Understanding conceptus–maternal interactions: what tools do we need to develop?
Zenab Butt A , Haidee Tinning A , Mary J O’Connell B , Jonathan Fenn B , Ramiro Alberio C and Niamh Forde A *A
B
C
Abstract
Communication between the maternal endometrium and developing embryo/conceptus is critical to support successful pregnancy to term. Studying the peri-implantation period of pregnancy is critical as this is when most pregnancy loss occurs in cattle. Our current understanding of these interactions is limited, due to the lack of appropriate in vitro models to assess these interactions. The endometrium is a complex and heterogeneous tissue that is regulated in a transcriptional and translational manner throughout the oestrous cycle. While there are in vitro models to study endometrial function, they are static and 2D in nature or explant models and are limited in how well they recapitulate the in vivo endometrium. Recent developments in organoid systems, microfluidic approaches, extracellular matrix biology, and in silico approaches provide a new opportunity to develop in vitro systems that better model the in vivo scenario. This will allow us to investigate in a more high-throughput manner the fundamental molecular interactions that are required for successful pregnancy in cattle.
Keywords: endometrium, gene expression, in vitro models, in silico models, interferon tau, pregnancy, progesterone, uterus.
Introduction
Pregnancy loss affects all mammalian species and occurs at critical developmental timepoints, particularly during early pregnancy. In cattle and other food-producing animals, reproduction needs to be as efficient as possible to decrease environmental impacts, increase food production to feed an ever-increasing global population, and to increase profit margins for the farmers. In beef cattle, 50% of pregnancies fail before day 30 (Reese et al. 2020), with that number estimated to be even higher in dairy cattle (Ealy and Seekford 2019). Furthermore, up to 60% of human pregnancies are lost in the early stages (before 12 weeks) (Larsen et al. 2013). Although many of these losses can be attributed to developmental failures with the pre-implantation embryo, one underexplored cause is thought to be errors in embryo–maternal bilateral communication (Sánchez et al. 2019). Therefore, it is essential to understand the critical embryo–maternal interactions that occur in vivo and how these contribute to pregnancy success or failure. Much of what we know about the fundamentals of embryo–maternal communication comes from in vivo studies (recently reviewed by Idelevich and Vilella 2020) as well as more static and traditional 2D culture systems. However, recent moves in certain countries including the UK (e.g. by NC3Rs) are looking to reduce the number of animals used for research (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/277942/bis-14-589-working-to-reduce-the-use-of_animals-in-research.pdf). This, combined with developments in novel in vitro and in silico methods (reviewed below), allow for an opportunity to examine and explore conceptus–maternal interactions in a more high-throughput manner. The aim of this review will be to set into context what we know about the endometrium itself, its critical interactions with the developing embryo/conceptus, where the gaps in our knowledge are, and what developments will allow us to develop more high-throughput approaches to understand and mitigate pregnancy loss in food-producing animals and broader array of mammals.
Structure and function of the endometrium
The fundamental role of the endometrium is to support growth and development of the embryo/conceptus prior to development of functional cotyledonary placental structures (Spencer et al. 2016). This heterogeneous tissue that lines the mammalian uterus and is the first point of cellular contact for the conceptus provides nutrients via histotrophic secretions (termed uterine luminal fluid, ULF), establishes receptivity to implantation for an appropriately developed conceptus, and maintains pregnancy. The endometrium is composed of luminal epithelia (LE) and glandular epithelia (GE) cells, stromal/mesenchymal cells, immune cells and a blood supply, all of which support different functions of the endometrium (Fig. 1).
Schematic diagram of the function of the bovine endometrium during the peri-implantation period of pregnancy. IFNT – interferon tau, ER1 – oestrogen receptor 1, OXTR – oxytocin receptor. Created with Biorender.com.
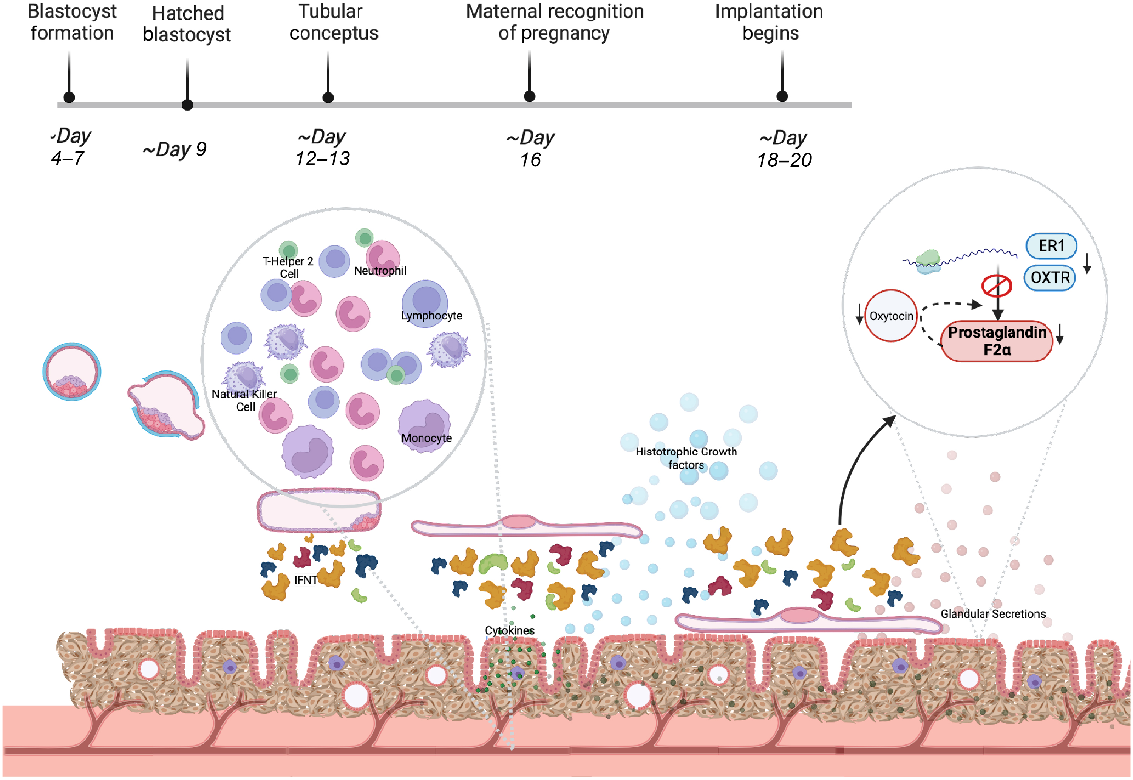
Factors driving conceptus elongation and embryonic nutritional cargo are secreted by LE and GE throughout elongation; during implantation, embryonic secretions to the glands induce specific gene expression changes, thought to be essential for successful pregnancy (Adhikari et al. 2022). In between these glandular areas are aglandular areas called caruncles – small protrusions on the surface of the endometrium (Chankeaw et al. 2021). Underlying this glandular region exists a supportive network of fibroblast-like stromal cells, holding vasculature, immune cells, and lymph vessels throughout an extracellular matrix (ECM) (Chankeaw et al. 2021).
Research has indicated that epithelial cell secretions include growth factors, hormones, cytokines, and exosome-encapsulated molecules that regulate uterine receptivity, stromal cell decidualisation (in species such as mouse and humans, which undergo this process) and blastocyst/conceptus implantation (Zhang et al. 2013; Filant and Spencer 2014; Cretoiu et al. 2016). The GE and LE provide nutritional sustenance to support the growth of the blastocyst prior to placental formation, during the elongation process through secretions into the histotroph in ruminants (Spencer 2014). This is evidenced by the fact that sheep blastocysts fail to elongate in sheep where uterine gland and luminal epithelia have been inhibited from developing (Gray et al. 2002; Spencer 2014).
Pre-implantation embryo development
In cattle and other mammals, fertilisation occurs in the oviduct (or fallopian tube) and this is where the process of embryonic genome activation occurs. There is conservation in terms of the morphological steps that occur, e.g. development to the morula and transition to the blastocyst stage of development. This is key for pregnancy success as this is when differentiation between the inner cell mass (which goes on to form the fetus) and the outer cell mass (the trophectoderm that forms the extraembryonic membranes) occurs (Idelevich and Vilella 2020). The trophectoderm is not only the site of the first physical interaction between the conceptus and the endometrium but also produces the species-specific pregnancy recognition signal (detailed below). Bovine embryos don’t depend on exposure to the maternal system until after blastocyst hatching, as evidenced by in vitro embryo culture and successful pregnancy after transfer at this point. In cattle, the blastocyst hatches from the zona pellucida around day 9, where the conceptus undergoes a period of rapid elongation into a filamentous conceptus (Spencer 2013), which begins implantation on day 16–20 after ovulation (Eozenou et al. 2020). This differs in humans, where the implantation processes of apposition occur immediately post-hatching (Kim and Kim 2017). The maternal endometrium provides nourishment for the conceptus prior to placenta formation via endometrial histotrophic secretions from endometrial glands (Forde and Lonergan 2012).
Successful implantation in all species relies on the maternal exchange of nutrition and sustenance. Ruminants characteristically have an extended period of conceptus development prior to implantation, called trophoblast elongation. This gives the conceptus a long thin appearance, beginning at a length of 2 mm and measuring at ~200 mm (20 cm) at the time of implantation (Brooks et al. 2014). At this point, the endometrium and uterine lumen are key to conceptus survival. During elongation, the conceptus is reliant on the histotrophic nutrition and growth factors provided mostly by the uterine glands (Forde and Lonergan 2012). This has not been successfully recapitulated in vitro and thus limits our understanding of pregnancy loss/success. It is therefore essential to explore the mechanisms by which uterine lumen and endometrial sensitivity are initiated and maintained. As bovine conceptus elongation is a maternally dependent process, it is currently extremely challenging to study the conceptus–maternal interaction in vivo. It is also necessary to develop new techniques and models accurately reflecting the start of this interaction (Lonergan and Fair 2014).
In vitro model development
Decreased endometrial receptivity is one of the major contributing factors to a failed pregnancy within the first 3 weeks of gestation. Studying the development of an embryo within the maternal uterus and consequential endometrial development is not only extremely expensive but also technologically challenging to replicate in vitro. Ideally, an in vitro model would be able to recapitulate the transcriptomics and structural changes that occur in the epithelium during the peri-implantation period of pregnancy and the differentiation of stromal cells following exposure to steroids such as progesterone and oestradiol, as well as various signalling molecules and growth factors such as interferon tau (IFNT) and insulin-like growth factor (Donofrio et al. 2008; Adhikari et al. 2022). Moving forward to begin the formation of a suitable model, a detailed insight into each cell type, its individual function, and its interactions with surrounding cell types is required. We must also consider biophysical structure, multicellular interactions, and the flow of components and signals between the trophoblast and endometrium (Table 1).
Most of our knowledge of endometrial function from in vivo studies has come from bulk RNA sequencing of endometrial tissues at different stages of pregnancy. Generating single-cell resolution maps of the endometrium and its response to cues important for early pregnancy and implantation would allow us to understand cell-specific changes that occur during successful pregnancy and allow us to map the in vitro systems to understand which are appropriate to answer specific functional questions of the endometrium. Single-cell sequences of other mammalian species, including humans (Lucas et al. 2020) and murines (Kirkwood et al. 2021), have resolved different subcellular phenotypes of the endometrium, but this technique has been used in a more limited way in bovines, with bovine placental single-cell sequencing only identifying different trophoblast cell lineages (Davenport et al. 2023). To develop effective in vitro systems, a better understanding of the single-cell resolution and the temporal changes of the endometrium in response to progesterone, oestradiol and IFNT is required.
One largely under investigated component of the endometrium is the contribution of the ECM to early pregnancy success. The ECM is the non-cellular component of any tissue, which supports the tissue in a variety of ways, including providing biophysical structure to the cells and supplying biochemical cues (Frantz et al. 2010). The ECM is mainly composed of proteins such as collagen (mostly secreted by fibroblasts; De Wever et al. 2008) elastin, fibronectin, some proteoglycans, water, and polysaccharides, although the exact composition is unique to each tissue and determines the properties of that tissue (Frantz et al. 2010). Cell adhesion and migration within the ECM is mediated by ECM receptors and cellular cytoskeletal adherence to the ECM (Schmidt and Friedl 2010). The 3D structure and properties of the ECM itself are controlled by enzymatic degradation (such as by metalloproteinases) or inhibitors of those enzymes (Cruz-Munoz and Khokha 2008). Changes to the ECM are known as ECM remodelling and have been implicated in disease states (Nallanthighal et al. 2019), tissue stiffness (Engler et al. 2006), and tissue morphology and structure (Chevalier et al. 2016).
The ECM also contains factors which are required for tissue homeostasis, cell differentiation, and tissue morphogenesis. It acts as a reservoir for growth factors (such as transforming growth factor-b), which are released from the ECM to act upon neighbouring cells when the ECM is remodelled (Taipale and Keski-Oja 1997). ECM-resident matrix-bound extracellular vesicles (EVs) have also recently been identified (Huleihel et al. 2016). EVs can contain coding and non-coding RNA species and proteins, among other cellular components, which can influence target cells after uptake (Tkach and Théry 2016). EVs can be targeted to specific cell types by the presence of surface receptors which interact with those expressed on the target cell membrane (Tkach and Théry 2016). Matrix-bound EVs have been shown to influence the ECM directly through vesicle surface factors (Sanderson et al. 2019) and indirectly by fusing with cells (Sajeesh et al. 2020).
The correct morphology, functionality, and remodelling of the endometrium are critical for endometrial receptivity and subsequent implantation, and change drastically during the oestrous cycle in species such as humans (Strowitzki et al. 2006). Therefore, the dynamics and regulation of the ECM contribute to early pregnancy success (O’Connor et al. 2020). In cattle, the transcription of genes involved in ECM remodelling is altered during the oestrous cycle (Mitko et al. 2008), and similarly the abundance of collagen in the endometrium (Scolari et al. 2016). Authors also found that reduced ECM remodelling on day 6 is correlated with pregnancy success in cattle (Scolari et al. 2016). By studying the endometrium in vitro using novel techniques, we can better model the endometrium to gain a greater understanding of early pregnancy processes and how the ECM contributes to their success.
Until recently, very few in vitro models successfully considered and incorporated the endometrial ECM. Work by Díez et al. (2023) utilised a 3D polystyrene scaffold populated with bovine endometrial stromal cells which deposited ECM proteins in vitro (Díez et al. 2023). The resulting tissue-like structure supported by ECM allowed the formation of an epithelial monolayer, thereby recapitulating the basic 3D structure of the endometrium in vitro (Díez et al. 2023). Similarly, an electrospun scaffold has been used to culture bovine endometrial cells in which ECM was deposited, although culture was limited to a shorter timeframe than the polystyrene scaffold more recently reported (MacKintosh et al. 2013). These scaffolds require an artificial scaffold into which fibroblasts are seeded, following which they can then secrete ECM. The scaffolds themselves cannot be altered through remodelling.
Although these advances in 3D cell culture and ECM deposition are a leap forward in research addressing ECM in in vitro culture systems, they still do not fully recapitulate in vivo endometrial ECM. A noteworthy method for addressing this utilises decellularisation of tissue to leave behind the natural ECM of that tissue. Cells can then be seeded or the remaining tissue ECM investigated. A recent example successfully used decellularisation of porcine endometria to produce an endometrial ECM ‘hydrogel’ which was then seeded with human primary endometrial cells for long-term culture (López-Martínez et al. 2021). Although the authors identified many points of optimisation for the technique, the method is an interesting example of utilising the in vivo–produced ECM in vitro to improve endometrial models. The ECM is a complex matrix which undergoes remodelling and contains EVs and other factors which can influence the cells of that tissue. The ECM deserves consideration when designing in vitro tissue models, especially when the composition and architecture of the tissue is critical for its function, such as in the endometrium.
Multicellular endometrial model approaches
Investigating tissue and cellular interactions and the functional understanding of tissues currently relies heavily on 2D in vitro models. Using static monolayers of cells in culture does not portray the intricacy of a 3D multicellular network and the interactions which take place in a physiologically accurate micro-environment. Three-dimensional cellular models are now increasingly popular in vitro models as they are able to replicate this multicellular network. Such models currently include organoid cultures and systems, scaffolds, various hydrogel-based assays, and microfluidic and organ-on-chip approaches.
Immortalised cell lines are often used as tools for preliminary data; however, they may not provide an accurate phenotype or genotype of the tissue of origin (Nallanthighal et al. 2019). Inter-species genetic variation cannot be accounted for with immortalised cultures; therefore, it is preferable to use primary cultures when possible (Li et al. 2016). Primary cells have allowed researchers to better observe cellular characteristics as they would exist in vivo. Isolated directly from host tissue samples taken via biopsies, resections, or post-mortem autopsies, these cell lines better represent both phenotypical and genotypical signatures as they exist within the host than immortalised secondary cultures. They prove particularly beneficial when observing factors within the cellular micro-environment, especially when co-cultured with systemically relevant primary cell lines in model systems incorporating microfluidic technology. A more representative micro-environment would produce responses more indicative of what occurs in vivo (Richter et al. 2021).
Bovine endometrial research regularly uses primary cells. These cells are routinely harvested from the bovine uterus, isolated, and cultured. Established protocols are followed for this process, as previously described in detail (Cronin et al. 2012). This allows the study of molecular properties, phenotypical characteristics, and cellular responses to treatments and conditions. These studies allow us to gain insight into cellular-specific properties, aiding our understanding of how the bovine endometrium aids implantation and gestation (Li et al. 2016; Tinning et al. 2020; Mathew et al. 2022). Previous studies have successfully isolated bovine endometrial epithelial cells and used these cells for further processing such as single-cell sequencing and immune factor investigation (Kelly et al. 2020; Chankeaw et al. 2021), and for further use in 3D co-cultured systems (Murillo and Muñoz 2021). Endometrial cells have also successfully been harvested from humans, pigs, sheep, horses, and rodents (Wang et al. 2000; De Clercq et al. 2017; Rink et al. 2017; Tavakol et al. 2018; Hu et al. 2022), with advances allowing the transformation of some primary cultures into immortalised-like cultures, able to maintain primary cell–like characteristics but for prolonged passages (Hu et al. 2022).
Although primary cultures prove beneficial and provide researchers with a platform for insight and data, there will still be differences in cellular characteristics depending on the tissue origin (Qadan et al. 2018), especially when taken from different animals. The lack of multicellular stimulation and therefore altering of cellular micro-environment will alter the molecular structure of cells (Lee et al. 2006). The process of endometrial and stromal cell isolation involves degradation of the endometrial structure as a whole, potentially causing cells to release damage associated with molecular patterns, altering downstream genetic profile and functioning of cells (Borges et al. 2012).
There can be concern over the methods of cell isolation used due to proteolytic and other digestive enzymes potentially altering cellular makeup. The next question which arises is the number of passages for which these cells will continue to represent cells in vivo. One way to combat some of these issues is using tissue explants, co-culturing, or 3D models of the various cell types involved in implantation. Furthermore, in vitro culturing often uses serum-based media with additional antibiotic components, which overtime likely influence cellular characteristics and morphology. This is evident by numerous studies displaying alterations in primary cell lines through serum starvation, e.g. shortening of microglial processes alongside increase in cell body size, cessation of proliferation in human endometrial mesenchymal cells and induction of autophagy in endometrial epithelial cells.
Explant cultures are another form of primary tissue culture and involve the culturing of the tissue or organ of interest until tissue-specific progenitor cells migrate into the culturing vessel and can be maintained. Progenitor cells can then differentiate into the relevant organ-specific cells while being reliant on the growth factors and conditions they are exposed to. This method can only be maintained for 2 to 3 weeks and requires a delicate balance of culturing. This ensures all components of the media are a precise replica of the in vivo environment (Lee et al. 2006).
The continuation of cells alongside host tissue proves hugely advantageous for mimicking the cellular micro-environment (Bedzhov and Zernicka-Goetz 2014). Factors such as the ECM, vesicular secretions, growth factors, cytokines and cellular contents are functionally active within the tissue explant once it has been established, which allows for cellular growth and stability (Borges et al. 2012). The stem cells subjected to less biological stress when this method is utilised will retain more in vivo–like characteristics (Hendijani 2017).
Endometrial tissue explants have proved advantageous in exploring the conceptus–maternal relationship across species. They have been successfully cultured and used for downstream applications such as determining mechanisms of innate immunity/inflammation, and the influence of blastocyst presence to cellular molecular profile (Borges et al. 2012; Passaro et al. 2018; Sánchez et al. 2019). Explanted endometrial tissue taken from various stages of the oestrous cycle has also shown changes in expression across the cycle (Ault-Seay et al. 2022). The same tissues showed significant differences in gene expression in response to lipopolysaccharide treatment, indicative of a difference in immune response dependent on the stage of oestrous cycle the host is in, potentially resulting in changes to endometrial function and therefore pregnancy success (Ault-Seay et al. 2022). Other factors influencing immune cell populations and consequential responses may include: immunological stresses present in the mother (e.g. infection) and fluctuations in hormone levels.
In another study, bovine endometrial tissue was cultured alongside long and short length embryos, all 15 days old. The observations showed a difference in molecular patterns in endometrial cells, which were dependent on which length conceptus they had been exposed to (Sánchez et al. 2019). This study showed the importance of how this relationship can influence the genotype of both the endometrium and conceptus. While two conceptuses may be the same age, growth rate may differ and consequentially influence the endometrial transcriptome (Sánchez et al. 2019). In 2005, Tan et al. successfully developed and optimised the culture conditions of a co-culturing system using mouse endometrial tissue explants taken alongside blastocysts on day 4 of pregnancy. Tissue taken at this specific time allowed the group to encapsulate the short lived ‘window of implantation’ in mice, lasting only a few hours on this day, thus providing a step in the right direction for research into implantation (Tan et al. 2005).
2D immortalised cell cultures provide an ideal baseline for disease modelling, and the development of both primary isolated cultures and explant cultures provides a closer mimicking cellular profile to that of host tissue. However, recent biological models are turning to 3D cell cultures such as spheroids and organoids for an increasingly physiologically accurate representation of in vivo conditions.
3D models of cell culture are most often grown using acellular scaffolds of either a biological or synthetic nature. They can be anchorage dependent (scaffold based), formed in biological or synthetic hydrogels, or independent (non-scaffold based) systems such as spheroids, formed using the hanging drop, low attachment plates or gels, formation through agitation, and magnetic levitation (Langhans 2018). One study successfully modelled formation of spheroidal cultures of trophoblasts, fibroblasts and epithelia. Through this they observed a decrease in the expression of polarisation proteins ezrin and CK18 in the presence of trophoblast spheroids, indicative of the depolarisation of epithelial lining at implantation (Haeger et al. 2015).
Another study developed a model of implantation in bovines using 3D trophoblast spheroids applied onto endometrial epithelial cells, aiming to investigate factors affecting attachment of trophoblast cells to uterine epithelium (Sakurai et al. 2012). Looking at the expression of IFNT in both these components, the authors attempted to mimic implantation by applying CT-1 trophoblast spheroids to endometrial epithelial cells, with the addition of concentrated uterine fluid from pregnant ewes. The attachment rate, alongside the expression of various other factors, was observed and quantified. Using this model they were able to conclude that IFNT showed a decreased expression in CT-1 trophoblast spheroids when applied onto endometrial epithelial cells at attachment, while no changes in IFNT expression were observed in the CT-1 spheroids with the absence of epithelial cells, proving that IFNT is a good indicator of successful attachment (Sakurai et al. 2012).
Although 3D cultures are a preferable model, they still lack the presence of vascular and supportive networks for waste removal and transport, similar to what is found in vivo. While a monolayer of cells can retrieve the nutrition and perform the gas exchange necessary, cells within the centre of a 3D spheroid or organoid will not have the same exposure to nutrients and gases as those on the surface. This is beneficial when studying tumour biology; however, it does not replicate tissue systems in vivo, where a vast network of exchange and communication would exist between cells (including immune cells), via the actions of cytokines, various microRNAs, and nutritional factors.
While the advances in cell culture models have been beneficial, they still do not mimic the physiological systemic flow under which all biological structures function. Advances in bioengineering have introduced intricate microfluidic models (Pun et al. 2021), which allow a two-way exchange of relevant nutritional material and waste removal alongside sufficient gas exchange. These microfluidic approaches allow us to mimic physiological flow in in vitro cell culture systems. The devices are made of a translucent plastic, polymer, or glass, and include a variable number of hollow microchannels within which cells grow. Under pump systems, channels conduct the flow of reagents, with volumes as small as 0.001 μL being required (Whitesides 2006)
Singular or multiple cell lines can be grown/layered in unison or under flow to recreate tissue-specific structures (Pun et al. 2021). Research conducted into complex systems such as neurological, cardiovascular, endometrial, and oncological physiologies are benefitting from microfluidic models ranging in complexity, allowing the recreation of such networks with the correct multicellular composition in a non-static state, and therefore a more physiologically realistic micro-environment (Nolan et al. 2023).
Within the study of uterine biology, hormonal fluctuations that occur throughout the oestrous cycle and their influence on endometrial function cannot be ignored. Recreation of endometrial biology encompassing the network of reproductive hormones has been successfully attempted, mimicking the menstrual cycle of humans using microfluidics modelling. These models have been used to better mimic the hormonal fluctuations that the reproductive tract experiences in vivo. The synergistic relationship between embryonic development and endometrial structure and function cannot be accurately represented using static in vitro models.
A recent study (Ahn et al. 2021) included a vascularised multicellular endometrium with constitutive endothelial cells, stromal fibroblasts and finally epithelial cells that were all grown under flow. Oestradiol and progesterone were incorporated into culture media in varying concentrations and combinations depending on menstrual cycle phase. Once established, the authors applied embryo-sized microbeads coated with either insulin-like growth factor binding protein 1 or heparin-binding EGF-like growth factor, both of which are known to be released by the embryo (Ahn et al. 2021). A successful model was indicated in doing so; researchers observed a marked increase in epithelial lining thickness and achieved successful attachment of the embryo, developing a model of implantation (Ahn et al. 2021).
De Bem et al. (2021) utilised microfluidic technology to replicate the bovine endometrial structure using primary isolated endometrial cells. By altering the flow with glucose and insulin-like growth factor 1 (IGF1) throughout the model, they were able to explore any concentration-dependent transcription changes to the endometrium and any resultant downstream proteomic changes within the surrounding media. Both glucose and IGF1 concentrations in circulation in vivo are associated with embryo development and are affected at times of metabolic stress, e.g. in dairy cows during negative energy balance (Forde et al. 2016; De Bem et al. 2021). Both factors could therefore could play a vital role in early pregnancy failure in bovines. Both endometrial and stromal cells responded to these factors with numerous changes in the protein secretome (De Bem et al. 2021).
Xiao et al. (2017) developed a unique microfluidic model capable of combining five different tissue structures: ovarian, fallopian, uterine, cervical, and hepatic (to determine tissue stability outside the reproductive system). Each of these tissue explants were incorporated into a microfluidic model with an individual recirculation of media. A second circulating system allowed for recirculation of media within and around the whole model. An individual follicular model was also incorporated, containing murine ovarian follicles, with sustained human chorionic gonadotrophin (hCG). Through the sustenance of hCG and development of these follicles within this complex system, the group observed identical hormonal fluctuations as seen in the human menstrual cycle (Xiao et al. 2017). This final model is an example of how microfluidic or organ-on-a-chip devices can be developed to interconnect various physiological systems and utilise cell-to-cell communication to produce an in vitro–like micro-environment.
Achieving a multicellular system in bovine reproductive physiology would allow for more accurate predictions and observations of which maternal factors influence embryonic development, and vice versa, in order to accurately predict factors which may be affecting implantation failure and therefore pregnancy loss in early pregnancy.
Investigating the conceptus side
While there has been progress made in modelling the in vitro endometrium, in order to understand pregnancy loss we must also consider models of trophoblast (TE) cells and how they interact with in vitro–produced endometria. Previous models have used TE cells (BT-1 cell line, Awad et al. 2020; CT-1 cell line, Talbot et al. 2000). More recently, bovine trophoblast stem cells (TSCs) have been isolated from blastocyst cultures (Wang et al. 2023). These cells can be maintained undifferentiated in long-term cultures and they can be induced to produce differentiated TE cells in ~5 days, as determined by the expression of trophoblast markers PTGS2, placental lactogen and IFNT. When injected into NOD-SCID mice, these cells differentiate in mature TE cells, evidenced by the presence of binucleated cells and expression of MMP2, normally detected in the trophoblast–endometrial interphase. These assays demonstrate the potential of these TSCs; however, more work is needed to demonstrate their ability to recapitulate the dynamic changes of TE cells during the complex process of TE elongation and show how these are related to the interaction with the maternal environment. Indeed, a recent transcriptomic study of bovine conceptuses between days 12 and 18 identified novel subpopulations of TE cells that may emerge during the transition from ovoid to tubular stage (Scatolin et al. 2023). Importantly, this study also identified possible signalling interactions between the epiblast, the TE and hypoblast during this critical period, highlighting the need to develop systems that include all the components of the conceptus in order to study the molecular interactions defining the embryo–maternal communication required for successful pregnancy establishment. A step towards achieving this objective was reported recently with the generation of bovine blastoids by using stem cells (Pinzón-Arteaga et al. 2023). TSCs were aggregated with expanded potential bovine stem cells (EPSC) and cultured under conditions favouring the differentiation of the hypoblast lineage from epiblast cells, while forming a blastocyst. Remarkably, these blastoids produce IFNT in recipient animals similar to levels seen in control embryos; however, later pregnancies were not reported. These blastoids need to be studied in more detail to determine their functionality, thus future experiments combining blastoids and 2D endometrial cultures will be instrumental in establishing the functional interactions between maternal–fetal interphase during the critical stage of embryo elongation and implantation.
In silico approaches
Using in silico and computational modelling approaches allows multicellular networks of a physiological or pharmacological system to be investigated. Computational approaches mean specific experimental conditions can be incorporated into models without being tested directly on experimental subjects. While most often used in pharmacologically based assays, in silico approaches are now being introduced to other complex physiological systems such as cardiovascular and endometrial biology (Colquitt et al. 2011). A major strength of in silico approaches is that multiple parameters (conditions) can be considered simultaneously, unlike in vivo or in vitro approaches. In silico approaches can also be combined with in vitro and in vivo studies to gain detailed mechanistic and functional insights. In silico studies are already helping researchers understand various cell states at different points throughout the reproductive cycle, with the multi-omic analysis of different stages of the cycle/developmental competency of embryos as well as validation of new in vitro model systems (recently reviewed in three review papers from the International Ruminant Reproduction conference; Huang et al. 2021; Yiğit et al. 2022; Cheredath et al. 2023). In recent years, the quality, quantity, and taxonomic breadth of available gene sequences and full genomes has increased dramatically. This has facilitated greater insight into the adaptive and evolutionary history of genomic elements associated with variation in reproductive phenotypes. Such approaches can inform and target subsequent in vitro and in vivo analyses by highlighting which regulatory elements and/or genes may contribute to reproductive pathologies, or reproductive strategies and phenotypes.
Many significant insights into pregnancy in placental mammals have been gained from these comparative evolutionary biology approaches. For example, seminal work by Lynch et al. (2015) set out to determine the evolution of uterine gene expression across tetrapods by characterising transcribed genes in the endometria of eutheria (dog, cow, horse, pig, and armadillo), marsupial (opossum) and monotreme (platypus), and combining these data with similar datasets from the pregnant uteri of other tetrapod species (frog, lizard, and chicken). They discovered thousands of genes evolved endometrial expression on the lineage leading to mammals, with roles in communication and immune tolerance. On the same lineage, thousands of cis-regulatory hormone-responsive elements emerged that were co-opted from ancient transposable elements, suggesting a major rewiring and functional innovation on this stem mammal lineage required to establish the regulatory networks needed for pregnancy success in mammals. Recently, taking a similar comparative transcriptomic approach with endometrium samples from several mammal species, genes have been identified that are hypothesised to be involved in determining human unique pregnancy-related traits (Mika et al. 2021, 2022), some of which, are heavily implicated in pregnancy complications. More broadly, these studies strongly implicate changes in uterine gene expression with several evolutionary innovations during the origin of eutherian pregnancy. Indeed, reconstructing the ancestral transcriptome of 23 species shows how the maternal gene expression profiles are correlated with the degree of placental invasion, suggesting maternal control on placental invasiveness (Mika et al. 2022).
Using a combination of transcriptomic data, morphological analyses, evolutionary and comparative genomics, the narrative around implantation has transformed in recent years from that of conflict between a ‘passive maternal side’ and an ‘aggressive embryo side’ to a model where cooperative inflammation and the role of endometrial receptivity in pregnancy are centre stage (Mika et al. 2022). The process of attachment in implantation is derived from an attachment reaction in the ancestral therian mammal (model = opossum), which leads to parturition (Mika et al. 2021). However, in eutheria a key shift to a non-inflammatory phase of pregnancy permits the extended period of placentation (Chavan et al. 2021). Comparative uterine transcriptomics revealed that this transition to a non-inflammatory phase of pregnancy in eutheria is underpinned by suppression of interleukin-17A (IL17A) from T helper cells by decidual stromal cells (Chavan et al. 2021)
The use of in silico approaches with high-quality genomes also helps to elucidate the role of non-coding regulatory elements of the genome. The human chromosome 19 microRNA cluster (C19MC) was identified by Bentwich et al. (2005) using computational methods, and then validated through in multiple tissues using in vitro methods. C19MC has since been shown to be expressed exclusively in the placenta during pregnancy and, once expressed, is trafficked to the maternal circulation via extracellular vesicles (Donker et al. 2012; Chang et al. 2017; Morales-Prieto et al. 2020). Furthermore, variations in expression of this cluster in humans have been associated with multiple pregnancy-related pathologies, including pre-eclampsia (Hromadnikova et al. 2013, 2017).
Importantly, variation in evolutionary rates of proteins doesn’t always correlate with functional shift as has been shown by comparative analysis (in silico and in vitro) of a sperm protein (CatSper). Despite differences in evolutionary history of the CatSper gene between marsupial and eutherian mammals, much of the function of the CatSper protein is conserved (Hwang et al. 2021).
Taylor et al. (2023) explored the pattern of microRNA presence and absence in the genomes of 10 therian mammals, representing a range of implantation methods, and identified a ‘core toolkit’ of 13 mammal-exclusive microRNAs present in all species therein. Many of these core toolkit microRNAs were implicated in placental function and pregnancy-related pathologies. Using computational predictions of the targets of these microRNAs, they found that genes predicted to be under positive selection (indicative of protein functional shift) on the ancestral eutherian mammal lineage are preferentially targeted by these microRNAs. In vitro experiments then demonstrated that expression of these microRNAs was affected by early pregnancy molecules. Furthermore, dysfunction of these microRNAs and their targets contributes to endometrial-derived recurrent pregnancy loss (Hume et al. 2023).
To better predict the factors determining success in implantation and pregnancy, many recent studies have used artificial intelligence (AI) and machine-learning (ML) methods such as random forest and neural networks (Uyar et al. 2015; VerMilyea et al. 2020; Siristatidis et al. 2021; Li et al. 2022). Typically, these approaches take a selection of maternal or conceptus phenotypes and assess the extent to which these factors predict developmental outcomes. Data is randomly subset into a training set, which is used to develop a predictive model, which is subsequently validated on the remaining data. These models are then run until their predictive power reaches an optimal value. ML approaches have been taken to better understand the factors underlying success in IVF implantation (Larsen et al. 2013; Luo et al. 2017; Akbulut et al. 2018; Miao and Miao 2018; Araya et al. 2021).
ML approaches have recently become popular as a method of analysing large, complex omics-based datasets. Rabaglino et al. (2023) combined Bayesian logistic regression and neural network models with bovine transcriptomic data to identify eight genes whose expression was predictive of conceptus competence. In addition, the development of ML approaches, such as in Christmas et al. (2023), have led to finding enhancer regions associated with phenotypic differences in mammals, with many of the most highly conserved regions associated with embryonic development. Novel ML approaches and applications such as these are set to be increasingly important in understanding the role of non-protein-coding regions of the genome, which are typically more difficult to determine. These two studies highlight the potential for ML in better predicting developmental outcomes, as they have been leveraged to handle complex datasets across different scales, identifying potential portions of the transcriptome and genome, respectively, which may be essential in healthy fetal development. As ML approaches become more robust and reliable, it is likely that they will become an increasingly useful tool in the effort to better understand pregnancy-associated risk factors and conceptus–maternal interactions, and to reduce the need for in vivo and in vitro studies.
Outlook and future goals
Investigating conceptus–maternal interactions is critical if we are to overcome pregnancy loss in cattle. Most of what we know comes from bulk RNA sequencing and the scientific community would benefit from single-cell sequencing to fine map components of the endometrium and how they respond to signals during the peri-implantation period of pregnancy. This would allow the in vitro model development to map to what is known in vivo. Creation of representative models to study implantation would allow us to physically and mathematically recreate in vivo scenarios in vitro in a system that is currently difficult to study.
Declaration of funding
Research in NF’s lab is supported by N8 agri-food pump priming, QR GCRF, as well as BBSRC grant numbers BB/R017522/1, as well as support from LTHT. NF and MO’C are funded jointly by BBSRC grant BB/X007332/1 and NF, MO’C and RA are funded by Wellcome Trust grant 227178/Z/23/Z.
References
Adhikari B, Lee CN, Khadka VS, et al. (2022) RNA-Sequencing based analysis of bovine endometrium during the maternal recognition of pregnancy. BMC Genomics 23, 494.
| Crossref | Google Scholar | PubMed |
Ahn J, Yoon MJ, Hong SH, et al. (2021) Three-dimensional microengineered vascularised endometrium-on-a-chip. Human Reproduction 36(10), 2720-2731.
| Crossref | Google Scholar | PubMed |
Akbulut A, Ertugrul E, Topcu V (2018) Fetal health status prediction based on maternal clinical history using machine learning techniques. Computer Methods and Programs in Biomedicine 163, 87-100.
| Crossref | Google Scholar | PubMed |
Araya J, Rodriguez A, Lagos-SanMartin K, et al. (2021) Maternal thyroid profile in first and second trimester of pregnancy is correlated with gestational diabetes mellitus through machine learning. Placenta 103, 82-85.
| Crossref | Google Scholar | PubMed |
Ault-Seay TB, Payton RR, Moorey SE, et al. (2022) Endometrial gene expression in response to lipopolysaccharide between estrous cycle phases and uterine horns in cattle. Frontiers in Animal Science 3, 939876.
| Crossref | Google Scholar |
Awad M, Kizaki K, Ishiguro-Oonuma T, et al. (2020) Secreted protein of Ly6 domain 1 enhanced bovine trophoblastic cell migration activity. In Vitro Cellular & Developmental Biology – Animal 56(10), 827-831.
| Crossref | Google Scholar | PubMed |
Bedzhov I, Zernicka-Goetz M (2014) Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156(5), 1032-1044.
| Crossref | Google Scholar | PubMed |
Bentwich I, Avniel A, Karov Y, et al. (2005) Identification of hundreds of conserved and nonconserved human microRNAs. Nature Genetics 37(7), 766-770.
| Crossref | Google Scholar | PubMed |
Borges ÁM, Healey GD, Sheldon IM (2012) Explants of intact endometrium to model bovine innate immunity and inflammation ex vivo. American Journal of Reproductive Immunology 67(6), 526-539.
| Crossref | Google Scholar | PubMed |
Brooks K, Burns G, Spencer TE (2014) Conceptus elongation in ruminants: roles of progesterone, prostaglandin, interferon tau and cortisol. Journal of Animal Science and Biotechnology 5(1), 53.
| Crossref | Google Scholar | PubMed |
Chang G, Mouillet JF, Mishima T, et al. (2017) Expression and trafficking of placental microRNAs at the feto-maternal interface. The FASEB Journal 31(7), 2760-2770.
| Crossref | Google Scholar | PubMed |
Chankeaw W, Lignier S, Richard C, et al. (2021) Analysis of the transcriptome of bovine endometrial cells isolated by laser micro-dissection (1): specific signatures of stromal, glandular and luminal epithelial cells. BMC Genomics 22(1), 451.
| Crossref | Google Scholar | PubMed |
Chavan AR, Griffith OW, Stadtmauer DJ, et al. (2021) Evolution of embryo implantation was enabled by the origin of decidual stromal cells in eutherian mammals. Molecular Biology and Evolution 38(3), 1060-1074.
| Crossref | Google Scholar |
Cheredath A, Uppangala S, Asha CS, et al. (2023) Combining machine learning with metabolomic and embryologic data improves embryo implantation prediction. Reproductive Sciences 30(3), 984-994.
| Crossref | Google Scholar | PubMed |
Chevalier NR, Gazquez E, Bidault L, et al. (2016) How tissue mechanical properties affect enteric neural crest cell migration. Scientific Reports 6(1), 20927.
| Crossref | Google Scholar |
Christmas MJ, Kaplow IM, Genereux DP, et al. (2023) Evolutionary constraint and innovation across hundreds of placental mammals. Science 380(6643), eabn3943.
| Crossref | Google Scholar |
Colquitt RB, Colquhoun DA, Thiele RH (2011) In silico modelling of physiologic systems. Best Practice & Research Clinical Anaesthesiology 25(4), 499-510.
| Crossref | Google Scholar | PubMed |
Cretoiu D, Xu J, Xiao J, Cretoiu SM (2016) Telocytes and their extracellular vesicles—evidence and hypotheses. International Journal of Molecular Sciences 17, 1322.
| Crossref | Google Scholar |
Cronin JG, Turner ML, Goetze L, et al. (2012) Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biology of Reproduction 86(2), 51.
| Crossref | Google Scholar | PubMed |
Cruz-Munoz W, Khokha R (2008) The role of tissue inhibitors of metalloproteinases in tumorigenesis and metastasis. Critical Reviews in Clinical Laboratory Sciences 45(3), 291-338.
| Crossref | Google Scholar | PubMed |
Davenport KM, Ortega MS, Liu H, et al. (2023) Single-nuclei RNA sequencing (snRNA-seq) uncovers trophoblast cell types and lineages in the mature bovine placenta. Proceedings of the National Academy of Sciences of the United States of America 120(12), e2221526120.
| Crossref | Google Scholar |
De Bem THC, Tinning H, Vasconcelos EJR, et al. (2021) Endometrium on-a-chip reveals insulin- and glucose-induced alterations in the transcriptome and proteomic secretome. Endocrinology 162(6), bqab054.
| Crossref | Google Scholar |
De Clercq K, Hennes A, Vriens J (2017) Isolation of mouse endometrial epithelial and stromal cells for in vitro decidualization. Journal of Visualized Experiments: JoVE [121] 55168.
| Crossref | Google Scholar |
De Wever O, Demetter P, Mareel M, et al. (2008) Stromal myofibroblasts are drivers of invasive cancer growth. International Journal of Cancer 123(10), 2229-2238.
| Crossref | Google Scholar | PubMed |
Donker RB, Mouillet JF, Chu T, et al. (2012) The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Molecular Human Reproduction 18(8), 417-424.
| Crossref | Google Scholar | PubMed |
Donofrio G, Franceschi V, Capocefalo A, et al. (2008) Bovine endometrial stromal cells display osteogenic properties. Reproductive Biology and Endocrinology: RB&E 6, 65.
| Crossref | Google Scholar |
Díez MC, Przyborski S, Del Cerro A, et al. (2023) Generation of a novel three-dimensional scaffold-based model of the bovine endometrium. Veterinary Research Communications 47(3), 1721-1733.
| Crossref | Google Scholar |
Ealy AD, Seekford ZK (2019) Symposium review: predicting pregnancy loss in dairy cattle. Journal of Dairy Science 102(12), 11798-11804.
| Crossref | Google Scholar | PubMed |
Engler AJ, Sen S, Sweeney HL, et al. (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4), 677-689.
| Crossref | Google Scholar | PubMed |
Eozenou C, Lesage-Padilla A, Mauffré V, et al. (2020) FOXL2 is a progesterone target gene in the endometrium of ruminants. International Journal of Molecular Sciences 21(4), 1478.
| Crossref | Google Scholar | PubMed |
Filant J, Spencer TE (2014) Uterine glands: biological roles in conceptus implantation, uterine receptivity and decidualization. International Journal of Developmental Biology 58(2–4), 107-116.
| Crossref | Google Scholar | PubMed |
Forde N, Lonergan P (2012) Transcriptomic analysis of the bovine endometrium: what is required to establish uterine receptivity to implantation in cattle? Journal of Reproduction and Development 58(2), 189-195.
| Crossref | Google Scholar | PubMed |
Forde N, O’Gorman A, Whelan H, Duffy P, O’Hara L, Kelly AK, Havlicek V, Besenfelder U, Brennan L, Lonergan P (2016) Lactation-Induced changes in metabolic status and follicular-fluid metabolomic profile in postpartum dairy cows. Reproduction, Fertility and Development 28, 1882-1892.
| Crossref | Google Scholar |
Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. Journal of Cell Science 123(24), 4195-4200.
| Crossref | Google Scholar | PubMed |
Gray CA, Burghardt RC, Johnson GA, et al. (2002) Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124(2), 289-300.
| Crossref | Google Scholar | PubMed |
Haeger J-D, Hambruch N, Dantzer V, et al. (2015) Changes in endometrial ezrin and cytokeratin 18 expression during bovine implantation and in caruncular endometrial spheroids in vitro. Placenta 36(8), 821-831.
| Crossref | Google Scholar | PubMed |
Hendijani F (2017) Explant culture: an advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Proliferation 50(2), e12334.
| Crossref | Google Scholar | PubMed |
Hromadnikova I, Kotlabova K, Ondrackova M, et al. (2013) Circulating C19MC MicroRNAs in preeclampsia, gestational hypertension, and fetal growth restriction. Mediators of Inflammation 2013, e186041.
| Crossref | Google Scholar | PubMed |
Hromadnikova I, Kotlabova K, Ivankova K, et al. (2017) First trimester screening of circulating C19MC microRNAs and the evaluation of their potential to predict the onset of preeclampsia and IUGR. PLoS ONE 12(2), e0171756.
| Crossref | Google Scholar | PubMed |
Hu G, Hao K, Ling F, et al. (2022) Establishment and characterization of a sheep endometrial epithelial cell line. Biochemical and Biophysical Research Communications 603, 63-68.
| Crossref | Google Scholar | PubMed |
Huang TTF, Kosasa T, Walker B, et al. (2021) Deep learning neural network analysis of human blastocyst expansion from time-lapse image files. Reproductive BioMedicine Online 42(6), 1075-1085.
| Crossref | Google Scholar | PubMed |
Huleihel L, Hussey GS, Naranjo JD, et al. (2016) Matrix-bound nanovesicles within ECM bioscaffolds. Science Advances 2(6), e1600502.
| Crossref | Google Scholar | PubMed |
Hume L, Edge JC, Tinning H, et al. (2023) MicroRNAs emerging coordinate with placental mammals alter pathways in endometrial epithelia important for endometrial function. iScience 26(4), 106339.
| Crossref | Google Scholar | PubMed |
Hwang JY, Maziarz J, Wagner GP, et al. (2021) Molecular evolution of CatSper in mammals and function of sperm hyperactivation in gray short-tailed opossum. Cells 10(5), 1047.
| Crossref | Google Scholar | PubMed |
Idelevich A, Vilella F (2020) Mother and embryo cross-communication. Genes 11(4), 376.
| Crossref | Google Scholar | PubMed |
Kelly P, Barry-Reidy A, Brewer A, et al. (2020) Improved filtration method to isolate pure populations of primary bovine endometrial epithelial and stromal cells for immunological studies. Veterinary Research Communications 44(1), 29-39.
| Crossref | Google Scholar | PubMed |
Kim S-M, Kim J-S (2017) A review of mechanisms of implantation. Development & Reproduction 21(4), 351-359.
| Crossref | Google Scholar | PubMed |
Kirkwood PM, Gibson DA, Smith JR, et al. (2021) Single-cell RNA sequencing redefines the mesenchymal cell landscape of mouse endometrium. FASEB Journal 35(4), e21285.
| Crossref | Google Scholar | PubMed |
Langhans SA (2018) Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Frontiers in Pharmacology 9, 6.
| Crossref | Google Scholar | PubMed |
Larsen EC, Christiansen OB, Kolte AM, et al. (2013) New insights into mechanisms behind miscarriage. BMC Medicine 11(1), 154.
| Crossref | Google Scholar |
Lee J, Kotliarova S, Kotliarov Y, et al. (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9(5), 391-403.
| Crossref | Google Scholar | PubMed |
Li X, Li Z, Hou D, et al. (2016) The bovine endometrial epithelial cells promote the differentiation of trophoblast stem-like cells to binucleate trophoblast cells. Cytotechnology 68(6), 2687-2698.
| Crossref | Google Scholar | PubMed |
Li B, Duan H, Wang S, et al. (2022) Gradient boosting machine learning model for defective endometrial receptivity prediction by macrophage-endometrium interaction modules. Frontiers in Immunology 13, 842607.
| Crossref | Google Scholar | PubMed |
Lonergan P, Fair T (2014) The ART of studying early embryo development: progress and challenges in ruminant embryo culture. Theriogenology 81(1), 49-55.
| Crossref | Google Scholar | PubMed |
Lucas ES, Vrljicak P, Muter J, et al. (2020) Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Communications Biology 3, 37.
| Crossref | Google Scholar |
Luo Y, Li Z, Guo H, et al. (2017) Predicting congenital heart defects: a comparison of three data mining methods. PLoS ONE 12(5), e0177811.
| Crossref | Google Scholar | PubMed |
Lynch VJ, Nnamani MC, Kapusta A, et al. (2015) Ancient transposable elements transformed the uterine regulatory landscape and transcriptome during the evolution of mammalian pregnancy. Cell Reports 10(4), 551-561.
| Crossref | Google Scholar | PubMed |
López-Martínez S, Campo H, de Miguel-Gómez L, et al. (2021) A natural xenogeneic endometrial extracellular matrix hydrogel toward improving current human in vitro models and future in vivo applications. Frontiers in Bioengineering and Biotechnology 9, 639688.
| Crossref | Google Scholar | PubMed |
MacKintosh SB, Schuberth HJ, Healy LL, et al. (2013) Polarised bovine endometrial epithelial cells vectorially secrete prostaglandins and chemotactic factors under physiological and pathological conditions. Reproduction 145(1), 57-72.
| Crossref | Google Scholar | PubMed |
Mathew DJ, Peterson KD, Senn LK, Oliver MA, Ealy AD (2022) Ruminant conceptus-maternal interactions: interferon-tau and beyond. Journal of Animal Science 100(7), skac123.
| Crossref | Google Scholar | PubMed |
Miao JH, Miao KH (2018) Cardiotocographic diagnosis of fetal health based on multiclass morphologic pattern predictions using deep learning classification. International Journal of Advanced Computer Science and Applications 9(5), 1-11.
| Crossref | Google Scholar |
Mika K, Marinić M, Singh M, et al. (2021) Evolutionary transcriptomics implicates new genes and pathways in human pregnancy and adverse pregnancy outcomes. eLife 10, e69584.
| Crossref | Google Scholar | PubMed |
Mika K, Whittington CM, McAllan BM, et al. (2022) Gene expression phylogenies and ancestral transcriptome reconstruction resolves major transitions in the origins of pregnancy. eLife 11, e74297.
| Crossref | Google Scholar |
Mitko K, Ulbrich SE, Wenigerkind H, Sinowatz F, Blum H, Wolf E, Bauersachs S (2008) Dynamic changes in messenger RNA profiles of bovine endometrium during the oestrous cycle. Reproduction 135(2), 225-240.
| Crossref | Google Scholar | PubMed |
Morales-Prieto DM, Favaro RR, Markert UR (2020) Placental miRNAs in feto-maternal communication mediated by extracellular vesicles. Placenta 102, 27-33.
| Crossref | Google Scholar | PubMed |
Murillo A, Muñoz M (2021) Isolation, Culture, and Characterization of Primary Bovine Endometrial, Epithelial, and Stromal Cells for 3D In Vitro Tissue Models. Methods in Molecular Biology 2273, 103-110.
| Crossref | Google Scholar | PubMed |
Nallanthighal S, Heiserman JP, Cheon D-J (2019) The role of the extracellular matrix in cancer stemness. Frontiers in Cell and Developmental Biology 7, 86.
| Crossref | Google Scholar |
Nolan J, Pearce OMT, Screen HRC, Knight MM, Verbruggen SW (2023) Organ-on-a-chip and microfluidic platforms for oncology in the UK. Cancers 15(3), 635.
| Crossref | Google Scholar | PubMed |
O’Connor BB, Pope BD, Peters MM, et al. (2020) The role of extracellular matrix in normal and pathological pregnancy: future applications of microphysiological systems in reproductive medicine. Experimental Biology and Medicine 245(13), 1163-1174.
| Crossref | Google Scholar | PubMed |
Passaro C, Tutt D, Mathew DJ, et al. (2018) Blastocyst-induced changes in the bovine endometrial transcriptome. Reproduction 156(3), 219-229.
| Crossref | Google Scholar | PubMed |
Pinzón-Arteaga CA, Wang Y, Wei Y, et al. (2023) Bovine blastocyst-like structures derived from stem cell cultures. Cell Stem Cell 30(5), 611-616.e7.
| Crossref | Google Scholar | PubMed |
Pun S, Haney LC, Barrile R (2021) Modelling human physiology on-chip: historical perspectives and future directions. Micromachines 12(10), 1250.
| Crossref | Google Scholar | PubMed |
Qadan MA, Piuzzi NS, Boehm C, Bova W, Moos M, Midura RJ, Hascall VC, Malcuit C, Muschler GF (2018) Variation in primary and culture-expanded cells derived from connective tissue progenitors in human bone marrow space, bone trabecular surface and adipose tissue. Cytotherapy 20(3), 343-360.
| Crossref | Google Scholar |
Rabaglino MB, Salilew-Wondim D, Zolini A, et al. (2023) Machine-learning methods applied to integrated transcriptomic data from bovine blastocysts and elongating conceptuses to identify genes predictive of embryonic competence. The FASEB Journal 37(3), e22809.
| Crossref | Google Scholar | PubMed |
Reese ST, Franco GA, Poole RK, et al. (2020) Pregnancy loss in beef cattle: a meta-analysis. Animal Reproduction Science 212, 106251.
| Crossref | Google Scholar | PubMed |
Richter M, Piwocka O, Musielak M, et al. (2021) From donor to the lab: a fascinating journey of primary cell lines. Frontiers in Cell and Developmental Biology 9, 711381.
| Crossref | Google Scholar |
Rink BE, Amilon KR, Esteves CL, et al. (2017) Isolation and characterization of equine endometrial mesenchymal stromal cells. Stem Cell Research & Therapy 8(1), 166.
| Crossref | Google Scholar | PubMed |
Sajeesh S, Broekelman T, Mecham RP, et al. (2020) Stem cell derived extracellular vesicles for vascular elastic matrix regenerative repair. Acta Biomaterialia 113, 267-278.
| Crossref | Google Scholar | PubMed |
Sakurai T, Bai H, Bai R, et al. (2012) Coculture system that mimics in vivo attachment processes in bovine trophoblast cells. Biology of Reproduction 87(3), 60.
| Crossref | Google Scholar | PubMed |
Sanderson RD, Bandari SK, Vlodavsky I (2019) Proteases and glycosidases on the surface of exosomes: newly discovered mechanisms for extracellular remodeling. Matrix Biology: Journal of the International Society for Matrix Biology 75–76, 160-169.
| Crossref | Google Scholar | PubMed |
Scatolin GN, Ming H, Wang Y, et al. (2023) Single-cell transcriptional landscapes of bovine peri-implantation development. bioRxiv 2023.06.13.544813.
| Crossref | Google Scholar | PubMed |
Schmidt S, Friedl P (2010) Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell and Tissue Research 339(1), 83-92.
| Crossref | Google Scholar | PubMed |
Scolari SC, Pugliesi G, Strefezzi RF, et al. (2016) Dynamic remodeling of endometrial extracellular matrix regulates embryo receptivity in cattle. Reproduction 153(1), 49-61.
| Crossref | Google Scholar |
Siristatidis C, Stavros S, Drakeley A, et al. (2021) Omics and artificial intelligence to improve in vitro fertilization (IVF) success: a proposed protocol. Diagnostics 11(5), 743.
| Crossref | Google Scholar | PubMed |
Spencer TE (2013) Early pregnancy: concepts, challenges, and potential solutions. Animal Frontiers 3(4), 48-55.
| Crossref | Google Scholar |
Spencer TE (2014) Biological roles of uterine glands in pregnancy. Seminars in Reproductive Medicine 32(5), 346-357.
| Crossref | Google Scholar | PubMed |
Spencer TE, Forde N, Lonergan P (2016) The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. Journal of Dairy Science 99(7), 5941-5950.
| Crossref | Google Scholar | PubMed |
Strowitzki T, Germeyer A, Popovici R, et al. (2006) The human endometrium as a fertility-determining factor. Human Reproduction Update 12(5), 617-630.
| Crossref | Google Scholar | PubMed |
Sánchez JM, Mathew DJ, Behura SK, et al. (2019) Bovine endometrium responds differentially to age-matched short and long conceptuses. Biology of Reproduction 101(1), 26-39.
| Crossref | Google Scholar | PubMed |
Taipale J, Keski-Oja J (1997) Growth factors in the extracellular matrix. The FASEB Journal 11(1), 51-59.
| Crossref | Google Scholar | PubMed |
Talbot NC, Caperna TJ, Edwards JL, et al. (2000) Bovine blastocyst-derived trophectoderm and endoderm cell cultures: interferon tau and transferrin expression as respective in vitro markers. Biology of Reproduction 62(2), 235-247.
| Crossref | Google Scholar | PubMed |
Tan Y, Tan D, He M, et al. (2005) A model for implantation: coculture of blastocysts and uterine endometrium in mice. Biology of Reproduction 72(3), 556-561.
| Crossref | Google Scholar | PubMed |
Tavakol S, Azedi F, Hoveizi E, et al. (2018) Human endometrial stem cell isolation from endometrium and menstrual blood. Bio-Protocol 8(2), e2693.
| Crossref | Google Scholar | PubMed |
Taylor AS, Tinning H, Ovchinnikov V, et al. (2023) A burst of genomic innovation at the origin of placental mammals mediated embryo implantation. Communications Biology 6(1), 459.
| Crossref | Google Scholar |
Tinning H, Taylor A, Wang D, Constantinides B, Sutton R, Oikonomou G, Velazquez MA, Thompson P, Treumann A, O’Connell MJ, Forde N (2020) The role of CAPG in molecular communication between the embryo and the uterine endometrium: is its function conserved in species with different implantation strategies? The FASEB Journal 34(8), 11015-11029.
| Crossref | Google Scholar | PubMed |
Tkach M, Théry C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164(6), 1226-1232.
| Crossref | Google Scholar | PubMed |
Uyar A, Bener A, Ciray HN (2015) Predictive modeling of implantation outcome in an in vitro fertilization setting: an application of machine learning methods. Medical Decision Making 35(6), 714-725.
| Crossref | Google Scholar | PubMed |
VerMilyea M, Hall JMM, Diakiw SM, et al. (2020) Development of an artificial intelligence-based assessment model for prediction of embryo viability using static images captured by optical light microscopy during IVF. Human Reproduction 35(4), 770-784.
| Crossref | Google Scholar | PubMed |
Wang G, Johnson GA, Spencer TE, et al. (2000) Isolation, immortalization, and initial characterization of uterine cell lines: an in vitro model system for the porcine uterus. In Vitro Cellular & Developmental Biology – Animal 36(10), 650-656.
| Crossref | Google Scholar | PubMed |
Wang Y, Ming H, Yu L, et al. (2023) Establishment of bovine trophoblast stem cells. Cell Reports 42(5), 112439.
| Crossref | Google Scholar | PubMed |
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442(7101), 368-373.
| Crossref | Google Scholar | PubMed |
Xiao S, Coppeta J, Rogers H, et al. (2017) A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nature Communications 8, 14584.
| Crossref | Google Scholar | PubMed |
Yiğit P, Bener A, Karabulut S (2022) Comparison of machine learning classification techniques to predict implantation success in an IVF treatment cycle. Reproductive BioMedicine Online 45(5), 923-934.
| Crossref | Google Scholar | PubMed |
Zhang S, Lin H, Kong S, et al. (2013) Physiological and molecular determinants of embryo implantation. Molecular Aspects of Medicine 34(5), 939-980.
| Crossref | Google Scholar |


