Changes in mononuclear immune cells during bovine pregnancy
Heloisa M. Rutigliano A B * , Kelsy A. Leppo B and Kira P. Morgado B
A B * , Kelsy A. Leppo B and Kira P. Morgado B
A School of Veterinary Medicine, Utah State University, Logan, UT 84322, USA.
B Department of Animal, Dairy and Veterinary Sciences, Utah State University, 4815 Old Main Hill, Logan, UT 84322, USA.
Reproduction, Fertility and Development 34(8) 608-618 https://doi.org/10.1071/RD21161
Published online: 20 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
This study aimed to determine the differences in gene expression between mononuclear cells derived from peripheral blood and endometrium during pregnancy in cattle and to determine the proportion of mononuclear cells in the endometrium of pregnant and diestrous cows. Endometrial tissue and peripheral blood were collected from Day 34 ± 2 pregnant cows, and mononuclear cell populations were quantified and sorted (n = 5). The relative mRNA levels of inflammatory mediators was assessed by quantitative real time polymerase chain reaction. During pregnancy, the proportion of CD8+, CD4+, CD4+CD25− and CD4+CD25dim cells among mononuclear cells was greater in blood than endometrium, and cells positive for CD14 and CD68 expressed greater mRNA amounts of interleukin (IL) 6, CXCL8 and IL10 in endometrium compared with blood. Cells positive for γ/δ-T cell receptor expressed greater amounts of IL1A transcript in the endometrium than in blood of diestrous cows, CD4+CD25bright cells expressed more CTLA4 mRNA in the endometrium compared with blood of diestrous cows, and endometrial natural killer cells expressed greater CXCL8 mRNA compared with blood of pregnant and diestrous cows. The percentages of CD21+, NCR1+, CD8+, FoxP3+, CD3+ and CD68+ cells were greater in the endometrium of Day 35 pregnant cows compared with diestrous cows.
Keywords: bovine gestation, diestrous, endometrium, gene expression, immune cell, leukocyte, lymphocyte, maternal–fetal interface, placenta, regulatory T cell.
Introduction
Reproductive performance is vital to the sustainability of animal production systems. Embryonic death accounts for most of the losses in reproductive efficiency in cattle. In bovine gestation, placental trophoblast cells are in direct contact with the maternal endometrium and dialogue between fetal and maternal cells must happen to promote successful fetal development. Changes to the maternal immune system contribute to this dialogue. The establishment of pregnancy requires the maternal immune system to adjust so it can tolerate the non-self-antigens encoded by paternal genes in the conceptus. Immune cell populations are present in significant numbers at the maternal–fetal interface during pregnancy in ruminants and are composed of macrophages, CD4+, CD8+, γ/δ+, B cells, natural killer (NK) cells and FoxP3+ T cells (Cobb and Watson 1995; Oliveira and Hansen 2009; Rutigliano et al. 2016; Vasudevan et al. 2017; Koroghli et al. 2018).
The maternal endometrium is composed of epithelial, glandular and immune cells. It is well established that the conceptus induces a shift in the numbers of endometrial immune cells and their functions in species with haemochorial placentation, such as humans and mice (Schumacher et al. 2018). In the endometrium, the role that a given immune cell population plays is changed during pregnancy. Immune cells are known to contribute to tissue remodeling and the development of the placenta during pregnancy. For instance, macrophages are involved in endometrial tissue remodelling (Lash et al. 2016), NK cells play an important function in spiral artery remodelling (Robson et al. 2012) and regulatory T cells have an essential role in inhibiting T cell responses to the fetal allograft (Aluvihare et al. 2004; Tilburgs et al. 2009).
While there are many studies investigating the roles of immune cell populations in species with haemochorial placentation, it is still unclear which immune cell populations are involved in promoting placental development in species with epitheliochorial placentas. In the endometrium of non-pregnant cattle, immune cells are scarce, and the presence of the conceptus in the maternal uterus leads to the expansion of endometrial macrophages, T cells (CD4+, CD8+, γ/δ+) and B cell populations in cattle, sheep, goats, pigs and horses (Cobb and Watson 1995; Grünig et al. 1995; Dimova et al. 2007; Oliveira and Hansen 2009; Rutigliano et al. 2016; Vasudevan et al. 2017; Koroghli et al. 2018). There is evidence that these cells modulate their protein and mRNA expression profiles, suggesting that their functions are altered during pregnancy (Kamat et al. 2016; Vasudevan et al. 2017). We have previously shown that T cell culture supernatant from pregnant cows leads to an increase in mRNA transcript expression of genes associated with placental growth in trophoblast cells (Leppo et al. 2021). Therefore, T cells play a role in placental development.
The objectives of this study were to assess the mRNA transcript levels of immune cells in peripheral blood and endometrium of pregnant cows at Day 34 ± 2 of gestation. We also aimed to determine the proportions of mononuclear cell populations in the endometrium of Day 35 pregnant cows and cows in diestrus. We hypothesised that endometrium-derived mononuclear cells would express less pro-inflammatory mediator transcripts, thereby regulating immune responses against placental antigens at the fetal–maternal interface during early placentation. We also hypothesised that certain mononuclear cell populations that have been described to be immunomodulatory in other species like FoxP3+ cells and macrophages would be enriched in the endometrium during pregnancy. This stage of pregnancy was selected because it is the beginning of the period when the embryo starts utilising nutrients transported through the placenta. The causes of embryonic loss during this phase of pregnancy are not well understood, and immune cells appear to play a role in placental development and function, thereby influencing embryonic development and survival.
Materials and methods
Animals
All animal procedures conducted in this study were reviewed and approved by the USU Institutional Animal Care and Use Committee. The Animal Welfare Act and USDA humane animal care and use policies and procedures were followed. Black Angus, multiparous, cyclic, 3- to 7-year-old cows in good health from the Utah State University beef cattle herd were used in this study.
The first experiment was conducted to determine the differences in gene expression of mononuclear cell populations between endometrium and peripheral blood of pregnant cows at Day 34 ± 2 of gestation (n = 5). Pregnancy was established by timed artificial insemination after a Presynch–Ovsynch estrous synchronisation protocol. Pregnancy was diagnosed via transrectal ultrasonography 34 ± 2 days post insemination. Immediately after pregnancy diagnosis, blood samples (30 mL) were collected from the coccygeal vein in acid citrate dextrose vacutainer tubes (Beckton, Dickinson and Company, Franklin Lakes, NJ, USA) and cows were humanely harvested at the Utah State University, USDA-inspected Meats Laboratory. Gestational age was confirmed to be approximately 34 ± 2 days based on embryonic crown to rump length. Endometrial tissue was harvested and processed immediately after the cows were sacrificed. Fresh endometrial tissue was transported to the laboratory in Dubelcco’s Modified Eagle Medium (DMEM) supplemented with 4 μg/mL penicillin/streptomycin and 10 μg/mL amphotericin B.
The second experiment was designed to determine the changes in endometrial immune cell populations between Day 35 pregnant and diestrous cows (n = 5/group). For the Day 35 pregnant cow group, pregnancy was established by timed artificial insemination after a Presynch–Ovsynch estrous synchronisation protocol, and pregnancy diagnosis and animal harvest were done 35 days post-insemination as described above. For the diestrous group, cows were verified to be non-pregnant at the beginning of the Presynch–Ovsynch estrous synchronisation protocol, and were harvested on Day 14 of the estrous cycle. Cows were verified to be in diestrus based on the presence of a corpus luteum in the uterus and serum progesterone concentrations of 1 ng/mL or greater. For immunohistochemistry, endometrium from two areas (3 × 2 cm) of the ipsilateral horn to the pregnancy (Day 35 pregnant cows) or to the corpus luteum (diestrous cows) were frozen in Tissue-Tek Optimal Cutting Temperature compound.
Blood mononuclear cell isolation
Unless otherwise stated, all laboratory supplies were obtained from Thermo Fisher Scientific. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation as described by Leppo et al. (2021). Briefly, blood samples were centrifuged at 800g at 20°C for 10 min to isolate buffy coat which was then diluted in Spinner Modification Minimum Essential Medium Eagle (5% fetal bovine serum (FBS), 4 μg/mL of penicillin/streptomycin), layered over Accu-Paque density gradient (Accurate Chemical and Scientific Corporation) and centrifuged at 1600g at 20°C for 20 min with the brake off. The interface containing PBMCs was collected, and erythrocytes were removed using an erythrocyte shock lysis solution. The remaining cells were washed twice, and cell concentration and viability were evaluated using a haemocytometer and trypan blue exclusion.
Endometrial tissue digestion and mononuclear cell isolation
Reproductive tracts were washed with phosphate-buffered saline (PBS) supplemented with 4 μg/mL penicillin/streptomycin and 10 μg/mL amphotericin. Endometrial tissue from intercaruncular areas was dissected, minced and washed in supplemented DMEM (DMEM with 4 μg/mL penicillin/streptomycin and 10 μg/mL amphotericin B). Approximately 100 g of tissue were used for digestion. Endometrial tissue was transferred to an Erlenmeyer flask containing 100 mL of warm DMEM containing 150 U/mL of collagenase type I (Millipore), and 10% FBS. Tissue was incubated twice at 37°C for 15 min on a shaker. After first 15 min incubation, tissue was strained using a sterile metal strained, and fresh DMEM and collagenase type I were added to remaining undigested tissue. After digestion, contents were strained in a 100 μm cell strainer, divided into 50 mL conical tubes and volume was brought to 45 mL with supplemented DMEM. Digested tissue was washed twice, cell concentration was assessed in a haemocytometer and adjusted to 5 × 105 cells/mL. Solution (10 mL/tube) was layered over 5 mL of Accu-Paque density gradient (Accurate Chemical and Scientific Corporation) and tubes were centrifuged at 800g at 20°C for 20 min with the brake off. Cell interface containing mononuclear cells was collected, put into 15 mL conical tubes and volume was brought to 12 mL with supplemented DMEM. Cells were washed twice with staining buffer (PBS with 0.1% (w/v) bovine serum albumin). Cell concentration and viability were assessed using a haemocytometer and trypan blue stain.
Flow cytometry and cell sorting
Blood and endometrium-derived mononuclear cells were stained for single or dual colour flow cytometry analysis using monoclonal antibodies followed by fluorescence labeled goat anti-mouse secondary antibodies. Cells were kept on ice during the staining procedure until cell analysis and sorting by fluorescence activated cell sorting (FACS). Primary antibodies against CD21, γ/δ-TCR, CD8, CD4, CD25, CD14, and their respective isotype-matched negative controls were purchased from the Washington State University Monoclonal Antibody Center. The antibody against NCR1 was purchased from AbD Serotec and the antibody against CD68 was purchased from Dako.
Primary antibody stock solutions were diluted to 15 μg/mL in staining buffer (PBS with 0.1% bovine serum albumin). Cell pellets with approximately 2 × 106 cells were resuspended in 100 μL of antibody solution and incubated for 15 min. Cells were washed twice in staining buffer and then resuspended in 100 μL of secondary antibody solutions at a 1:100 dilution and incubated for 15 min. The secondary antibody used for single staining of CD4, CD8, γ/δ-TCR, NCR1, CD21, CD14, CD68 and their respective negative controls was goat anti-mouse IgG conjugated with R-phycoerythrin (R-PE; Jackson ImmunoResearch). For dual staining of CD4/CD25 populations goat anti-mouse IgG2a conjugated with R-PE and goat anti-mouse IgG1 conjugated with allophycocyanin were used to identify CD4 and CD25 markers, repectively.
After incubation, cells were washed twice with staining buffer. Stained cells were resuspended in 1 mL of staining buffer and transferred to sterile 12 × 75 mm Falcon tubes. Mononuclear cells were sorted via flow cytometry using a FACS Aria II equipped with FACS Diva software (Beckton, Dickinson and Company). These cells were first gated based on the forward and side scatter characteristics. Cell populations CD21, γ/δ+, NCR1, CD8+, CD4+CD25−, CD4+CD25dim, CD4+CD25bright, CD14, and CD68 were then sorted based on their fluorescence intensity. Approximately 2000 cells were sorted into the wells of 96-well plates containing 6 μL of 2 × reaction mix from the Cell Direct One-Step real time quantitative polymerase chain reaction (qRT-PCR) kit. Cells were frozen at −80°C for later use in gene expression analyses. The proportions of the immune cell subpopulations within the parent population were quantified using the BD FACS Diva software (Beckton, Dickinson and Company).
Gene expression profiling of mononuclear cells
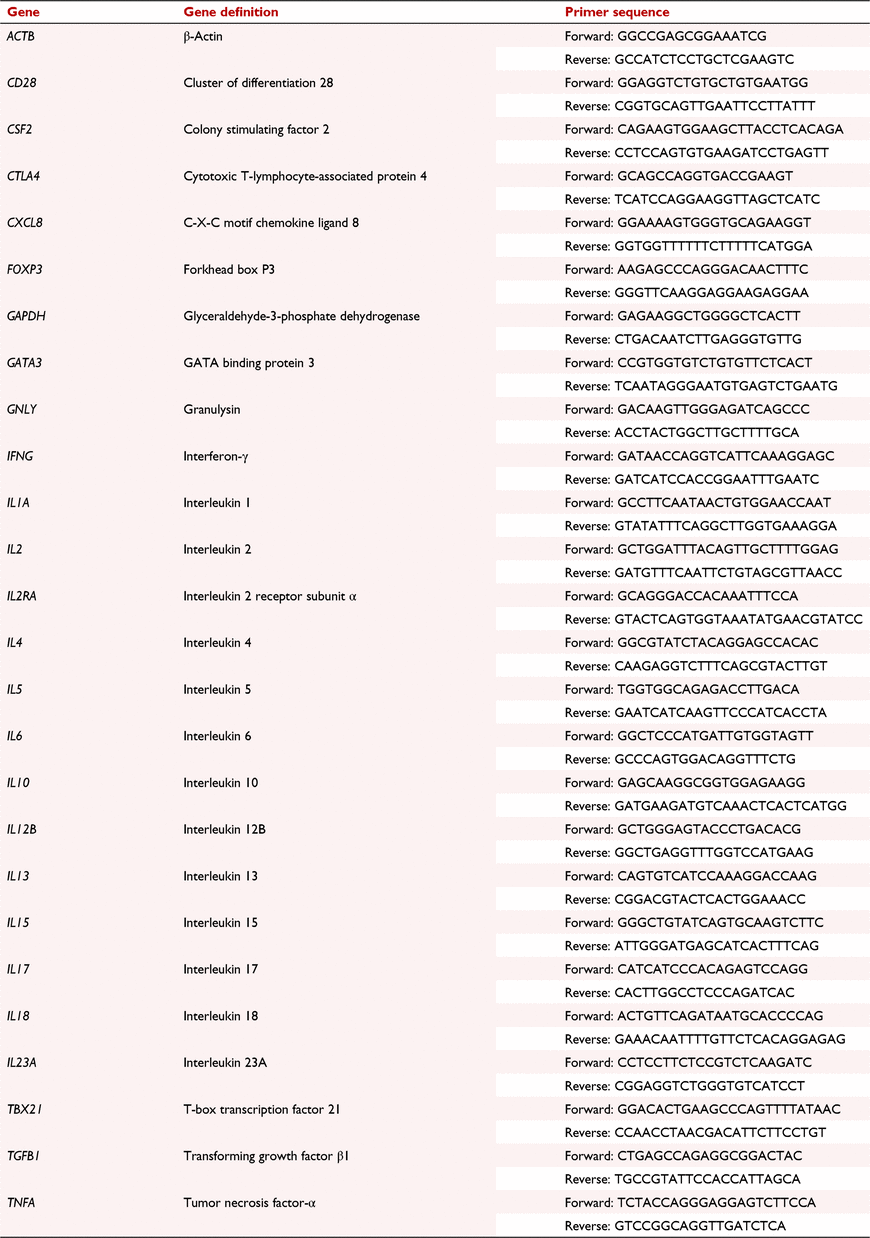
Sorted mononuclear cell populations were subjected to qRT-PCR assay conducted using 48.48 qRT-PCR Dynamic Array Integrated Fluidic Circuits (IFC; Fluidigm Corporation). The single-cell gene expression protocol was used. This protocol is described in detail by (Yang et al. 2016). Briefly, reverse transcription and specific target amplification were performed and the relative mRNA levels of genes encoding the following cytokines, transcription factors and cluster of differentiation (CD) markers were assessed: interleukin 2 receptor (CD25), cluster of differentiation 28 (CD28), colony stimulating factor 2 (CSF2), cytotoxic T-lymphocyte-associated protein 4 (CTLA4), chemokine (C-X-C motif) ligand 8 (CXCL8), forkhead box P3 (FOXP3), GATA binding protein 3 (GATA3), granulysin (GNLY), interferon-γ (IFNG), interleukin (IL) 1A, IL2, IL4, IL5, IL6, IL10, IL12B, IL13, IL15, IL17, IL18, IL23A, T-box 21 (TBX21), transforming growth factor-β1 (TGFB1) and tumor necrosis factor-α (TNFA). Primer sets for glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) and β-actin (ACTB) were used as reference genes (Table 1). All primers were designed and produced by Fluidigm Corporation. Relative gene expression was determined by the 2−ΔΔCt method using the average of the CT values of the reference genes GAPDH and ACTB for normalisation using the Fluidigm Real-Time PCR Analysis Software (Fluidigm Corporation). Each sample was run in four replicates. Values presented in this study are the fold change in gene expression for each treatment group compared with expression values of control samples. Intra-assay coefficient of variation ranged from 0.3 to 1.6%, inter-assay coefficient of variation ranged from 0.5 to 1.8%, and primer efficiencies ranged from 83 to 97% for each primer used.
|
Because it was challenging to isolate sufficient numbers (2000 cells) for qRT-PCR of each cell population from the uterus of diestrous cows, mononuclear cell populations from peripheral blood from animals in diestrus served as control samples for the gene expression analysis (n = 5).
Mononuclear leukocyte populations in the endometrium of pregnant and diestrous cows
Immunohistochemistry was conducted to compare the proportion of endometrial tissue occupied by certain mononuclear cells in pregnant and diestrous cows as described previously (Rutigliano et al. 2016; Koroghli et al. 2018). Endometrial tissue (3 × 2 cm) from the ipsilateral horn to the pregnancy or corpus luteum were frozen in Tissue-Tek optimal cutting temperature compound. Tissue sections (8 μm thick) were acquired using a cryostat microtome, put on microscope glass slides, fixed in cold acetone for 5 min, and air-dried. Slides were frozen at −80°C for long term storage. Each sample was replicated twice with each antibody.
Tissue sections were allowed to thaw and were rehydrated in PBS and treated with 0.3% H2O2 diluted in PBS. A PBS solution containing 1% bovine serum albumin and 2% normal goat serum was used to block nonspecific binding sites. Primary monoclonal antibodies were added to the tissue sections immediately after treatment with blocking solution and incubated for 1 h. Tissue sections were treated with biotinylated anti-mouse IgG secondary antibody, streptavidin peroxidase, aminoethyl carbazole (AEC), and then counterstained with haematoxylin. Finally, slides were mounted using Fluoromount-G mounting medium (Southern Biotech), analysed using a Zeiss Axio Observer microscope (Zeiss) and images were acquired using a high-resolution AxioCam HRC digital camera and the AxioVision software (Zeiss). The percentage of the surface area occupied by cells positive for CD4, CD21, NCR1, CD8, FoxP3, γ/δ-TCR, CD3 and CD68 was assessed in two tissue sections and 16 ± 2 images per section were acquired. A region of interest was drawn around the endometrial stroma and the AutoMeasure module of the AxioVision software was used to set colour intensity threshold values and measure the area occupied by the cell populations listed above. Area percentage of positive cells was calculated by dividing the area occupied by stained cells by the area of the region of interest. The same person conducted all measurements without prior knowledge of treatment group. The whole endometrial stroma was imaged to avoid site selection bias.
Statistical analyses
Flow cytometry data on the percentages of mononuclear cells in the endometrium and peripheral blood of pregnant cows were analysed by paired sample t-test using GraphPad Prism (GraphPad Software). Immunohistochemistry data were analysed by independent sample t-test using GraphPad Prism. Levels of mRNA transcript were analysed using the mixed command of SPSS Statistics (version 26.0, IBM Corporation). The ΔΔCt values were used to compare differences between mRNA levels in mononuclear cells derived from endometrium and blood of pregnant cows and blood of diestrous cows. Homogeneity of variance was assessed using the Levene’s test and normality was assessed by the Shapiro–Wilk test. When data were not normally distributed, numbers were square root or log transformed. Tissue of origin was considered a fixed factor and cow was considered a random factor in the model. Significant differences between samples were reported at false discovery rate adjusted P-values (Benjamini and Hochberg 1995) equal to or below 0.05.
Results
Mononuclear cell analysis and sorting
The proportions of CD21+, γ/δ+, NCR1+, CD8+, CD4+, CD4+CD25−, CD4+CD25dim, CD4+CD25bright, CD14+ and CD68+ cells among mononuclear cells was assessed in peripheral blood and endometrium at 34 ± 2 days of gestation by flow cytometry. Flow cytometry analysis of mononuclear cells based on forward and side scatter defined one parent population composed of small cells with low granularity, which is defined as the lymphocyte population, and of larger, more granular cells, which is defined as the monocyte/macrophage population. Cells were further characterised by their fluorescence intensity, which represents the expression of surface markers CD21, γ/δ, NCR1, CD8, CD4, CD25, CD14 and CD68. Cells positive for CD4 were grouped into CD4+CD25−, CD4+CD25dim and CD4+CD25bright based on the fluorescence intensity of the CD25 marker.
Percentages of blood and endometrial mononuclear cell populations in pregnant cows
Cells positive for CD4 and CD14 had the greatest proportion of mononuclear cells in peripheral blood and endometrium of pregnant cows (Fig. 1a and c). The proportions of the CD8+, CD4+, CD4+CD25− and CD4+CD25dim cell populations were significantly greater (P < 0.01) in blood compared with the endometrium (Fig. 1a and b). The percentages of CD21+, γ/δ-TCR+, NCR1+, CD14+, CD68+ and CD4+CD25bright cells did not differ between tissues.
Gene expression profiles of circulating and endometrial mononuclear cells
Of the nine mononuclear cell populations and 24 genes investigated, four genes were differentially expressed between blood and endometrial-derived mononuclear cells of pregnant cows. In CD68+ cells, the mRNA expression levels of IL1A were 6.9-fold greater in blood (P = 0.002) and 47.2-fold greater in endometrium (P = 0.01) of pregnant cows compared with blood from diestrous cows (control samples used for normalisation). The mRNA expression of this gene was not significantly different between blood and endometrium-derived CD68+ cells during pregnancy. The mRNA expression of CXCL8 was greater (P < 0.001) in endometrium than blood of pregnant cows. Expression of this gene was 24.3-fold greater in blood of pregnant cows compared with blood of diestrous cows (P < 0.001), and 455-fold greater in endometrium compared with blood of diestrous cows (P < 0.001). The mRNA expression of IL6 and IL10 was greater in the endometrium compared with blood of pregnant cows (P < 0.001), and the mRNA expression of these genes was 9.2- and 13.5-fold greater (P < 0.01) in the endometrium compared with blood of diestrous cows (Fig. 2a), respectively. The IL6 and IL10 mRNA expression of blood from pregnant cows was not significantly different from blood of diestrous cows.
For the CD14+cell population, the patterns of expression were similar to the CD68+ cell population. Endometrium-derived CD14+ cells expressed greater amounts of mRNA transcripts for IL1A (P = 0.018), IL6 (P < 0.001), CXCL8 (P = 0.003) and IL10 (P = 0.03) than blood-derived CD14+ cells from pregnant cows. In endometrial CD14+ cells, the expression of IL1A, IL6, CXCL8 and IL10 mRNA was 46.5-, 15.5-, 724- and 19.4-fold greater than in blood of diestrous cows (P < 0.01). In addition, transcript expression of CXCL8 was 26.5-fold greater (P = 0.005) in blood of pregnant cows compared with blood of diestrous cows (Fig. 2b).
In γ/δ+ cells, IL1A mRNA expression was 4.9-fold greater (P = 0.021) in endometrium of pregnant cows compared with blood of diestrous cows. In CD4+CD25bright cells, CTLA4 mRNA levels were 3.7-fold greater (P = 0.044) in endometrium of pregnant cows compared with blood of diestrous cows (Fig. 3b). The mRNA levels of IL1A in γ/δ+ cells and CTLA4 in CD4+CD25bright cells did not differ between blood and endometrium during pregnancy (Fig. 3a and b). In NCR1+ cells, CXCL8 mRNA transcripts were greater (P < 0.001) in the endometrium compared to blood of pregnant cows and were 33.4-fold greater (P = 0.005) in endometrium compared with blood of diestrous cows (Fig. 3c).
Proportion of endometrial mononuclear cell populations in pregnant and diestrous cows
Immunohistochemistry results show that CD3+ and CD68+ cells were the most abundant cells in the endometrium of pregnant and diestrous cows (Fig. 4c). Cells positive for CD21, NCR1, CD8, FoxP3, CD3 and CD68 were more abundantly present in the endometrium of pregnant cows compared to diestrous cows (Fig. 4).
Discussion
This study investigated the gene expression profile changes between mononuclear cells derived from peripheral blood and endometrium in Day 34 ± 2 pregnant cows. We also investigated the differences in the proportion of mononuclear cell populations in the endometrium of Day 35 pregnant cows and diestrous cows. This gestational period was selected because it is when early placentation and placentome development occur. With these changes, immune cells may play a particular role in tissue remodelling and establishing tolerance to fetal antigens. Here we observed a difference in the proportions of mononuclear cells in the endometrium of cows in diestrus and Day 35 pregnant cows. In addition, we observed that the mRNA profiles of mononuclear cells derived from endometrium and peripheral blood are different during early pregnancy.
To assess the gene expression profiles of macrophages in the endometrium and monocytes in blood, cells were identified and sorted based on their expression of cell surface markers CD14 and CD68. Macrophages, monocytes and neutrophils express CD14 (Paape et al. 1996) and this molecule is a co-receptor for lipopolysaccharides in bacteria (Triantafilou and Triantafilou 2002). The lysosomal-associated protein, CD68, is expressed in macrophages, monocytes and dendritic cells. Compared to cyclic cows, cells positive for CD14 are present in greater numbers in the pregnant endometrium as early as Day 13 of gestation (Mansouri-Attia et al. 2012), and cells positive for CD68 are increased in the pregnant endometrium at 54–240 days of gestation (Oliveira and Hansen 2008). In the endometrium of pregnant cows, CD14+ cells co-express CD68 (Oliveira and Hansen 2009) suggesting that these cells are in fact, macrophages. In addition, transcript levels for major histocompatibility complex (MHC)-II, CD80 and CD86 were greater in the endometrium of Day 17 and 20 pregnant cows compared with cyclic cows. Cells positive for MHC-II and SIRPA were also increased during gestation (Kamat et al. 2016) suggesting that the number of macrophages and/or dendritic cells expands during early pregnancy. In our study the proportion of CD68+ cells was greater in the endometrium of Day 35 pregnant cows than in cows in diestrus. It is, therefore, possible that macrophages expand in numbers as early as Day 13 of gestation and their numbers remain increased until the end of gestation.
Cells positive for CD14 were more abundant than CD68+ cells in blood and endometrium of pregnant cows. Oliveira and Hansen (2009) noted that several CD14+ cells in the stroma were either negative or weakly stained for CD68, which may explain why CD68+ cells were less abundant than CD14+ cells in our study. Upon morphological observation of haematoxylin–eosin-stained histology sections of pregnant endometrium it was determined that there were not many neutrophils present; therefore, it seems unlikely that these CD14+ cells are neutrophils.
Of the nine leukocyte subpopulations and 24 genes investigated, most immune-related genes had similar expression profiles between blood and endometrium. We identified four differentially expressed genes between immune cells derived from endometrial tissue and peripheral blood. Oliveira et al. (2010) collected CD14+ cells from peripheral blood and endometrium from cows in mid to late gestation and evaluated their gene expression profiles by microarray hybridisation. They observed that most genes were similarly expressed in peripheral blood and endometrium derived CD14+ cells. Nevertheless, they identified 1364 differentially regulated genes. Several of these genes were related to immune regulation, angiogenesis, apoptosis and tissue remodelling (Oliveira et al. 2010). Our results along with Oliveira et al. (2010) suggest that the transcriptional changes that occur when immune cells migrate from blood to the endometrium during pregnancy are not generalised, but rather, targeted to specific genes and biological functions.
The macrophage population is heterogeneous, and their functions are regulated by the micro-environment of the tissue where they reside. Macrophages may play a critical role during early pregnancy due to their involvement in tissue remodelling. There are two general subsets of macrophages: M1 macrophages, which produce Th1 and pro-inflammatory cytokines such as IL-1, IL-6, TNF-α and IFN-γ among others; and M2 macrophages, which are cells that promote tissue repair and immunomodulation by secreting Th2 and anti-inflammatory cytokines such as IL-10 and TGF-β. Interleukin 10 is a potent immunoregulator that inhibits the release of pro-inflammatory cytokines like IL-1, IL-6, IL-12 and TNF-α (Saraiva and O’Garra 2010), decreasing antigen presentation and the expression of co-stimulatory molecules such as CD80 and CD86 (Moore et al. 2001). This cytokine regulates dendritic cell phenotype, which is critical for preventing pregnancy loss (Blois et al. 2017). Vasudevan et al. (2017) determined that CD45+ cells sorted from Day 17 pregnant endometrium expressed greater amounts of IL-10 than their counterparts in non-pregnant endometrial tissue at Day 17 of the estrous cycle.
The balance between M1 and M2 macrophages is important to maintain tissue hemostasis. Here, endometrium derived CD68+ and CD14+ cells expressed greater levels of pro-inflammatory cytokines such as IL1A, IL6 and CXCL8, and anti-inflammatory cytokine IL10 compared with their counterparts in peripheral blood. It is possible that CD14+ and CD68+ cell populations represent a combination of M1 and M2 macrophages, thereby activating local immune responses and leukocyte recruitment but also regulating immune cell activity. Alternatively, these cells could be transitioning from an M1 to an M2 phenotype. Oliveira et al. (2010) demonstrated that during cattle pregnancies at least a subset of CD68+ and CD14+ cells differentiate into M2 macrophages that not only inhibit inflammation, but also promote angiogenesis, tissue remodelling and immunoregulation. In the second and third trimesters of gestation in cattle, endometrial CD14+ cells express greater amounts of chemokines CXCL14 and CCL22 (Oliveira et al. 2010), which provides further evidence that macrophages and/or dendritic cells are actively involved in the recruitment of other leukocytes to the pregnant endometrium. Future studies are needed to determine the phenotype and function of different subsets of macrophages throughout the gestational period in cattle.
The percentages of CD4+CD25− and CD4+CD25dim cells in the blood of pregnant cows were greater than in the pregnant endometrium. Conversely, the percentage of CD4+CD25bright cells was not affected by tissue of origin. We also observed an increase in FoxP3+ cells in the endometrium during pregnancy. T regulatory cells are defined as CD4+CD25+ and FoxP3+ and they suppress T cell activity (Campbell 2015). Although CD25 is an activation marker of T cells, studies in humans have shown that T regulatory cells primarily reside in the CD4+CD25bright T cell population (Kuniyasu et al. 2000; Baecher-Allan et al. 2001). In the decidua of pregnant women, the number of CD4+CD25bright cells, but not of CD4+CD25dim cells, increased compared with women undergoing spontaneous abortions (Sasaki et al. 2004). These data combined demonstrate that T regulatory cells are enriched in the endometrium during pregnancy in species with haemochorial and epitheliochorial placentation.
We observed that CD4+CD25bright cells in the endometrium of pregnant cows expressed greater amounts of CTLA4 mRNA transcripts compared to blood of non-pregnant cows. This molecule, also known CD152, competes with CD28 for binding to B7 on antigen presenting cells, preventing T cell activation and proliferation (Chambers et al. 2001). Sasaki et al. (2004) showed that T cells in the decidua express greater levels of surface CTLA4 compared with their counterparts in peripheral blood of women during early pregnancy. In addition, Vasudevan et al. (2017) observed greater mRNA expression of inhibitory molecules CD274 and CTLA4 in the endometrium of pregnant heifers. The greater expression of CTLA4 in CD4+CD25bright cells observed in our study suggests that these cells may be differentiating into regulatory T cells in the endometrium of pregnant cows.
Despite being of part of the lymphoid lineage, γ/δ-T cells are presumed to be part of the first line of defence of the innate immune system. These cells recognise antigens in an MHC independent manner and in general, γ/δ-T cells are cytotoxic, have Th1 activity and express many NK receptors (Wang et al. 2001; Holtmeier and Kabelitz 2005; Hayday 2009). Also, these cells are involved in maintaining tissue homeostasis and repair (Munoz et al. 2020). Although the function of γ/δ-T cells at the maternal–fetal interface is not clear, studies investigating the cytokine profiles of these cells as well as their in vitro functions suggest they are immunoregulatory during pregnancy (Mincheva-Nilsson 2003; Hoek et al. 2009). Others have found an enrichment of terminally differentiated pro-inflammatory γ/δ-T cells at the fetal–maternal interface during early human pregnancy (Terzieva et al. 2019). Although we did not observe a difference in numbers nor in the mRNA expression between blood and endometrium of pregnant cows, others have shown that their numbers expand in the endometrium during mid-gestation in sheep (Liu et al. 1997) and pigs (Dimova et al. 2007). This discrepancy could be due to differences in day of gestation, species, small sample size in our study or the methods used to select these cell populations.
Here we found that γ/δ-T cells expressed greater levels of IL1A mRNA in the endometrium of pregnant cows compared with blood of diestrous cows. This cytokine has potent pro-inflammatory properties such as induction of vasodilation, and attraction of monocytes and neutrophils to site of tissue damage or stress. This further supports the idea that these cells are activating immune responses at the fetal–maternal interface by recruiting leukocytes and promoting inflammation, which may be necessary for proper implantation and tissue remodelling.
Cluster of differentiation 21 is a protein that together with CD19, CD81 and CD225 form the B cell co-receptor complex. We have found increased amounts of CD21+ cells in the endometrium during pregnancy while the percentage of these cells did not differ between blood and endometrium in pregnant cows. Although the specific function of B cells at the fetal–maternal interface is unclear, B cells are known for their antigen presenting and immunoglobulin secretion functions. The regulatory functions of B cells have also been described to be involved in immunoregulation in autoimmune diseases, cancer and pregnancy in humans and murine animal models. Its regulatory effects are mediated through the anti-inflammatory effects of IL-10, which inhibits TNF-α production (Fettke et al. 2014). Further studies are necessary to investigate the specific functions of these cells in the pregnant endometrium.
NK (NCR1+) cell numbers significantly increased in the pregnant compared with the diestrous endometrium, which is consistent with findings that the proportions of these cells increase in the endometrium of heifers at Day 17 and 20 of gestation (Vasudevan et al. 2017). In humans and rodents, these cells do not have cytolytic activity and seem to contribute toward the development of an immunoregulatory environment by producing Th2 cytokines (Mori et al. 2016). NK cells are prevalent during pregnancy and play an important role in vascularisation by modifying spiral vascular arteries (Kane et al. 2009; Lash et al. 2016).
We observed that in the endometrium of pregnant cows, NK cells expressed greater levels of CXCL8 mRNA transcripts. This suggests that along with macrophages, NK cells contribute to the recruitment of leukocytes to the pregnant endometrium as evidenced by the increase in immune cells observed in the endometrium of pregnant cows by immunohistochemistry. Bovine NK cells in Day 17 pregnant endometrium have greater Granzyme A mRNA levels than NK cells derived from the non-pregnant endometrium at Day 17 of the estrous cycle, suggesting that NK cells in the pregnant endometrium have greater capacity for degranulation than NK cells in the non-pregnant endometrium. Interestingly, at least 80% of uterine lymphocytes that express NKp46 are also positive for CD8 (Vasudevan et al. 2017). In the present study, however, we did not investigate co-expression of cell surface markers.
Future studies are warranted to determine how global mRNA transcript levels change in mononuclear cells throughout pregnancy to predict the specific roles that different immune cell populations play. In addition, protein abundance and functional studies are needed to determine the specific roles of each of these mononuclear cell populations at the fetal–maternal interface.
To the best of our knowledge, this is the first study to demonstrate that the gene expression profiles of mononuclear cells in peripheral blood and endometrium of pregnant cows are distinct. Contrary to our initial hypothesis, we observed that mRNA transcription levels of pro-inflammatory mediators such as IL1A, IL6 and CXCL8 are greater in endometrium-derived mononuclear cells than their peripheral blood counterparts. We also provide evidence that the numbers of mononuclear cell populations are different in the endometrium of pregnant and diestrous cows with B cells, T cells, NK cells and cells expressing FoxP3 being more abundant in the pregnant endometrium. Our data suggest that mononuclear cells are actively involved in the process of tissue remodelling during early placentome development. Identifying the changes in endometrial mononuclear cells in response to pregnancy is the first step in determining potential strategies to prevent immune-mediated pregnancy loss in cattle.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research was supported by the Utah Agricultural Experiment Station (Project #1459) and approved as journal paper number 9482.
Acknowledgements
The authors thank Lynnette Harris for the editorial revisions.
References
Aluvihare, VR, Kallikourdis, M, and Betz, AG (2004). Regulatory T cells mediate maternal tolerance to the fetus. Nature Immunology 5, 266–271.| Regulatory T cells mediate maternal tolerance to the fetus.Crossref | GoogleScholarGoogle Scholar | 14758358PubMed |
Baecher-Allan, C, Brown, JA, Freeman, GJ, and Hafler, DA (2001). CD4+CD25high regulatory cells in human peripheral blood. The Journal of Immunology 167, 1245–1253.
| CD4+CD25high regulatory cells in human peripheral blood.Crossref | GoogleScholarGoogle Scholar | 11466340PubMed |
Benjamini, Y, and Hochberg, Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological) 57, 289–300.
| Controlling the false discovery rate: a practical and powerful approach to multiple testing.Crossref | GoogleScholarGoogle Scholar |
Blois, SM, Freitag, N, Tirado-González, I, Cheng, S-B, Heimesaat, MM, Bereswill, S, Rose, M, Conrad, ML, Barrientos, G, and Sharma, S (2017). NK cell-derived IL-10 is critical for DC-NK cell dialogue at the maternal–fetal interface. Scientific Reports 7, 2189.
| NK cell-derived IL-10 is critical for DC-NK cell dialogue at the maternal–fetal interface.Crossref | GoogleScholarGoogle Scholar | 28526846PubMed |
Campbell, DJ (2015). Control of regulatory T cell migration, function, and homeostasis. The Journal of Immunology 195, 2507–2513.
| Control of regulatory T cell migration, function, and homeostasis.Crossref | GoogleScholarGoogle Scholar | 26342103PubMed |
Chambers, CA, Kuhns, MS, Egen, JG, and Allison, JP (2001). CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual Review of Immunology 19, 565–594.
| CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy.Crossref | GoogleScholarGoogle Scholar | 11244047PubMed |
Cobb, SP, and Watson, ED (1995). Immunohistochemical study of immune cells in the bovine endometrium at different stages of the oestrous cycle. Research in Veterinary Science 59, 238–241.
| Immunohistochemical study of immune cells in the bovine endometrium at different stages of the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 8588099PubMed |
Dimova, T, Mihaylova, A, Spassova, P, and Georgieva, R (2007). Establishment of the porcine epitheliochorial placenta is associated with endometrial T-cell recruitment. American Journal of Reproductive Immunology 57, 250–261.
| Establishment of the porcine epitheliochorial placenta is associated with endometrial T-cell recruitment.Crossref | GoogleScholarGoogle Scholar | 17362386PubMed |
Fettke, F, Schumacher, A, Costa, S-D, and Zenclussen, AC (2014). B cells: the old new players in reproductive immunology. Frontiers in Immunology 5, 285.
| B cells: the old new players in reproductive immunology.Crossref | GoogleScholarGoogle Scholar | 25002862PubMed |
Grünig, G, Triplett, L, Canady, LK, Allen, WR, and Antczak, DF (1995). The maternal leucocyte response to the endometrial cups in horses is correlated with the developmental stages of the invasive trophoblast cells. Placenta 16, 539–559.
| The maternal leucocyte response to the endometrial cups in horses is correlated with the developmental stages of the invasive trophoblast cells.Crossref | GoogleScholarGoogle Scholar | 8570575PubMed |
Hayday, AC (2009). γδ T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196.
| γδ T cells and the lymphoid stress-surveillance response.Crossref | GoogleScholarGoogle Scholar | 19699170PubMed |
Hoek, A, Rutten, VPMG, Kool, J, Arkesteijn, GJA, Bouwstra, RJ, Van Rhijn, I, and Koets, AP (2009). Subpopulations of bovine WC1+ γδ T cells rather than CD4+CD25high Foxp3+ T cells act as immune regulatory cells ex vivo. Veterinary Research 40, 6.
| Subpopulations of bovine WC1+ γδ T cells rather than CD4+CD25high Foxp3+ T cells act as immune regulatory cells ex vivo.Crossref | GoogleScholarGoogle Scholar | 18928784PubMed |
Holtmeier, W, and Kabelitz, D (2005). γδ T cells link innate and adaptive immune responses. Chemical Immunology and Allergy 86, 151–183.
| γδ T cells link innate and adaptive immune responses.Crossref | GoogleScholarGoogle Scholar | 15976493PubMed |
Kamat, MM, Vasudevan, S, Maalouf, SA, Townson, DH, Pate, JL, and Ott, TL (2016). Changes in myeloid lineage cells in the uterus and peripheral blood of dairy heifers during early pregnancy. Biology of Reproduction 95, 68.
| Changes in myeloid lineage cells in the uterus and peripheral blood of dairy heifers during early pregnancy.Crossref | GoogleScholarGoogle Scholar | 27512154PubMed |
Kane, N, Kelly, R, Saunders, PTK, and Critchley, HOD (2009). Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology 150, 2882–2888.
| Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor.Crossref | GoogleScholarGoogle Scholar | 19196802PubMed |
Koroghli, JA, Floyd, E, Regouski, M, Rood, K, Gash, K, Panter, K, Stott, R, Davies, CJ, Polejaeva, IA, and Rutigliano, HM (2018). Gene expression and lymphocyte population at the fetal–maternal interface in sheep pregnancies established by somatic cell nuclear transfer. Reproduction, Fertility and Development 30, 1011–1020.
| Gene expression and lymphocyte population at the fetal–maternal interface in sheep pregnancies established by somatic cell nuclear transfer.Crossref | GoogleScholarGoogle Scholar |
Kuniyasu, Y, Takahashi, T, Itoh, M, Shimizu, J, Toda, G, and Sakaguchi, S (2000). Naturally anergic and suppressive CD25+CD4+ T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. International Immunology 12, 1145–1155.
| Naturally anergic and suppressive CD25+CD4+ T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation.Crossref | GoogleScholarGoogle Scholar | 10917889PubMed |
Lash, GE, Pitman, H, Morgan, HL, Innes, BA, Agwu, CN, and Bulmer, JN (2016). Decidual macrophages: key regulators of vascular remodeling in human pregnancy. Journal of Leukocyte Biology 100, 315–325.
| Decidual macrophages: key regulators of vascular remodeling in human pregnancy.Crossref | GoogleScholarGoogle Scholar | 26819320PubMed |
Leppo, KA, Collins, PA, Morgado, KP, Silva, AC, Thomas, A, and Rutigliano, HM (2021). Lymphocyte soluble factors from pregnant cows modulate mRNA transcript abundances encoding for proteins associated with trophoblast growth and development. Animal Reproduction Science 228, 106747.
| Lymphocyte soluble factors from pregnant cows modulate mRNA transcript abundances encoding for proteins associated with trophoblast growth and development.Crossref | GoogleScholarGoogle Scholar | 33838589PubMed |
Liu, W-J, Gottshall, SL, and Hansen, PJ (1997). Increased expression of cell surface markers on endometrial γδ T-cell receptor+ intraepithelial lymphocytes induced by the local presence of the sheep conceptus. American Journal of Reproductive Immunology 37, 199–205.
| Increased expression of cell surface markers on endometrial γδ T-cell receptor+ intraepithelial lymphocytes induced by the local presence of the sheep conceptus.Crossref | GoogleScholarGoogle Scholar | 9083618PubMed |
Mansouri-Attia, N, Oliveira, LJ, Forde, N, Fahey, AG, Browne, JA, Roche, JF, Sandra, O, Reinaud, P, Lonergan, P, and Fair, T (2012). Pivotal role for monocytes/macrophages and dendritic cells in maternal immune response to the developing embryo in cattle. Biology of Reproduction 87, 123.
| Pivotal role for monocytes/macrophages and dendritic cells in maternal immune response to the developing embryo in cattle.Crossref | GoogleScholarGoogle Scholar | 23034158PubMed |
Mincheva-Nilsson, L (2003). Pregnancy and γ/δ T cells: taking on the hard questions. Reproductive Biology and Endocrinology: RB&E 1, 120.
| Pregnancy and γ/δ T cells: taking on the hard questions.Crossref | GoogleScholarGoogle Scholar |
Moore, KW, de Waal Malefyt, R, Coffman, RL, and O’Garra, A (2001). Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology 19, 683–765.
| Interleukin-10 and the interleukin-10 receptor.Crossref | GoogleScholarGoogle Scholar | 11244051PubMed |
Mori, M, Bogdan, A, Balassa, T, Csabai, T, and Szekeres-Bartho, J (2016). The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Seminars in Immunopathology 38, 635–649.
| The decidua-the maternal bed embracing the embryo-maintains the pregnancy.Crossref | GoogleScholarGoogle Scholar | 27287066PubMed |
Munoz, LD, Sweeney, MJ, and Jameson, JM (2020). Skin resident γδ T cell function and regulation in wound repair. International Journal of Molecular Sciences 21, 9286.
| Skin resident γδ T cell function and regulation in wound repair.Crossref | GoogleScholarGoogle Scholar |
Oliveira, LJ, and Hansen, PJ (2008). Deviations in populations of peripheral blood mononuclear cells and endometrial macrophages in the cow during pregnancy. Reproduction 136, 481–490.
| Deviations in populations of peripheral blood mononuclear cells and endometrial macrophages in the cow during pregnancy.Crossref | GoogleScholarGoogle Scholar | 18635742PubMed |
Oliveira, LJ, and Hansen, PJ (2009). Phenotypic characterization of macrophages in the endometrium of the pregnant cow. American Journal of Reproductive Immunology 62, 418–426.
| Phenotypic characterization of macrophages in the endometrium of the pregnant cow.Crossref | GoogleScholarGoogle Scholar | 19895377PubMed |
Oliveira, LJ, McClellan, S, and Hansen, PJ (2010). Differentiation of the endometrial macrophage during pregnancy in the cow. PLoS ONE 5, e13213.
| Differentiation of the endometrial macrophage during pregnancy in the cow.Crossref | GoogleScholarGoogle Scholar | 20949061PubMed |
Paape, MJ, Lilius, EM, Wiitanen, PA, Kontio, MP, and Miller, RH (1996). Intramammary defense against infections induced by Escherichia coli in cows. American Journal of Veterinary Research 57, 477–482.
| 8712510PubMed |
Robson, A, Harris, LK, Innes, BA, Lash, GE, Aljunaidy, MM, Aplin, JD, Baker, PN, Robson, SC, and Bulmer, JN (2012). Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. The FASEB Journal 26, 4876–4885.
| Uterine natural killer cells initiate spiral artery remodeling in human pregnancy.Crossref | GoogleScholarGoogle Scholar | 22919072PubMed |
Rutigliano, HM, Thomas, AJ, Wilhelm, A, Sessions, BR, Hicks, BA, Schlafer, DH, White, KL, and Davies, CJ (2016). Trophoblast major histocompatibility complex class I expression is associated with immune-mediated rejection of bovine fetuses produced by cloning. Biology of Reproduction 95, 39.
| Trophoblast major histocompatibility complex class I expression is associated with immune-mediated rejection of bovine fetuses produced by cloning.Crossref | GoogleScholarGoogle Scholar | 27385783PubMed |
Saraiva, M, and O’Garra, A (2010). The regulation of IL-10 production by immune cells. Nature Reviews Immunology 10, 170–181.
| The regulation of IL-10 production by immune cells.Crossref | GoogleScholarGoogle Scholar | 20154735PubMed |
Sasaki, Y, Sakai, M, Miyazaki, S, Higuma, S, Shiozaki, A, and Saito, S (2004). Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Molecular Human Reproduction 10, 347–353.
| Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases.Crossref | GoogleScholarGoogle Scholar | 14997000PubMed |
Schumacher, A, Sharkey, DJ, Robertson, SA, and Zenclussen, AC (2018). Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development. The Journal of Immunology 201, 325–334.
| Immune cells at the fetomaternal interface: how the microenvironment modulates immune cells to foster fetal development.Crossref | GoogleScholarGoogle Scholar | 29987001PubMed |
Terzieva, A, Dimitrova, V, Djerov, L, Dimitrova, P, Zapryanova, S, Hristova, I, Vangelov, I, and Dimova, T (2019). Early pregnancy human decidua is enriched with activated, fully differentiated and pro-inflammatory gamma/delta T cells with diverse TCR repertoires. International Journal of Molecular Sciences 20, 687.
| Early pregnancy human decidua is enriched with activated, fully differentiated and pro-inflammatory gamma/delta T cells with diverse TCR repertoires.Crossref | GoogleScholarGoogle Scholar |
Tilburgs, T, Scherjon, SA, van der Mast, BJ, Haasnoot, GW, Voort-Maarschalk, MV, Roelen, DL, van Rood, JJ, and Claas, FHJ (2009). Fetal–maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. Journal of Reproductive Immunology 82, 148–157.
| Fetal–maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells.Crossref | GoogleScholarGoogle Scholar | 19631389PubMed |
Triantafilou, M, and Triantafilou, K (2002). Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends in Immunology 23, 301–304.
| Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster.Crossref | GoogleScholarGoogle Scholar | 12072369PubMed |
Vasudevan, S, Kamat, MM, Walusimbi, SS, Pate, JL, and Ott, TL (2017). Effects of early pregnancy on uterine lymphocytes and endometrial expression of immune-regulatory molecules in dairy heifers. Biology of Reproduction 97, 104–118.
| Effects of early pregnancy on uterine lymphocytes and endometrial expression of immune-regulatory molecules in dairy heifers.Crossref | GoogleScholarGoogle Scholar | 28633489PubMed |
Wang, L, Kamath, A, Das, H, Li, L, and Bukowski, JF (2001). Antibacterial effect of human Vγ2Vδ2 T cells in vivo. Journal of Clinical Investigation 108, 1349–1357.
| Antibacterial effect of human Vγ2Vδ2 T cells in vivo.Crossref | GoogleScholarGoogle Scholar |
Yang, M, Hall, J, Fan, Z, Regouski, M, Meng, Q, Rutigliano, HM, Stott, R, Rood, KA, Panter, KE, and Polejaeva, IA (2016). Oocytes from small and large follicles exhibit similar development competence following goat cloning despite their differences in meiotic and cytoplasmic maturation. Theriogenology 86, 2302–2311.
| Oocytes from small and large follicles exhibit similar development competence following goat cloning despite their differences in meiotic and cytoplasmic maturation.Crossref | GoogleScholarGoogle Scholar | 27650944PubMed |


