Fertilization potential of cold-stored Fowler’s toad (Anaxyrus fowleri) spermatozoa: temporal changes in sperm motility based on temperature and osmolality
Lucia Arregui A C , Andy J. Kouba B C , Jennifer M. Germano C D , Laura Barrios E , Marian Moore C and Carrie K. Kouba
A C , Andy J. Kouba B C , Jennifer M. Germano C D , Laura Barrios E , Marian Moore C and Carrie K. Kouba  F *
F *
A Department of Biology, Universidad Autónoma de Madrid, Madrid 28049, Spain.
B Wildlife, Fisheries and Aquaculture, Mississippi State University, Mississippi State, MS 39762, USA.
C Conservation and Research Department, Memphis Zoological Society, Memphis, TN 38112, USA.
D New Zealand Department of Conservation, Hamilton, New Zealand.
E Department of Statistics, CTI, Consejo Superior Investigaciones Científicas, 28006 Madrid, Spain.
F Biochemistry, Molecular Biology, Entomology and Plant Pathology, Mississippi State University, Mississippi State, MS 39762, USA.
Reproduction, Fertility and Development 34(5) 461-469 https://doi.org/10.1071/RD21037
Published online: 1 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Asynchrony of gamete release is problematic in amphibian captive breeding programs but can be overcome by short-term storage of spermatozoa. Hormonally induced sperm from the model species Anaxyrus fowleri were used to determine storage conditions for optimal fertilisation capacity. Sperm motility was measured over time, as a function of storage temperature (4°C or 22°C) and solution osmolality (7–40 mOsm/kg). Sperm at 40 mOsm/kg (spermic urine) stored at 4°C exhibited higher motility compared to 22°C. Also, sperm stored at 40 mOsm/kg retained higher motility compared to sperm stored below 15 mOsm/kg at both temperatures. Under optimal storage conditions (40 mOsm and 4°C) a 30% decrease in sperm motility occurred within 24 h, however, subsequent loss of sperm motility was lower (<10%/day) for days 2–8 thereafter. Sperm samples stored for 1–8 days under optimal conditions were tested for fertilising capacity by conducting in vitro fertilisation trials. Sperm stored for 8 days yielded 48% neurula development, similar to sperm stored for 1 day, which produced 60% neurula development. Overall, sperm stored for up to 8 days at 4°C as spermic urine retained fertilising capacity and thus can be used to circumvent asynchronous gamete release in assisted breeding efforts for amphibians.
Keywords: amphibian, anuran, bufonid, captive breeding, embryo, human chorionic gonadotrophin, in vitro fertilisation, reproduction.
Introduction
Amphibians are important components of both aquatic and terrestrial communities and are highly sensitive to modification of habitat due to their complex life cycles (Duellman and Trueb 1986; Wells 2007). Of the more than 8345 amphibian species worldwide, nearly 43% are in decline (IUCN 2020). To combat declines of severely at-risk amphibian species, ex situ assurance colonies have been put in place. Difficulties in maintaining and propagating the population under captive conditions frequently arise from poorly understood natural history and reproductive biology. To circumvent this lack of knowledge, captive breeding programs are increasingly implementing assisted reproductive technologies (ARTs) as conservation tools for overcoming the significant challenges of managing these small and isolated populations of endangered amphibians (Kouba and Vance 2009; Kouba et al. 2012; Clulow et al. 2014; Browne et al. 2019; Della Togna et al. 2020).
Asynchrony of gamete release is a common problem in many breeding programs that leads to low fertilisation rates and reduced numbers of tadpoles for reintroduction programs (Kouba et al. 2012). The number of animals in a captive breeding centre is limited by space and resources, while the exchange of animals between centres is prohibitive due to concerns of animal welfare and disease transmission. Using ARTs for short and long-term storage of gametes provides a means to circumvent such problems by allowing gamete exchange among populations such that in vitro fertilisation (IVF) can be conducted without time or spatial constraints. Captive and wild populations of amphibians can benefit from enhanced offspring production that arises from implementing ART practices both in vivo and ex vivo.
Amphibian spermatozoa obtained from testes macerates can be stored short-term (days) or cryopreserved for long-term biobanking (Browne et al. 2001, 2002a, 2002b; Silla et al. 2015). Spermatozoa collected in this manner remain inactive while in a high osmotic environment (>280 mOsm/kg) provided by the cellular components of the maceration process and subsequently, the various storage solutions containing buffers, extenders, or cryoprotectants (Clulow and Clulow 2016). Spermatozoa from testes maceration maintains fertilising capacity after several days when kept at cold temperatures (Wolf and Hedrick 1971; Browne et al. 2001), producing embryos in early IVF attempts with Rhinella marina (Browne et al. 1998). Testes maceration is a lethal technique and inappropriate for critically endangered species, but can provide important information and serve as a gamete recovery approach post-mortem.
Hormonally induced spermatozoa pass from the testes into the cloaca, undergoing final maturation and activation in the hypo-osmotic environment (<100 mOsm/kg) of the urine (Inoda and Morisawa 1987; Kouba et al. 2003). Sperm metabolism and physiology differs after exposure to low osmotic pressure as the spermatozoa consume existing ATP stores for active movement and to maintain integrity of the acrosome and plasma membrane against the external osmotic pressure difference. With energy stores actively being depleted, the maintenance of motility and fertilising capacity during short-term storage fertilisation may be compromised compared to spermatozoa from testes macerates held in an iso-osmotic environment. Hormonally induced spermatozoa can be inactivated by adding hypertonic solutions, and reactivated by adding water to decrease the osmolality (Kouba et al. 2003). Unfortunately, spermatoazoa still undergo a significant reduction in motility and forward progression within an hour of expression (Kouba et al. 2003). Another consequence of manipulating osmolality is that the sperm concentration potentially becomes too low for effective fertilisation (Kouba et al. 2003), especially considering that sperm concentrations start off much lower in spermic urine than when obtained from testes macerates. In R. marina, 0.5–1 × 109 sperm/mL were obtained from testes macerates and after serially diluting the sperm below 1 × 105 sperm/mL, less than 20% fertility was achieved (Browne et al. 1998). Altogether, a balance between the osmolality and sperm concentration is needed in order to maintain sperm viability and motility for fertilisation efforts. Moreover, these conditions must support sperm fertilisability over extended time periods (days–weeks) in order to coordinate fertilisation when eggs become available.
Amphibian spermatozoa can maintain viability at room temperature for up to 1–3 h showing high variation in longevity across individuals (Waggener and Carroll 1998; Hettyey and Roberts 2007; Sherman et al. 2008), but when stored at cold temperatures amphibian spermatozoa has the potential to survive for days, or even weeks (Silla et al. 2015; Langhorne et al. 2021). Extending the viability and motility of sperm collected from testes macerates has been in practice for decades but has also revealed species variations. For instance, when kept at 0°C, testicular spermatozoa from Rana temporaria was motile for 6 h, but spermatozoa from R. marina retained motility for 10 days, and Litoria Booroolongensis for 21 days when the sperm sample was oxygenated (Browne et al. 2001; Mansour et al. 2010; Silla et al. 2015). Spermatozoa collected from Ambystoma mexicanum as spermatophores remained viable for 28 days when stored at 0–6°C (Figiel 2020). In other species, hormonally-induced spermatozoa from Epidalea calamita, Pleurodeles walt, and Litoria peronii remains viable up to 4–5 days when stored near 4°C (Sherman et al. 2008; Kouba et al. 2011; Uteshev et al. 2015; Arregui et al. 2019). Cold storage does not suspend sperm metabolic activity indefinitely in the same manner as cryopreservation, and sperm continue to deplete their energy stores in an effort to retain motility and membrane integrity. In the case of hormone-induced spermatozoa released as spermic urine, extended exposure to the hypo-osmotic environment risks compromising the integrity of cellular features such as the acrosome membrane or DNA and could render the spermatozoa incapable of fertilisation, despite exhibiting motility. How long sperm retain fertilising capacity under conditions of cold-storage and low osmotic environment becomes a critical question for breeding amphibians, particularly when access to eggs may be significantly delayed for days.
This study examined the motility and fertilisation potential of hormone-induced spermatozoa stored at 4°C in a hypo-osmotic environment for up to 8 days post-collection. Using the Fowler’s toad (Anaxyrus fowleri) as a model, the objectives were to: (1) determine the relationship between osmolality and temperature (4°C vs 22°C) on sperm motility initially after release; (2) evaluate motility of spermatozoa stored at 4°C over 2 weeks under the optimised conditions from Objective 1; and (3) establish the fertilisation potential of cold-stored sperm over time. The overarching goal is to extend the lifespan and cellular integrity of sperm collected through non-invasive hormone therapy in order to overcome the problems of both temporal (delayed egg laying) and spatial (transport of sperm) asynchronous gamete availability.
Methods and experimental design
Animals
Fowler’s toads were wild caught in Shelby County Tennessee during the breeding season (Tennessee Wildlife Resources Agency Permit #3506) and transported to the Memphis Zoo (Memphis, TN, USA). All experiments were approved by the Memphis Zoo Institutional Animal Care and Use Committee (#2008-09). Toads were housed in single sex groups (10–15 toads/group) in 178 cm × 81 cm × 36 cm Waterland tubs with mesh covering and kept under ambient lighting (skylights) and additional UV lamps on timers to simulate natural light cycles. Sphagnum moss substrate (5–7 cm) was provided in the dry section of the tub and 5–7 cm of water in the water pool. The toads were fed adult crickets, dusted with Reptocal (Tetrafauna, Melle, Germany) one to two times a week.
Collection and evaluation of sperm samples
For sperm collection, males were administered 300 IU of human chorionic gonadotrophin (hCG; Sigma CG5, St. Louis, MO, USA) diluted in 200 μL of phosphate-buffered saline solution (PBS) by intraperitoneal injection and placed in holding boxes (34 cm × 20 cm × 12 cm), with 1 cm of tap water to induce water uptake. Spermic urine was collected in a Petri-dish as a result of a natural defence mechanism by the toads upon handling.
Sperm samples were assessed for total motility (TM), forward progressive motility (FPM) and quality of forward movement (QFM) with an Olympus CX41 microscope (200X magnification, under phase contrast). Percent TM was calculated as the proportion of spermatozoa moving (exhibiting tail motion or forward swimming) out of 100 spermatozoa counted. Percent FPM was calculated as the proportion of spermatozoa swimming in a linear direction out of 100 spermatozoa counted. QFM is a measure of the sperm sample as a whole and is subjectively scored on a scale of 0 (no forward movement) to 5 (maximum forward movement), as previously described (Kouba and Vance 2009; Kouba et al. 2012). Sperm concentration (sperm/mL) was calculated using a Neubauer hemocytometer by diluting the sample 1:10 in saline to stop motility. Osmolality was measured with a Vapro 5520 osmometer (Wescor, Inc., Logan, UT, USA).
Induction of ovulation and spawning of females for IVF
To induce ovulation and spawning, females (n = 3) were primed with 100 IU of hCG 72 h and a second priming dose of 100 IU hCG was given 24 h before the resolving dose of 500 IU of hCG + 15 μg of Luteinising Hormone-Releasing Hormone (LHRH; Sigma L4513) in 200 μL of PBS was administered intraperitoneally. Ovulation occurred within 14–18 h.
Experiment 1: temperature and osmolality effect on sperm motility
Male toads (n = 10) were administered hCG to produce spermic urine, which was immediately evaluated for sperm parameters TM, FPM and QFM. The effects of temperature and osmolality on the sperm motility were evaluated using a randomised block design with sperm samples assigned to one of two temperature treatments (4°C or 22°C). Within each block, subsamples of spermic urine were randomly assigned to one of four dilution treatments (undiluted control, 1:5, 1:10 and 1:20). Subsamples were diluted with distilled water to hypo-osmotic environments as follows: undiluted control (39.8 ± 1.3 mOsm/kg), 1:5 (10.3 ± 0.8 mOsm/kg), 1:10 (7.9 ± 0.7 mOsm/kg) or 1:20 (6.9 ± 0.7 mOsm/kg). All subsamples within each temperature block were subsequently analysed at 15, 30, 60, 120, 240 or 360 min for sperm motility parameters as described.
Experiment 2: sperm motility when stored at 4°C for up to 2 weeks
Spermic urine from seven males was collected and evaluated for TM and QFM and then subsequently stored undiluted at 4°C. Sperm samples were assessed for motility parameters on days 1, 2, 4, 7, 11 and 14 post-collection.
Experiment 3: fertilisation capacity of cold-stored spermatozoa
The IVF experiments with sperm stored for 1–8 days at 4°C were compared to fertilisation rates using fresh sperm (day 0) from two males. Over the course of 3 days, spermic urine samples were collected from 15 males (5 males/day) and stored at 4°C. Motility parameters and concentration of sperm were determined upon initial collection of spermic urine. As stored sperm sample volume declined over time from being used for IVF, we lost the ability to balance the number of males used for IVF each day; hence, the unbalanced male numbers observed in the experimental design (Tables 1 and 2). The non-normal distribution was corrected for in the statistical analysis.

|
IVF was conducted by placing 50.6 ± 2.0 (range 35–85 eggs/dish) eggs in the centre of 150 mm Petri dishes, and immediately pipetting an aliquot of spermatozoa onto the eggs and allowing 5 min for ’dry’ fertilisation before flooding the dish with distilled water. Fertilisations occurred using a sperm to egg ratio of 5 × 105 sperm/mL for 30–100 eggs per dish, which produced a high rate of fertilisation in fresh controls (>80% cleavage). Fertilisation rate was calculated based on the number of eggs, and as the percentage of cleaved embryos observed 4–6 h after IVF (Gosner stages 3–6), and as the percentage of embryos with neural crests (stages 14–16) 48 h after fertilisation (Gosner 1960). As a parthenogenic control, 60.3 ± 4.4 eggs from the females (n = 3) were ‘fertilised’ with 50 μL of distilled prior to flooding.
Statistical analysis
Statistical analysis was performed using SPSS 26 for Windows (SPSS Inc., Chicago, IL, USA). Assumptions of normality and homogeneity of variance were checked using the Shapiro–Wilk test. In Experiment 1, the effects of temperature, osmolality and time of incubation on sperm motility during storage was evaluated using a linear mixed effects models (LMM) introducing the three variables as a within subject variables and considering the time of incubation as a covariable. As a main effect of temperature was identified, a reanalysis of the effect of osmolality and time of incubation was conducted using an LMM that incorporated the time of incubation as a fixed factor and the toad as a random factor. A Bonferroni multiple-comparison correction was performed prior to pairwise comparisons. Osmolality was analysed with a one-way ANOVA for related samples. In Experiment 3 a one-way ANOVA and post hoc Tukey test were used for fresh sperm parameters and a generalised estimating equation (GEE) for differences across days. Pearson’s correlations were used to look at the relationship between the number of days that sperm samples were stored, sperm motility parameters and the percentage of embryos. Data are expressed as means ± s.e.m. and P < 0.05 was considered statistically significant.
Results
Experiment 1: temperature and osmolality effect on sperm motility
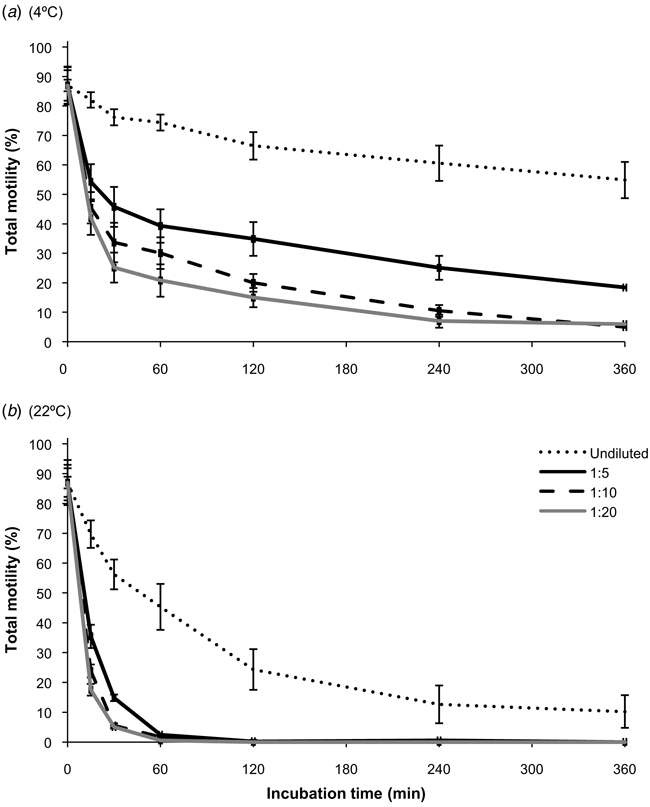
Initial spermic urine samples had an average TM of 87 ± 2.3% (range 76–96%). This initial motility changed dramatically over the 6 h incubation period depending on temperature and dilution. The model showed that temperature, osmolality, time of incubation and the interactions of these three variables were significant (P < 0.01) predictors for TM, with temperature having the greatest effect (higher F value). Temperature, time of incubation, and the interaction of these two factors were all significant (P ≤ 0.001) for undiluted sperm. Within 15 min, the temperature effect on sperm TM was significant (P = 0.034) and became more significant after 6 h (P ≤ 0.004) for undiluted samples (Fig. 1).
Sperm TM declined significantly faster (P ≤ 0.009) in the first 15 min at osmolalities below 15 mOsm/kg compared to spermic urine (39.8 ± 1.3 mOsm/kg) regardless of storage temperature. When stored at 4°C, osmolality and time of incubation were significant effects on sperm TM (P < 0.001), but not the interaction (P > 0.05). Sperm TM at 4°C was higher (P = 0.001) when diluted only 1:5 (10.3 mOsm/kg) than when diluted either by 1:10 (7.9 mOsm/kg) or by 1:20 (6.9 mOsm/kg). The immediate effect of osmolality on sperm TM was even more pronounced (P < 0.001) for sperm held at 22°C. After 2 h of incubation at 22°C only the undiluted control contained motile spermatozoa (>20%) (Fig. 1).
The initial QFM of freshly collected sperm was 4.4 ± 0.10 (range 3.5–4.5). The complete model for QFM was assessed only for the first hour, because sperm at 22°C ceased moving. Temperature, osmolality, time of incubation, and their interactions were significant predictors of QFM (P ≤ 0.005). Sperm temperature, time of incubation, and their interaction were significant (P ≤ 0.013) predictors for sperm QFM for sperm at 40 mOsm/kg. At 4°C, osmolality and time of incubation were significantly different (P < 0.002), yet their interaction was not (P > 0.05) for the first 60 min, whereas at 22°C the interaction was significant (P < 0.001). Thus, by lowering the external osmolality the QFM decreased faster for diluted sperm samples than in undiluted samples when stored at 22°C, indicating that temperature has a dramatic effect on the quality of sperm forward progressive movement.
Experiment 2: sperm motility when stored at 4°C for up to 2 weeks
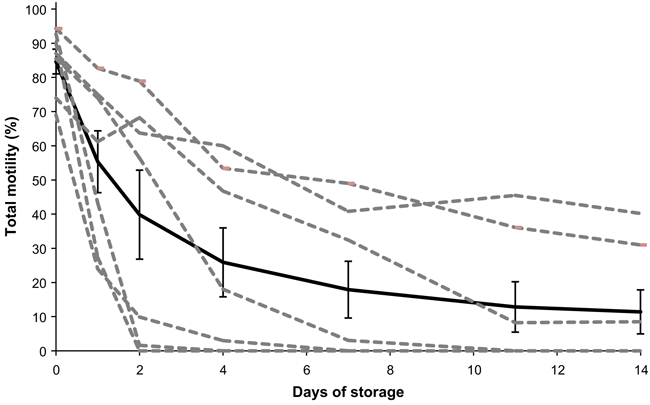
Here, we discuss the results of short-term cold storage on sperm motility across 14 days in an effort to understand the quality of sperm that could be used for IVF should asynchrony occur in the release of gametes during assisted breeding. Initial sperm samples (day 0) had TM of 84.6 ± 3.6% (range 69–94%) and a QFM of 4.93 ± 0.07 (range 4.5–5). After 24 h of storage at 4°C, average sperm TM decreased almost 30% (55.3 ± 9.1%, Fig. 2) and average sperm QFM decreased by 40% (to 2.79 ± 0.5%). The rate of sperm TM slowed to <10% per day, on average, over the next 13 days. Sperm motility was observed in 57% of spermic urine samples after 1 week of storage at 4°C, with variation across samples from individual males (Fig. 2).
Experiment 3: fertilisation capacity of cold-stored spermatozoa
Experiment 3 aimed to test whether spermic urine stored at cold temperatures could successfully fertilise eggs and at what rate of success. On average, freshly collected samples (day 0) had sperm TM = 85.5 ± 1.9%, FPM = 60.9 ± 3.4%, QFM; 4.09 ± 0.14, and sperm concentration = 11.0 ± 4.8 × 106 sperm/mL. Samples stored at 4°C had lower sperm TM (P ≤ 0.015), FPM (P < 0.003) and QFM (P ≤ 0.025) than fresh sperm samples (Table 1).
Spermic urine kept at 4°C showed fertilising capacity for 8 days and no correlation was found between the number of stored days and percentage of cleaved embryos or neurulas. The parthenogenic controls showed a cleavage rate of 10.7 ± 9.1% with most eggs asymmetrical in the first division, and none achieving neurula stage. Sperm stored at 4°C for up to 8 days fertilised a total of 1193 of 1989 eggs (60%), of which 1005 (50%) advanced to neurula (Table 2). Cleaved embryos ranged 35–76%, while neurulation ranged 27–62. Interestingly, sperm stored for 8 days at 4°C had the fourth highest cleavage rate and the second highest neurulation rate. Sperm TM directly correlated with both the rate of fertilisation (P = 0.002, R2 = 0.235) and neurulation (P = 0.005, R2 = 0.197).
Discussion
Conducting IVF in amphibians can be challenging due to asynchronous release of gametes and the rapid loss of fertilisation potential in female eggs upon oviposition. Developing a means to store sperm over a period of days would facilitate better fertilisation outcomes and greater production of offspring. To develop a method for short-term storage of anuran sperm, the impacts of temperature and osmolality on the motility of toad sperm over 6 h was investigated. These results showed that by diluting spermic urine in water to mimic what would occur during natural fertilisation, resulted in a rapid decline in sperm motility. Additionally, the rate of decline was impacted by temperature, with sperm stored at 4°C able to maintain motility at a higher level for longer compared to sperm stored at 22°C, and even up to 14 days. These results show a clear impact of lowering the osmolality on sperm motility, which is compounded by keeping the sperm at room temperature vs holding the sperm at 4°C. Since sperm motility could be preserved when left in spermic urine and held at cold temperatures, the next step was to determine if the sperm could still fertilise eggs. Here, sperm fertilisation potential was maintained across 8 days with little drop in cleavage or embryo development rates.
Temperature, time of storage, and the interaction between both were significant predictors of sperm motility showing that sperm samples stored at 4°C decreased in total percentage of motile spermatozoa at a slower rate than those held at room temperature. Similar results have been found for several other species of anurans, Anaxyrus boreas, Lithobates sevosa, Rana temporaria, and Litoria booroolongensis, where sperm were found to remain motile for longer periods when stored at 4°C than at 20–23°C (Mansour et al. 2010; Kouba et al. 2011; Silla et al. 2015; Langhorne et al. 2021). In contrast, sperm from Atelopus zeteki showed no difference in sperm motility between samples maintained at 4°C vs 22°C for over 46 min (Della Togna et al. 2018), albeit this incubation period was much shorter than tested here. There was significant variability in sperm longevity across individuals, with some males producing sperm samples that quickly lost motility, while other males produced sperm that persisted over the entire duration of the study. In every instance, fresh sperm had a higher sperm motility, forward motility and quality of forward motility than cold-stored sperm, regardless of temperature or osmolality. The rate of decline was greater for forward motility and QFM than for total motility, suggesting that the sperm are transitioning through a process of first slowing their rate of swimming, and then eventually stopping movement altogether. This action reflects a consumption of energy resources and is different from other potential causes of sperm inactivation, such as the abrupt dislodging of the mitochondrial vesicle or fracturing of the tail membrane (Kouba et al. 2003). Although differences were not found for sperm parameters among freshly collected samples, the rate of decline over time in cold-storage varied greatly among individuals. This range in sperm quality may simply be natural within the population and can be observed for other wild amphibians (Sherman et al. 2008). Lowering the temperature of sperm samples has been shown to decrease cell metabolism and sperm velocity, extending the duration of motility when energetic resources for spermatozoa in urine are limited (Alavi and Cosson 2005).
Anuran sperm are immotile within the testes where they are immersed in an isotonic environment; however, motility is activated by the hypotonic environment of urine or following expression into the external aqueous environment (Inoda and Morisawa 1987; Kouba et al. 2003; Alavi and Cosson 2006). The hypotonic stress caused by this severe change in osmotic pressure can be tolerated just long enough for fertilisation to take place shortly after gamete release yet causes intense cell damage within a short time. In Rana sylvatica, sperm obtained from testes macerates and then exposed to an osmotic pressure below 55 mOsm/kg showed a decreased motility, while concentration also decreased due to the lysis of sperm cells (Costanzo et al. 1998). In comparison, the species Crinia signifera express sperm that are tolerant to a broad range of osmolalities (10–100 mOsm/kg) in the environment which may be associated with differences in reproductive modes (Byrne et al. 2015). The short-lived motility of Fowler’s toad sperm upon dilution in water may reflect a strategy that aquatic breeders use to avoid cross-fertilisation of eggs from other males, especially in species where aquatic reproduction takes place in the presence of dozens or more pairs in amplexus. Short-term sperm functionality may have been selected for in external fertilising species that reproduce in an aquatic environment, especially in the presence of sexual competitors.
Spermic urine samples from Fowler’s toads retained motility for up to 14 days when stored at 4°C. These results are similar to those previously reported for the frog L. peronii (Sherman et al. 2008) and Anaxyrus boreas boreas (Langhorne et al. 2021), where viability and motility can last up to 12 days when stored in a similar manner as described. These data suggest that under cold-storage conditions, the persistence of motile spermatozoa in spermic urine is comparable to that obtained from testicular sperm in other bufonids. For instance, in Rana marina, sperm extracted from excised testes stored at 4°C retained motility for up to 12 days, with the greatest drop in motility occurring within the first 2 days of storage (Browne et al. 2001). Moreover, sperm motility has been observed in anuran testis excised from carcasses stored in the refrigerator for 7 days (Shishova et al. 2013).
The fertilising capacity of sperm stored over 8 days was demonstrated here by comparing rates of early embryo production from cold-stored sperm vs fresh sperm, following IVF. Although the average percent cleavage rate and neurula rate varied between days of sperm storage (range 34–80% and 27–62%, respectively), there was not a downward trend over time, with some of the highest fertilisation rates occurring after sperm had been 8 days in cold-storage.
There may be a high intraspecific variation in sperm longevity in anurans. For example, some sperm samples were able to fertilise a high percentage of eggs (40–70% cleaved) after being stored for 8 days, while others were not able to fertilise any eggs after only 2 days of storage. Similar observations have been noted in fertilising variability for the Natterjack toad using cryopreserved sperm (Arregui et al. 2020). Total motility at the time of IVF was a predictor for embryos to achieve cleavage and reach the neurula stage, yet the predicting capability of this variable was low.
In several anuran species, sperm samples collected by hormonal induction methods and cryopreserved for long-term storage have recovered live and motile sperm post-thaw (Shishova et al. 2011; Langhorne et al. 2013; Uteshev et al. 2013; Hinkson et al. 2019; Arregui et al. 2020). Similarly, viable sperm from caudate species have also been recovered (Peng et al. 2011; Figiel 2013; Unger et al. 2013; Marcec et al. 2014; Guy et al. 2020). Although sperm forward motility after thawing has been achieved in anurans, fertilisation success has been highly variable. For instance, moderate to high hatching rates were achieved in R. temporaria (>70%) and E. calamita (<40%) for embryos fertilised using cryopreserved sperm (Shishova et al. 2011; Arregui et al. 2020). However, in other species such as Pelophylax lessonae (<15%) and L. sevosa (<6%) the embryo hatch rates are extremely low (Uteshev et al. 2013; Langhorne et al. 2013). The use of cryopreserved sperm is one solution to overcoming the problem of asynchronous gamete production yet keeping sperm samples chilled for several days as demonstrated here, may be a low-tech solution that doesn’t require the sophisticated expertise, chemicals or equipment needed for proper cryopreservation. Proof of concept for shipping cold-stored sperm for genetic management has already been accomplished in the endangered Lithobates sevosa, where sperm was shipped overnight between two captive holding institutions, followed by IVF and the production of hundreds of offspring (Kouba et al. 2011). The success of this IVF procedure using cooled sperm allowed for a genetic exchange between captive populations without the normal disease risks and stress to individuals when animals are transported or undergo quarantine.
In conclusion, bufonid sperm quickly lose motility when kept at room temperature and that loss of motility is exacerbated when the osmolality is dropped by diluting the sperm in water. Thus, keeping the sample in spermic urine without further dilution, and immediately storing the sample at 4°C until evaluation, IVF or long-term biobanking procedures can be implemented. In this cold storage environment, toad sperm remained motile for up to 14 days and showed a relatively high rate of fertilisation for up to 8 days. These results provide additional knowledge on amphibian sperm physiology relative to environmental triggers and fertilisation, yet also provide a pathway for exploitation by conservation biologists for assisted breeding, genetic management and reintroduction of threatened species.
Data availability
Data are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors have no competing interests to report.
Declaration of funding
This study was supported by a grant from Morris Animal Foundation Grant (D09ZO-032) for developing assisted reproductive technologies for threatened amphibians awarded to AJK and CKK which supported JG. In addition, CKK and AJK are supported by the Mississippi Agricultural and Forestry Experiment Station and CKK by the USDA-ARS Biophotonics Initiative grant #58-6402-018 and National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under the accession number W3173. LA was a recipient of a travel award funded by the Research service of Universidad Autónoma de Madrid. The funding agencies had no role in the project design, data collection, analysis, interpretation of results or construction of the written document.
Acknowledgements
The authors are grateful to all the keepers and volunteers at the Memphis Zoo that assisted with the animal husbandry.
References
Alavi, SMH, and Cosson, J (2005). Sperm motility in fishes. (I). Effects of temperature and pH: a review. Cell Biology International 29, 101–110.| Sperm motility in fishes. (I). Effects of temperature and pH: a review.Crossref | GoogleScholarGoogle Scholar |
Alavi, SMH, and Cosson, J (2006). Sperm motility in fishes. (II). Effects of ions and osmolality: a review. Cell Biology International 30, 1–14.
| Sperm motility in fishes. (II). Effects of ions and osmolality: a review.Crossref | GoogleScholarGoogle Scholar |
Arregui, L, Diaz-Diaz, S, Alonso-López, E, and Kouba, AJ (2019). Hormonal induction of spermiation in a Eurasian bufonid (Epidalea calamita. Reproductive Biology and Endocrinology 17, 92.
| Hormonal induction of spermiation in a Eurasian bufonid (Epidalea calamita.Crossref | GoogleScholarGoogle Scholar | 31711511PubMed |
Arregui, L, Bóveda, P, Gosálvez, J, and Kouba, AJ (2020). Effect of seasonality on hormonally induced sperm in Epidalea calamita (Amphibia, Anura, Bufonidae) and its refrigerated and cryopreservated storage. Aquaculture 529, 735677.
| Effect of seasonality on hormonally induced sperm in Epidalea calamita (Amphibia, Anura, Bufonidae) and its refrigerated and cryopreservated storage.Crossref | GoogleScholarGoogle Scholar |
Browne, RK, Clulow, J, Mahony, M, and Clark, A (1998). Successful recovery of motility and fertility of cryopreserved Cane toad (Bufo marinus) sperm. Cryobiology 37, 339–345.
| Successful recovery of motility and fertility of cryopreserved Cane toad (Bufo marinus) sperm.Crossref | GoogleScholarGoogle Scholar | 9917350PubMed |
Browne, RK, Clulow, J, and Mahony, M (2001). Short-term storage of cane toad (Bufo marinus) gametes. Reproduction 121, 167–173.
| Short-term storage of cane toad (Bufo marinus) gametes.Crossref | GoogleScholarGoogle Scholar | 11226040PubMed |
Browne, RK, Clulow, J, and Mahony, M (2002a). The short-term storage and cryopreservation of spermatozoa from hylid and myobatrachid frogs. CryoLetters 23, 129–136.
| 12050781PubMed |
Browne, RK, Davis, J, Pomering, M, and Clulow, J (2002b). Storage of cane toad (Bufo marinus) sperm for 6 days at 0°C with subsequent cryopreservation. Reproduction, Fertility and Development 14, 267–273.
| Storage of cane toad (Bufo marinus) sperm for 6 days at 0°C with subsequent cryopreservation.Crossref | GoogleScholarGoogle Scholar |
Browne, RK, Silla, AJ, Upton, R, Della-Togna, G, Marcec-Greaves, R, Shishova, NV, Uteshev, VK, Proaño, B, Pérez, OD, Mansour, N, Kaurova, SA, Gakhova, EN, Cosson, J, Dyzuba, B, Kramarova, LI, McGinnity, D, Gonzalez, M, Clulow, J, and Clulow, S (2019). Sperm collection and storage for the sustainable management of amphibian biodiversity. Theriogenology 133, 187–200.
| Sperm collection and storage for the sustainable management of amphibian biodiversity.Crossref | GoogleScholarGoogle Scholar | 31155034PubMed |
Byrne, PG, Dunne, C, Munn, AJ, and Silla, AJ (2015). Environmental osmolality influences sperm motility activation in an anuran amphibian. Journal of Evolutionary Biology 28, 521–534.
| Environmental osmolality influences sperm motility activation in an anuran amphibian.Crossref | GoogleScholarGoogle Scholar | 25586700PubMed |
Clulow, J, and Clulow, S (2016). Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: bringing the ARTs up to speed. Reproduction, Fertility and Development 28, 1116–1132.
| Cryopreservation and other assisted reproductive technologies for the conservation of threatened amphibians and reptiles: bringing the ARTs up to speed.Crossref | GoogleScholarGoogle Scholar |
Clulow J, Trudeau V, Kouba A (2014) Amphibian declines in the twenty-first century: why we need assisted reproductive technologies. In ‘Reproductive sciences in animal conservation. Vol. 753.’ (Eds WV Holt, JL Brown, P Comizzoli) pp. 275–316. (Springer: New York, NY, USA)
| Crossref |
Costanzo, JP, Mugnano, JA, Wehrheim, HM, and Lee, RE (1998). Osmotic and freezing tolerance in spermatozoa of freeze-tolerant and -intolerant frogs. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 275, R713–R719.
Della Togna, G, Gratwicke, B, Evans, M, Augustine, L, Chia, H, Bronikowski, E, Murphy, JB, and Comizzoli, P (2018). Influence of extracellular environment on the motility and structural properties of spermatozoa collected from hormonally stimulated Panamanian Golden Frog (Atelopus zeteki. Theriogenology 108, 153–160.
| Influence of extracellular environment on the motility and structural properties of spermatozoa collected from hormonally stimulated Panamanian Golden Frog (Atelopus zeteki.Crossref | GoogleScholarGoogle Scholar | 29216539PubMed |
Della Togna, G, Howell, LG, Clulow, J, Langhorne, CJ, Marcec-Greaves, R, and Calatayud, NE (2020). Evaluating amphibian biobanking and reproduction for captive breeding programs according to the Amphibian Conservation Action Plan objectives. Theriogenology 150, 412–431.
| Evaluating amphibian biobanking and reproduction for captive breeding programs according to the Amphibian Conservation Action Plan objectives.Crossref | GoogleScholarGoogle Scholar | 32127175PubMed |
Duellman WE, Trueb L (1986) ‘Biology of amphibians’. (The Johns Hoppkins University Press)
Figiel, CR (2013). Cryopreservation of sperm from the axolotl Ambystoma mexicanum: implications for conservation. Herpetological Conservation and Biology 8, 748.
Figiel, CR (2020). Cold storage of sperm from the Axolotl, Ambystoma mexicanum. Herpetological Conservation and Biology 15, 367–371.
Gosner, KL (1960). A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190.
Guy, EL, Gillis, AB, Kouba, AJ, Barber, D, Poole, V, Marcec-Greaves, RM, and Kouba, CK (2020). Sperm collection and cryopreservation for threatened newt species. Cryobiology 94, 80–88.
| Sperm collection and cryopreservation for threatened newt species.Crossref | GoogleScholarGoogle Scholar | 32437677PubMed |
Hettyey, A, and Roberts, JD (2007). Sperm traits in the quacking frog (Crinia georgiana), a species with plastic alternative mating tactics. Behavioral Ecology and Sociobiology 61, 1303–1310.
| Sperm traits in the quacking frog (Crinia georgiana), a species with plastic alternative mating tactics.Crossref | GoogleScholarGoogle Scholar |
Hinkson, KM, Baecher, JA, and Poo, S (2019). Cryopreservation and hormonal induction of spermic urine in a novel species: the smooth-sided toad (Rhaebo guttatus. Cryobiology 89, 109–111.
| Cryopreservation and hormonal induction of spermic urine in a novel species: the smooth-sided toad (Rhaebo guttatus.Crossref | GoogleScholarGoogle Scholar | 31078579PubMed |
Inoda, T, and Morisawa, M (1987). Effect of osmolality on the initiation of sperm motility in Xenopus laevis. Comparative biochemistry and physiology. A, Comparative Physiology 88, 539–542.
| Effect of osmolality on the initiation of sperm motility in Xenopus laevis.Crossref | GoogleScholarGoogle Scholar | 2892629PubMed |
IUCN (2020) The IUCN red list of threatened species. Summary tables. Version 2020-1. http://www.iucnredlist.org
Kouba, AJ, and Vance, CK (2009). Applied reproductive technologies and genetic resource banking for amphibian conservation. Reproduction, Fertility and Development 21, 719–737.
| Applied reproductive technologies and genetic resource banking for amphibian conservation.Crossref | GoogleScholarGoogle Scholar |
Kouba, AJ, Vance, CK, Frommeyer, MA, and Roth, TL (2003). Structural and functional aspects of Bufo americanus spermatozoa: effects of inactivation and reactivation. Journal of Experimental Zoology 295A, 172–182.
| Structural and functional aspects of Bufo americanus spermatozoa: effects of inactivation and reactivation.Crossref | GoogleScholarGoogle Scholar |
Kouba, A, Willis, E, Vance, C, Hasenstab, S, Reichling, S, Krebs, J, Linhoff, L, Snoza, M, Langhorne, C, and Germano, J (2011). Development of assisted reproduction technologies for the endangered Mississippi Gopher frog (Rana sevosa) and sperm transfer for in vitro fertilization. Reproduction, Fertility and Development 24, 170–170.
| Development of assisted reproduction technologies for the endangered Mississippi Gopher frog (Rana sevosa) and sperm transfer for in vitro fertilization.Crossref | GoogleScholarGoogle Scholar |
Kouba A, Vance C, Calatayud N, Rowlison T, Langhorne C, Willard S (2012) Assisted reproductive technologies (ART) for amphibians. In ‘Amphibian husbandry resource guide’. (Eds VA Poole, S Grow). (AZA’s Amphibian Taxon Advisory Group)
Langhorne, CJ, Calatayud, NE, Kouba, AJ, Feugang, JM, Vance, CK, and Willard, ST (2013). Cryoconservation: Successful sperm cryopreservation and developmental outcomes using endangered North American amphibians. Cryobiology 67, 405.
| Cryoconservation: Successful sperm cryopreservation and developmental outcomes using endangered North American amphibians.Crossref | GoogleScholarGoogle Scholar |
Langhorne, C, Calatayud, NE, Kouba, CK, Willard, ST, Smith, T, Ryan, PL, and Kouba, AJ (2021). Efficacy of hormone stimulation on sperm production in an alpine amphibian (Anaxyrus boreas boreas) and the impact of short-term storage on sperm quality. Zoology 146, 125912.
| Efficacy of hormone stimulation on sperm production in an alpine amphibian (Anaxyrus boreas boreas) and the impact of short-term storage on sperm quality.Crossref | GoogleScholarGoogle Scholar | 33743452PubMed |
Mansour, N, Lahnsteiner, F, and Patzner, RA (2010). Motility and cryopreservation of spermatozoa of European common frog, Rana temporaria. Theriogenology 74, 724–732.
| Motility and cryopreservation of spermatozoa of European common frog, Rana temporaria.Crossref | GoogleScholarGoogle Scholar | 20537698PubMed |
Marcec, R, Langhorne, C, Vance, C, Kouba, A, and Willard, S (2014). C-1013: cryopreservation of spermic milt in the model species Ambystoma tigrinum (Tiger salamander) for application in endangered salamanders. Cryobiology 69, 515.
| C-1013: cryopreservation of spermic milt in the model species Ambystoma tigrinum (Tiger salamander) for application in endangered salamanders.Crossref | GoogleScholarGoogle Scholar |
Peng, L-Y, Xiao, Y-M, and Liu, Y (2011). Effect of cryopreservation and short-term storage of Chinese Giant salamander sperm. Acta Hydrobiologica Sinica 35, 325–332.
| Effect of cryopreservation and short-term storage of Chinese Giant salamander sperm.Crossref | GoogleScholarGoogle Scholar |
Sherman, C, Uller, T, Wapstra, E, and Olsson, M (2008). Within-population variation in ejaculate characteristics in a prolonged breeder, Peron’s tree frog, Litoria peronii. Naturwissenschaften 95, 1055–1061.
| Within-population variation in ejaculate characteristics in a prolonged breeder, Peron’s tree frog, Litoria peronii.Crossref | GoogleScholarGoogle Scholar | 18618091PubMed |
Shishova, NR, Uteshev, VK, Kaurova, SA, Browne, RK, and Gakhova, EN (2011). Cryopreservation of hormonally induced sperm for the conservation of threatened amphibians with Rana temporaria as a model research species. Theriogenology 75, 202–232.
| Cryopreservation of hormonally induced sperm for the conservation of threatened amphibians with Rana temporaria as a model research species.Crossref | GoogleScholarGoogle Scholar |
Shishova, NR, Uteshev, VK, Sirota, NP, Kuznetsova, EA, Kaurova, SA, Browne, RK, and Gakhova, EN (2013). The quality and fertility of sperm collected from European common frog (Rana temporaria) carcasses refrigerated for up to 7 days. Zoo Biology 32, 400–406.
| The quality and fertility of sperm collected from European common frog (Rana temporaria) carcasses refrigerated for up to 7 days.Crossref | GoogleScholarGoogle Scholar |
Silla, AJ, Keogh, LM, and Byrne, PG (2015). Antibiotics and oxygen availability affect the short-term storage of spermatozoa from the critically endangered booroolong frog, Litoria booroolongensis. Reproduction, Fertility and Development 27, 1147–115.
| Antibiotics and oxygen availability affect the short-term storage of spermatozoa from the critically endangered booroolong frog, Litoria booroolongensis.Crossref | GoogleScholarGoogle Scholar |
Unger, S, Mathis, A, and Wilkinson, R (2013). A comparison of sperm health in declining and stable populations of Hellbenders (Cryptobranchus alleganiensis alleganiensis and C.a. bishopi). The American Midland Naturalist 170, 382–392.
| A comparison of sperm health in declining and stable populations of Hellbenders (Cryptobranchus alleganiensis alleganiensis and C.a. bishopi).Crossref | GoogleScholarGoogle Scholar |
Uteshev, VK, Shishova, NR, Kaurova, SA, Manokhin, AA, and Gakhova, EN (2013). Collection and cryopreservation of hormonally induced sperm of pool frog (Pelophylax lessonae. Russian Journal of Herpetology 20, 105–109.
Uteshev, VK, Kaurova, SA, Shishova, NV, Stolyarov, SD, Browne, RK, and Gakhova, EN (2015). In vitro fertilization with hormonally induced sperm and eggs from sharp-ribbed newts Pleurodeles waltl. Russian Journal of Herpetology 22, 35–40.
Waggener, WL, and Carroll, EJ (1998). Spermatozoon structure and motility in the anuran Lepidobatrachus laevis. Development, Growth & Differentiation 40, 27–34.
| Spermatozoon structure and motility in the anuran Lepidobatrachus laevis.Crossref | GoogleScholarGoogle Scholar |
Wells KD (2007) ‘The ecology and behaviour of amphibians’. (The University of Chicago Press: Chicago, IL, USA and London, UK)
Wolf, DP, and Hedrick, JL (1971). A molecular approach to fertilization: II. Viability and artificial fertilization of Xenopus laevis gametes. Developmental Biology 25, 348–359.
| A molecular approach to fertilization: II. Viability and artificial fertilization of Xenopus laevis gametes.Crossref | GoogleScholarGoogle Scholar | 5567826PubMed |