 ± 1.7 | 51.0 ± 11.6 | 27.2 ± 6.4 | |
| HSP | 11.0 ± 4.1 | 0.8 ± 0.4 | 95.5 ± 9.2 | 5.2 ± 1.4 | 68.5 ± 2.5 | 39.5 ± 1.3 | |
The post-thaw effects of ‘good’ seminal plasma (GSP) and mixed seminal plasma (MSP), at high or low concentration, absence of seminal plasma (NOSP), and horse seminal plasma (HSP) on Asian elephant sperm kinematic parameters as assessed by CASA. Within the parameters presented, there were no significant differences between seminal plasma treatments (P > 0.05).
pMOT, progressive motility; VCL, curvilinear velocity; ALH, amplitude of lateral sperm head displacement; STR, straightness; LIN, linearity.
No differences in sperm motility, plasma membrane integrity, and acrosome and DNA integrity were detected (P > 0.05; Table 5) between spermatozoa exposed to different SP quality groups (GSP and MPS) or HSP. Spermatozoa subjected to HSP displayed significantly higher SMI than those exposed to GSP-low at both pre-freeze and 2 h post-thaw (P = 0.011; Fig. 3a). No differences in response between exposure to Asian elephant GSP and MSP was detected with SMI evaluation (P > 0.05; Fig. 3a). At 2 h post-thaw, VAP was significantly higher with HSP compared to both GSP-low and GSP-high (P = 0.013; Fig. 3b). With Asian elephant SP, both MSP-low and MSP-high resulted in higher VAP than GSP-low by 2 h post-thaw (P = 0.013; Fig. 3b). Immediately after thawing, MSP-high and MSP-low showed higher VSL than HSP; however, by 2 h post-thaw MPS-high, GSP-high, and GSP-low all had significantly lower VSL than HSP (P = 0.004; Fig. 3c). Within elephant SP, after 2 h post-thaw MSP-low displayed higher VSL than GSP-low (P = 0.004; Fig. 3c). Sperm BCF was not different immediately at thawing, but it was higher in HSP than in GSP-low, GSP-high and MSP-low after 2 h post-thaw (P = 0.008; Fig. 3d). Within elephant SP, MSP-high demonstrated higher BCF than GSP-low (P = 0.008). No significant effects of SP treatments were found with the other sperm kinematic parameters as measured by CASA (P > 0.05; Table 6).
Discussion
This study describes two steps in the identification of possible positive effects of SP proteins on the post-thaw quality of Asian Elephant spermatozoa. The initial part provides valuable knowledge about the SP proteome of Asian elephants, while the latter component elucidates some preliminary information about the effects of differing SP profiles on Asian elephant spermatozoa during cryopreservation.
Seminal plasma proteomics provides a tool to understand the interactions of SP proteins with spermatozoa and how this may affect sperm functionality, fertilisation, and in vitro preservation. Determining the presence and abundance of selected SP proteins can be used for fertility assessment and breeding soundness of males within domestic species (Moura et al. 2006), or as markers of poor fertility in males (Jobim et al. 2011; Vilagran et al. 2015). Similarly, certain SP proteins have been identified in bulls with ejaculates of poor freezability (Jobim et al. 2004). The presence of lactotransferrin, one of the multifunctional proteins found in the current study, has been suggested to positively correlate with the fresh seminal quality of Asian elephant ejaculates (Kiso et al. 2013). However, the influence of SP proteins on Asian elephant spermatozoa cryotolerance and on potential differences between males remains to be elucidated.
In the current proteomic study of Asian elephant SP, 155 total proteins were matched in the UniProt databases. In another previously published study (Wattananit et al. 2023), 597 proteins (nearly four times more) were similarly identified in Asian elephant SP using similar tandem-mass spectrometry techniques. Greater total numbers of proteins have also been observed in other mammalian species’ SP (Soleilhavoup et al. 2014; Perez-Patiño et al. 2016). However, the total number of proteins detected is dependent on the proteomic analysis techniques used and the degree of sensitivity for protein detection. The greater representation of samples and males in the proteomic analysis performed by Wattananit et al. (2023) may have also determined the number of detected proteins, suggesting the uniqueness of males and sample quality.
An interesting similarity between Wattananit et al. (2023) and this study was the relatively low proportion of mapped proteins recognised in reproductive processes (29 and 6, respectively). This emphasises the diverse, multifunctional, and ubiquitous nature of most proteins found in SP, and the great difficulty in identifying single proteins that correlate with and protect sperm parameters. The protein identification process in both studies differed. Whereas our study had peptides searched against the completed African elephant (L. africana) genome first, Wattananit et al. (2023) searched against a broader mammal protein database, likely yielding different proteins. Regardless, Asian elephant SP proteins identified by both studies have been matched with SP proteins from other species (Druart et al. 2013), but inter- and intraspecies comparisons show that most of them differ in terms of isoforms or subunits. To maximise the identification of proteins in Asian elephant SP, ejaculates with varying sperm parameter qualities were included in our analysis. Through gene ontology evaluation, we observed a large variety of proteins and protein groups, reflected in a diverse array of molecular functions, protein classes, and associated biological processes. One notable group of proteins identified in Asian elephant SP is the heat shock family proteins, including HSP90AA1, HSP90B1, HSPA1A, HSPA5, HSPA4L, and HSPA9. In other species, these proteins have been shown to have beneficial effects, including aspects of reproduction such as improving the viability of ram spermatozoa (Lloyd et al. 2012). Additionally, they have been correlated with high sperm motility, normal morphology, and viability, and have been used as predictors of freezability in boar spermatozoa (Turba et al. 2007; Casas et al. 2009). Spermadhesins are another group of proteins that can make up a large proportion of all SP proteins in other species. Their multifunctional roles include aspects of reproduction such as different stages of fertilisation (Töpfer-Petersen et al. 1998). Studies have suggested that spermadhesins, which are associated with binding to the sperm surface, also play a role in protecting ram spermatozoa against cold shock (Barrios et al. 2005). However, the specific roles of spermadhesins and many other identified proteins in Asian elephant SP remain undetermined. While functions can be surmised from studies in other species, these roles may be species-specific and not directly translatable. Furthermore, as aforementioned, proteins can be expressed as different isoforms or subunits across species (Pérez-Patiño et al. 2018; Wattananit et al. 2023). Mass spectrometry analysis allows for a deeper exploration of the proteomic landscape of Asian elephant SP, which may advance assisted reproductive technologies in the species and enhance our comprehension of male elephant fertility.
The second component of this study aimed to understand the effect of SP proteins on spermatozoa during the cryopreservation process. It appears that there is no consistent freezing protocol for Asian elephant spermatozoa, including whether to remove or dilute seminal plasma, despite several other publications in the area (Saragusty et al. 2009; Kiso et al. 2012; Arnold et al. 2017). This sort of preliminary cryopreservation research is warranted as it may help improve the post-thaw quality and fertility of Asian elephant spermatozoa. This is necessary due to the low success rate of AI with frozen-thawed spermatozoa (Thongtip et al. 2009) and the generally poor sperm cryosurvival observed across most males and ejaculates (Buranaamnuay et al. 2013; Imrat et al. 2013; Arnold et al. 2017).
Sperm velocity and kinematic characteristics, as determined by CASA, can provide valuable information about sperm activation and fertilising potential (Farrell et al. 1998). While this study was limited by the inability to undertake CASA assessment of the spermatozoa prior to freezing, there was still value in the ability to compare these parameters between the different treatment groups post-thaw. In the present study, total sperm motility and membrane-associated parameters did not differ between SP treatments, but some sperm kinematic parameters (CASA) did. The presence of SP resulted in elevated kinematics post-thaw when compared to the absence of SP. This finding is consistent with the corresponding patterns with SMI, which integrates subjective assessment of sperm forward progressive movement and total motility. Sperm velocity parameters VAP and VSL have been found to have a strong correlation with post-thawed bull sperm fertility (Nagy et al. 2015), suggesting that straight line speed may predict the likelihood of spermatozoa reaching the site of fertilisation. However, this data only applies to the motile spermatozoa, which made up only a small proportion of the total sperm population after cryopreservation in Asian elephant. The low total motility makes it problematic to extrapolate these findings for Asian elephants with confidence, and thus further work is warranted to improve the post-thaw total motility.
Within the same quality type group, the concentration of SP during the freezing process showed no difference. However higher concentration of SP (25% v/v) displayed greater sperm kinematics when compared to the absence of SP. Interestingly, the current study did not show a difference in the cryoprotective capabilities with the addition of SP from a pool of high motility ejaculates compared the absence of SP. Our assumption that the high motility samples would contain SP components which would enhance or protect spermatozoa was not confirmed. Similar findings have been documented with horse spermatozoa whereby SP had no effect on spermatozoa post-thaw even when sourced from males with good sperm cryotolerance (Al-Essawe et al. 2018). The difference in categorising and quantifying a ‘good’ sample from which to obtain SP could explain these differences between studies. There have been few studies on the direct effects of SP on the freezing and thawing of Asian elephant spermatozoa. The addition of 10% (v/v) autologous SP to Asian elephant spermatozoa post-thaw did not improve sample quality parameters (Saragusty et al. 2009). Despite the complexity of SP and its many potential influencing constituents, our preliminary study is the first to show the potential protective effects of SP during cooling and thawing in this species.
The potential enhancing and protective benefits of heterospecific SP on spermatozoa are often overlooked. In this study, HSP showed a positive effect on Asian elephant spermatozoa in terms of sperm velocity parameters post-thaw. These enhanced effects of HSP were also found with SMI at pre-freeze assessment. These findings coincide with previous studies reporting that HSP supplemented in the extender during chilled liquid storage of Asian elephant spermatozoa provided greater protection of sperm motility and velocities (Pinyopummin et al. 2017). More specifically, HSP has been shown to increase sperm velocity parameters measured by CASA with no effect on Asian elephant sperm viability and acrosomal integrity (Pinyopummin et al. 2018). Interestingly, the effects of HSP on stallion spermatozoa preservation can be detrimental (Jasko et al. 1991; Love et al. 2005), though they vary depending on the ejaculate and male source (Aurich et al. 1996). Further investigations are needed to understand the effects of individual male Asian elephants. However, given the widespread issue of inconsistent ejaculate quality in Asian elephants (Kiso et al. 2013), achieving this is challenging, and there may be more noticeable effects of individual samples due to the varying seminal plasma constitution between samples (Sivilaikul et al. 2010; Kiso et al. 2013).
The component in HSP that supported elephant sperm motility has not been identified. The proteomic profile of HSP is different from other domestic species (Druart et al. 2013) and Asian elephant SP. The three major groups of proteins identified in HSP are fibronectin type 2, cysteine-rich secretory proteins, and spermadhesins (Töpfer-Petersen et al. 2005). The majority of HSP proteins (70–80% of the total proteins) belong to the fibronectin group (Calvete et al. 1995), known for its specific interaction with the phospholipids of the sperm membrane and their heparin-binding ability (Calvete et al. 1994), indicating a potential role in early fertilisation processes. However, it is challenging to identify these specific causative SP components, particularly proteins, across different species, as the predominance of each type of heparin-binding protein varies between species (Druart et al. 2013) as well as between different protein families mediating heparin-binding roles (Töpfer-Petersen et al. 2005).
Whilst this cryopreservation study was limited due to the inconsistencies in collecting high quality ejaculates from Asian elephants, the preliminary results have shown the potential benefits that SP has on the post-thaw velocities of Asian elephant spermatozoa. Conspecific SP sourced from a large variety of elephant bulls and heterospecific HSP may provide protection of sperm velocity parameters during post-thaw incubation compared to the absence of SP. Identifying the protective components in conspecific and heterospecific HSP should be a priority for future studies. The current study has also contributed to the characterisation of the Asian elephant SP proteome, a relatively new and unexplored area. Due to limited samples, we were unable to identify the differences in the proteomic profile between high and low motility samples. Therefore, additional studies are warranted which should further investigate the impacts of SP on preservation techniques of Asian elephant spermatozoa. Nonetheless, the two reported studies have served as a foundational steppingstone in understanding Asian elephant SP and its potential role in improved sperm cryopreservation. Ultimately, further investigations in this field may aid in advancing reproductive technologies for this endangered species.
Data availability
Any data supporting this study not already included in the article or supplementary material will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that the research was conducted in the absence of any financial or non-financial (political, personal, professional) interests/relationships that may be interpreted to have influenced the manuscript.
Acknowledgements
The authors would like to express gratitude for the support and assistance of collections and providing research space from the following institutes that participated in this study: Thai Elephant Conservation Centre, Taronga Conservation Society Australia, Zoos Victoria, and Perth Zoo. The authors would also like to thank the Bioanalytical Mass Spectrometry Facility at the University of New South Wales for assistance in proteomic analyses. This paper forms part of the PhD Thesis of Negus (2021).
References
Al-Essawe EM, Wallgren M, Wulf M, Aurich C, Macias-Garcia B, Sjunnesson Y, Morrell JM (2018) Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology 115, 99-107.
| Crossref | Google Scholar | PubMed |
Aquino-Cortez A, Pinheiro BQ, Lima DBC, Silva HVR, Mota-Filho AC, Martins JAM, Rodriguez-Villamil P, Moura AA, Silva LDM (2017) Proteomic characterization of canine seminal plasma. Theriogenology 95, 178-186.
| Crossref | Google Scholar | PubMed |
Arnold DM, Gray C, Roth TL, Mitchell S, Graham LH (2017) A simple, field-friendly technique for cryopreserving semen from Asian elephants (Elephas maximus). Animal Reproduction Science 182, 84-94.
| Crossref | Google Scholar | PubMed |
Aurich JE, Kühne A, Hoppe H, Aurich C (1996) Seminal plasma affects membrane integrity and motility of equine spermatozoa after cryopreservation. Theriogenology 46(5), 791-797.
| Crossref | Google Scholar | PubMed |
Azerêdo GA, Esper CR, Resende KT (2001) Evaluation of plasma membrane integrity of frozen–thawed goat spermatozoa with or without seminal plasma. Small Ruminant Research 41(3), 257-263.
| Crossref | Google Scholar |
Barrios B, Fernández-Juan M, Muiño-Blanco T, Cebrián-Pérez JA (2005) Immunocytochemical localization and biochemical characterization of two seminal plasma proteins that protect ram spermatozoa against cold shock. Journal of Andrology 26(4), 539-549.
| Crossref | Google Scholar | PubMed |
Ben WX, Fu MT, Mao LK, Ming ZW, Xiong WW (1997) Effects of various concentrations of native seminal plasma in cryoprotectant on viability of human sperm. Archives of Andrology 39(3), 211-216.
| Crossref | Google Scholar | PubMed |
Björndahl L, Söderlund I, Kvist U (2003) Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Human Reproduction 18(4), 813-816.
| Crossref | Google Scholar | PubMed |
Buranaamnuay K, Mahasawangkul S, Saikhun K (2013) The in vitro quality of frozen-thawed Asian elephant (Elephas maximus) spermatozoa in semen supplemented with Equex STM paste and oxytocin during and after cryopreservation. Reproductive Biology 13(2), 169-171.
| Crossref | Google Scholar | PubMed |
Calvete JJ, Nessau S, Mann K, Sanz L, Sieme H, Klug E, Töpfer-Petersen E (1994) Isolation and biochemical characterization of stallion seminal-plasma proteins. Reproduction in Domestic Animals 29(5), 411-426.
| Crossref | Google Scholar |
Calvete JJ, Mann K, Schäfer W, Sanz L, Reinert M, Nessau S, Raida M, Töpfer-Petersen E (1995) Amino acid sequence of HSP-1, a major protein of stallion seminal plasma: effect of glycosylation on its heparin- and gelatin-binding capabilities. Biochemical Journal 310(2), 615-622.
| Crossref | Google Scholar |
Casas I, Sancho S, Briz M, Pinart E, Bussalleu E, Yeste M, Bonet S (2009) Freezability prediction of boar ejaculates assessed by functional sperm parameters and sperm proteins. Theriogenology 72(7), 930-948.
| Crossref | Google Scholar | PubMed |
Druart X, Rickard JP, Mactier S, Kohnke PL, Kershaw-Young CM, Bathgate R, Gibb Z, Crossett B, Tsikis G, Labas V, Harichaux G, Grupen CG, de Graaf SP (2013) Proteomic characterization and cross species comparison of mammalian seminal plasma. Journal of Proteomics 91, 13-22.
| Crossref | Google Scholar | PubMed |
Farrell PB, Presicce GA, Brockett CC, Foote RH (1998) Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenology 49(4), 871-879.
| Crossref | Google Scholar | PubMed |
Gatlin CL, Kleemann GR, Hays LG, Link AJ, Yates JR, III (1998) Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography–microspray and nanospray mass spectrometry. Analytical Biochemistry 263(1), 93-101.
| Crossref | Google Scholar | PubMed |
Henault MA, Killian GJ (1996) Effect of homologous and heterologous seminal plasma on the fertilizing ability of ejaculated bull spermatozoa assessed by penetration of zona-free bovine oocytes. Journal of Reproduction and Fertility 108(2), 199-204.
| Crossref | Google Scholar | PubMed |
Hernández M, Roca J, Calvete JJ, Sanz L, Muiño-Blanco T, Cebrián-Pérez JA, Vázquez JM, Martínez EA (2007) Cryosurvival and in vitro fertilizing capacity postthaw is improved when boar spermatozoa are frozen in the presence of seminal plasma from good freezer boars. Journal of Andrology 28(5), 689-697.
| Crossref | Google Scholar | PubMed |
Hildebrandt TB, Hermes R, Pratt NC, Fritsch G, Blottner S, Schmitt DL, Ratanakorn P, Brown JL, Rietschel W, Göritz F (2000) Ultrasonography of the urogenital tract in elephants (Loxodonta africana and Elephas maximus): an important tool for assessing male reproductive function. Zoo Biology 19(5), 333-345.
| Crossref | Google Scholar |
Hildebrandt TB, Göritz F, Hermes R, Reid C, Dehnhard M, Brown JL (2006) Aspects of the reproductive biology and breeding management of Asian and African elephants Elephas maximus and Loxodonta africana. International Zoo Yearbook 40(1), 20-40.
| Crossref | Google Scholar |
Howard J, Wildt DE (1990) Ejaculate-hormonal traits in the leopard cat (Felis bengalensis) and sperm function as measured by in vitro penetration of zona-free hamster ova and zona-intact domestic cat oocytes. Molecular Reproduction & Development 26(2), 163-174.
| Crossref | Google Scholar | PubMed |
Imrat P, Suthanmapinanth P, Saikhun K, Mahasawangkul S, Sostaric E, Sombutputorn P, Jansittiwate S, Thongtip N, Pinyopummin A, Colenbrander B, Holt WV, Stout TAE (2013) Effect of pre-freeze semen quality, extender and cryoprotectant on the post-thaw quality of Asian elephant (Elephas maximus indicus) semen. Cryobiology 66(1), 52-59.
| Crossref | Google Scholar | PubMed |
Jasko DJ, Moran DM, Farlin ME, Squires EL (1991) Effect of seminal plasma dilution or removal on spermatozoal motion characteristics of cooled stallion semen. Theriogenology 35(6), 1059-1067.
| Crossref | Google Scholar |
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJD (1984) Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. Journal of Reproduction and Fertility 70(1), 219-228.
| Crossref | Google Scholar | PubMed |
Jobim MIM, Oberst ER, Salbego CG, Souza DO, Wald VB, Tramontina F, Mattos RC (2004) Two-dimensional polyacrylamide gel electrophoresis of bovine seminal plasma proteins and their relation with semen freezability. Theriogenology 61(2-3), 255-266.
| Crossref | Google Scholar | PubMed |
Jobim MIM, Trein C, Zirkler H, Gregory RM, Sieme H, Mattos RC (2011) Two-dimensional polyacrylamide gel electrophoresis of equine seminal plasma proteins and their relation with semen freezability. Theriogenology 76(4), 765-771.
| Crossref | Google Scholar | PubMed |
Juyena NS, Stelletta C (2012) Seminal plasma: an essential attribute to spermatozoa. Journal of Andrology 33(4), 536-551.
| Crossref | Google Scholar | PubMed |
Kawano N, Shimada M, Terada T (2004) Motility and penetration competence of frozen-thawed miniature pig spermatozoa are substantially altered by exposure to seminal plasma before freezing. Theriogenology 61(2-3), 351-364.
| Crossref | Google Scholar | PubMed |
Kiso WK, Asano A, Travis AJ, Schmitt DL, Brown JL, Pukazhenthi BS (2012) Pretreatment of Asian elephant (Elephas maximus) spermatozoa with cholesterol-loaded cyclodextrins and glycerol addition at 4°C improves cryosurvival. Reproduction, Fertility and Development 24(8), 1134-1142.
| Crossref | Google Scholar | PubMed |
Kiso WK, Selvaraj V, Nagashima J, Asano A, Brown JL, Schmitt DL, Leszyk J, Travis AJ, Pukazhenthi BS (2013) Lactotransferrin in Asian elephant (Elephas maximus) seminal plasma correlates with semen quality. PLoS ONE 8(8), e71033.
| Crossref | Google Scholar | PubMed |
Larson JL, Miller DJ (1999) Simple histochemical stain for acrosomes on sperm from several species. Molecular Reproduction & Development 52(4), 445-449.
| Crossref | Google Scholar | PubMed |
Lloyd RE, Fazeli A, Watson PF, Holt WV (2012) The oviducal protein, heat-shock 70-kDa protein 8, improves the long-term survival of ram spermatozoa during storage at 17°C in a commercial extender. Reproduction, Fertility and Development 24(4), 543-549.
| Crossref | Google Scholar | PubMed |
Love CC, Brinsko SP, Rigby SL, Thompson JA, Blanchard TL, Varner DD (2005) Relationship of seminal plasma level and extender type to sperm motility and DNA integrity. Theriogenology 63(6), 1584-1591.
| Crossref | Google Scholar | PubMed |
Martínez-Pastor F, Anel L, Guerra C, Álvarez M, Soler AJ, Garde JJ, Chamorro C, de Paz P (2006) Seminal plasma improves cryopreservation of Iberian red deer epididymal sperm. Theriogenology 66(8), 1847-1856.
| Crossref | Google Scholar | PubMed |
Mataveia GA, Terblanche SJ, Nothling JO, Gerber D (2010) Effect of heterologous seminal plasma and semen extenders on motility of frozen-thawed ram spermatozoa. Journal of the South African Veterinary Association 81(3), 139-142.
| Crossref | Google Scholar | PubMed |
Matson P, Kappelle W, Malecki I (2009) The use of a hypo-osmotic swelling (HOS) test on sperm of the pig (Sus scrofa domesticus), emu (Dromaius novaehollandiae), Asian elephant (Elephas maximus), hamadryas baboon (Papio hamadryas hamadryas), and central rock rat (Zyzomys pedunculatus). Reproductive Biology 9(2), 181-187.
| Crossref | Google Scholar | PubMed |
Mogielnicka-Brzozowska M, Kordan W (2011) Characteristics of selected seminal plasma proteins and their application in the improvement of the reproductive processes in mammals. Polish Journal of Veterinary Sciences 14(3), 489-499.
| Crossref | Google Scholar | PubMed |
Moore AI, Squires EL, Graham JK (2005) Effect of seminal plasma on the cryopreservation of equine spermatozoa. Theriogenology 63(9), 2372-2381.
| Crossref | Google Scholar | PubMed |
Moura AA, Koc H, Chapman DA, Killian GJ (2006) Identification of proteins in the accessory sex gland fluid associated with fertility indexes of dairy bulls: a proteomic approach. Journal of Andrology 27(2), 201-211.
| Crossref | Google Scholar | PubMed |
Nagy Á, Polichronopoulos T, Gáspárdy A, Solti L, Cseh S (2015) Correlation between bull fertility and sperm cell velocity parameters generated by computer-assisted semen analysis. Acta Veterinaria Hungarica 63(3), 370-381.
| Crossref | Google Scholar | PubMed |
O’Brien JK, Steinman KJ, Montano GA, Love CC, Saiers RL, Robeck TR (2013) Characteristics of high-quality Asian elephant (Elephas maximus) ejaculates and in vitro sperm quality after prolonged chilled storage and directional freezing. Reproduction, Fertility and Development 25(5), 790-797.
| Crossref | Google Scholar | PubMed |
Pappin DJC, Hojrup P, Bleasby AJ (1993) Rapid identification of proteins by peptide-mass fingerprinting. Current Biology 3(6), 327-332.
| Crossref | Google Scholar | PubMed |
Perez-Patiño C, Barranco I, Parrilla I, Valero ML, Martinez EA, Rodriguez-Martinez H, Roca J (2016) Characterization of the porcine seminal plasma proteome comparing ejaculate portions. Journal of Proteomics 142, 15-23.
| Crossref | Google Scholar | PubMed |
Peña A, Linde-Forsberg C (2000) Effects of Equex, one- or two-step dilution, and two freezing and thawing rates on post-thaw survival of dog spermatozoa. Theriogenology 54(6), 859-875.
| Crossref | Google Scholar | PubMed |
Pérez-Patiño C, Parrilla I, Barranco I, Vergara-Barberán M, Simó-Alfonso EF, Herrero-Martínez JM, Rodriguez-Martínez H, Martínez EA, Roca J (2018) New in-depth analytical approach of the porcine seminal plasma proteome reveals potential fertility biomarkers. Journal of Proteome Research 17(3), 1065-1076.
| Crossref | Google Scholar | PubMed |
Pinyopummin A, Mahasawangkul S, Kornkaewrat K, Rattanapirom S, Leartsang W, Kitkha S (2017) The presence of seminal plasma, especially derived from stallion semen, helps preserve chilled Asian elephant (Elephas maximus) sperm motility. Andrologia 49(6), e12690.
| Crossref | Google Scholar |
Pinyopummin A, Mahasawangkul S, Nunklang G, Kornkaewrat K, Laopiem S, Koonjaenak S, Wattananit P (2018) Supplemented stallion seminal plasma can improve impaired motility due to the dilution effect in chilled Asian elephant sperm. Reproduction in Domestic Animals 53(2), 525-533.
| Crossref | Google Scholar | PubMed |
Pozor MA, McDonnell SM (2002) Ultrasonographic measurements of accessory sex glands, ampullae, and urethra of normal stallions of various size types. Theriogenology 58(7), 1425-1433.
| Crossref | Google Scholar | PubMed |
Rickard JP, Leahy T, Soleilhavoup C, Tsikis G, Labas V, Harichaux G, Lynch GW, Druart X, de Graaf SP (2015) The identification of proteomic markers of sperm freezing resilience in ram seminal plasma. Journal of Proteomics 126, 303-311.
| Crossref | Google Scholar | PubMed |
Rodríguez-Martínez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ (2011) Seminal plasma proteins: what role do they play? American Journal of Reproductive Immunology 66(S1), 11-22.
| Crossref | Google Scholar |
Saragusty J, Hildebrandt TB, Behr B, Knieriem A, Kruse J, Hermes R (2009) Successful cryopreservation of Asian elephant (Elephas maximus) spermatozoa. Animal Reproduction Science 115(1-4), 255-266.
| Crossref | Google Scholar | PubMed |
Schmitt DL, Hildebrandt TB (1998) Manual collection and characterization of semen from Asian elephants (Elephas maximus). Animal Reproduction Science 53(1–4), 309-314.
| Crossref | Google Scholar | PubMed |
Sivilaikul S, Jitprom A, Kularb A, Kornkaewrut K, Suthanmaphinuth P, Mahasawangkul S, Saikhun K, Wajjwalku W, Thongtipsiridech S (2010) Relationship between seminal and serum calcium concentration with semen quality in the Asian elephant (Elephas maximus). The Thai Journal of Veterinary Medicine 40(3), 251-255.
| Crossref | Google Scholar |
Soleilhavoup C, Tsikis G, Labas V, Harichaux G, Kohnke PL, Dacheux JL, Guerin Y, Gatti JL, de Graaf SP, Druart X (2014) Ram seminal plasma proteome and its impact on liquid preservation of spermatozoa. Journal of Proteomics 109, 245-260.
| Crossref | Google Scholar | PubMed |
Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S (1984) A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertility and Sterility 42(1), 87-91.
| Crossref | Google Scholar | PubMed |
Thongtip N, Saikhun J, Damyang M, Mahasawangkul S, Suthunmapinata P, Yindee M, Kongsila A, Angkawanish T, Jansittiwate S, Wongkalasin W, Wajjwalkul W, Kitiyanant Y, Pavasuthipaisit K, Pinyopummin A (2004) Evaluation of post-thaw Asian elephant (Elephas maximus) spermatozoa using flow cytometry: the effects of extender and cryoprotectant. Theriogenology 62(3–4), 748-760.
| Crossref | Google Scholar | PubMed |
Thongtip N, Saikhun J, Mahasawangkul S, Kornkaewrat K, Suthanmapinanh P, Pinyopummin A (2008) Effect of pentoxifylline on the motility characteristics and viability of spermatozoa in Asian elephants (Elephas maximus) with low semen quality. The Thai Journal of Veterinary Medicine 38(3), 37-45.
| Crossref | Google Scholar |
Thongtip N, Mahasawangkul S, Thitaram C, Pongsopavijitr P, Kornkaewrat K, Pinyopummin A, Angkawanish T, Jansittiwate S, Rungsri R, Boonprasert K, Wongkalasin W, Homkong P, Dejchaisri S, Wajjwalku W, Saikhun K (2009) Successful artificial insemination in the Asian elephant (Elephas maximus) using chilled and frozen-thawed semen. Reproductive Biology and Endocrinology 7(1), 75.
| Crossref | Google Scholar |
Töpfer-Petersen E, Romero A, Varela PF, Ekhlasi-Hundrieser M, Dostàlovà Z, Sanz L, Calvete JJ (1998) Spermadhesins: a new protein family. Facts, hypotheses and perspectives. Andrologia 30(4-5), 217-224.
| Crossref | Google Scholar | PubMed |
Töpfer-Petersen E, Ekhlasi-Hundrieser M, Kirchhoff C, Leeb T, Sieme H (2005) The role of stallion seminal proteins in fertilisation. Animal Reproduction Science 89(1-4), 159-170.
| Crossref | Google Scholar | PubMed |
Turba ME, Fantinati P, Bernardini C, Gentilini F, Bacci ML, Forni M (2007) Relationships between innovative and traditional parameters to investigate semen quality in pigs. Animal Reproduction Science 99(1-2), 72-81.
| Crossref | Google Scholar | PubMed |
Ustuner B, Alcay S, Toker MB, Nur Z, Gokce E, Sonat FA, Gul Z, Duman M, Ceniz C, Uslu A, Sagirkaya H, Soylu MK (2016) Effect of rainbow trout (Oncorhynchus mykiss) seminal plasma on the post-thaw quality of ram semen cryopreserved in a soybean lecithin-based or egg yolk-based extender. Animal Reproduction Science 164, 97-104.
| Crossref | Google Scholar | PubMed |
Vilagran I, Yeste M, Sancho S, Castillo J, Oliva R, Bonet S (2015) Comparative analysis of boar seminal plasma proteome from different freezability ejaculates and identification of Fibronectin 1 as sperm freezability marker. Andrology 3(2), 345-356.
| Crossref | Google Scholar | PubMed |
Wattananit P, Yingchutrakul Y, Kornkaewrat K, Mahasawangkul S, Roytrakul S, Pinyopummin A (2023) Non-targeted proteomic analysis of Asian elephant (Elephas maximus) seminal plasma using an in-solution digestion technique and liquid chromatography tandem-mass spectrometry. Frontiers in Veterinary Science 10, 1174078.
| Crossref | Google Scholar | PubMed |
Weber JA, Geary RT, Woods GL (1990) Changes in accessory sex glands of stallions after sexual preparation and ejaculation. Journal of the American Veterinary Medical Association 196(7), 1084-1089.
| Crossref | Google Scholar | PubMed |
Williams C, Tiwari SK, Goswami VR, de Silva S, Kumar A, Baskaran N, Yoganand K, Menon V (2020) Elephas maximus. The IUCN red list of threatened species 2020: e.T7140A45818198. Available at https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T7140A45818198.en [accessed 24 June 2024]
Zahn FS, Papa FO, Melo CM, Brisola ML (2005) Protein profile of equine seminal plasma: correlation to semen freezability. Animal Reproduction Science 89(1-4), 313-315.
| Google Scholar | PubMed |


