Maternal periconceptional and first trimester protein restriction in beef heifers: effects on placental parameters and fetal and neonatal calf development
K. J. Copping A , J. Hernandez-Medrano B , A. Hoare C , K. Hummitzsch A , I. C. McMillen D , J. L. Morrison E , R. J. Rodgers
A , J. Hernandez-Medrano B , A. Hoare C , K. Hummitzsch A , I. C. McMillen D , J. L. Morrison E , R. J. Rodgers  A and V. E. A. Perry
A and V. E. A. Perry  A F
A F
A The University of Adelaide, Robinson Research Institute, School of Medicine, Adelaide, SA 5005, Australia.
B Department of Obstetrics and Gynaecology, School of Medicine, University of Nottingham, Queen’s Medical Centre, Derby Road, NG7 2UH, UK.
C South East Vets, 314 Commercial Street, Mount Gambier, SA 5290, Australia.
D The Chancellery, University of Newcastle, Callaghan, NSW 2308, Australia.
E School of Pharmacy and Medical Sciences, Sansom Institute for Health Research, University of South Australia, SA 5001, Australia.
F Corresponding author. Email: viv.perry@adelaide.edu.au
Reproduction, Fertility and Development 32(5) 495-507 https://doi.org/10.1071/RD19017
Submitted: 10 January 2019 Accepted: 14 August 2019 Published: 7 February 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Few studies have investigated the effects of nutrition during the periconception and early gestation periods on fetal and placental development in cattle. In this study, nulliparous yearling heifers (n = 360) were individually fed a diet high or low in protein (HPeri and LPeri) beginning 60 days before conception. From 24 to 98 days after conception, half of each treatment group was changed to the alternative high- or low-protein diet (HPost and LPost) yielding four groups in a 2 × 2 factorial design. A subset of heifers (n = 46) was necropsied at 98 days after conception and fetoplacental development assessed. Placentome number and volume decreased in response to LPeri and LPost diets respectively. Absolute lung, pancreas, septum and ventricle weights decreased in LPost versus HPost fetuses, whereas the post-conception diet altered absolute and relative liver and brain weights depending on sex. Similarly, changes in fetal hepatic gene expression of factors regulating growth, glucose output and lipid metabolism were induced by protein restriction in a sex-specific manner. At term, neonatal calf and placental measures were not different. Protein restriction of heifers during the periconception and early gestation periods alters fetoplacental development and hepatic gene expression. These changes may contribute to functional consequences for progeny, but this may not be apparent from gross morphometry at birth.
Additional keywords: beef cattle, fetal programming.
Introduction
Maternal nutrition during gestation has been reported by many studies to affect fetal development, postnatal growth, metabolism and reproduction in species including humans, laboratory animals and domestic animals of agricultural importance (McMillen and Robinson 2005; Long et al. 2009; Sullivan et al. 2009a; Micke et al. 2010a). The effects of episodes of maternal undernutrition and/or overnutrition have been reported to vary depending on the timing, severity and length of nutrient restriction, as well as the sex of the fetus (Micke et al. 2015). Critical windows of vulnerability during fetal development differ for specific tissues and organs (McMillen et al. 2001). These periods of vulnerability include folliculogenesis and the preimplantation period (Velazquez 2015), placental development (Fowden et al. 2006) and organogenesis (McMillen et al. 2001). During these critical windows, a stimulus or insult such as altered maternal nutrition can result in long-term consequences for the progeny.
In sheep, nutritional restriction during the periconception period has been reported to result in impaired blastocyst development (Borowczyk et al. 2006), altered development of the hypothalamic–pituitary–adrenal axis (Edwards and McMillen 2002) and, more recently, altered hepatic insulin signalling and glucocorticoid regulation of hepatic glucose output, as well as hepatic fatty acid metabolism (Nicholas et al. 2013; Lie et al. 2014). Similarly, it has been established in cattle that the periconception diet affects oocyte quality (Adamiak et al. 2005; Adamiak et al. 2006) and embryo development (Kruse et al. 2017), with implantation occurring between 18 and 22 days post conception (dpc) (Wathes and Wooding 1980). Fetal organogenesis is complete by 42 dpc (DesCôteaux et al. 2010) and occurs simultaneously with placental development, preceding the development of bone, muscle and fat tissues (Hubbert et al. 1972). Because the growth trajectories for these tissues vary, each tissue is susceptible to maternal dietary perturbations at different gestational stages (McMillen et al. 2001; Symonds et al. 2012). Although maternal nutrition in mid to late pregnancy may affect fetal growth (Taylor et al. 2018) and birthweight (Larson et al. 2009; Micke et al. 2010b; LeMaster et al. 2017), nutritional insults during the first trimester have been reported to affect fetal and postnatal organ development (Long et al. 2009, 2010) and tissue gene expression (Long et al. 2010; Micke et al. 2011). Subsequent effects upon productivity include altered postnatal growth (Micke et al. 2010a) and reproductive development (Sullivan et al. 2009a; Mossa et al. 2013). However, it is acknowledged that the effects of maternal nutrition during the periconception period and early gestation on fetoplacental development in cattle are poorly understood (Mossa et al. 2015; Sinclair et al. 2016).
The aim of this study was to evaluate the effects of maternal dietary protein intake during the periconception and first trimester periods on fetal and placental development and hepatic gene expression in the offspring of nulliparous adolescent heifers. We hypothesised that restricted maternal protein during the periconception period and early gestation would reduce fetal and placental development, with subsequent deleterious effects on fetal morphometry at 98 dpc and at term. Further, protein restriction would decrease liver growth and mitogenic gene expression, but increase the expression of genes regulating hepatic glucose output and lipid accumulation, thus potentially contributing to the development of altered carcass traits and metabolic dysfunction.
Materials and methods
Ethics approval
The use of animals and the procedures performed in this study were in compliance with the Australian Code for the Care and Use of Animals for Scientific Purposes (NHMRC 2004) and were approved by the University of South Australia Institute of Medical and Veterinary Science Animal Ethics Committee, Australia (Approval no. 18/11) and The University of Adelaide Animal Ethics Committee, Australia (Approval no. S2012-249).
Experimental design and animal management
The purpose of this study was to evaluate the effects of maternal dietary protein during the periconception (PERI; −60 to 23 dpc) and first trimester (POST; 24 to 98 dpc) periods on fetal and placental development in nulliparous beef heifers. The maternal dietary protein levels reflected pasture conditions in Australian rangelands without (Low) and with (High) protein supplement.
The study was a two-by-two factorial design. The animals were the heifers and their progeny that have previously been described (Copping et al. 2014) with management and diet composition reported in detail by Copping et al. (2018).
Briefly, 360 nulliparous Santa Gertrudis (Bos taurus × Bos indicus) heifers underwent a 60-day acclimatisation period. At 12 months of age, 60 days before AI, heifers were assigned to two equal periconception (PERI; −60 to 23 dpc) treatment groups: high and low protein (HPeri and LPeri). Heifers were fed either a high-protein diet (71 MJ metabolisable energy (ME) and 1.18 kg crude protein (CP) heifer−1 day−1) or a low-protein diet (63 MJ ME, 0.62 kg CP heifer−1 day−1) consisting of a pelleted portion that was measured and fed individually in stalls each day with straw (5% CP) available ad libitum in pens. Heifers were oestrous synchronised using a progesterone-based program (Hernandez-Medrano et al. 2015) and were inseminated with frozen semen from one Santa Gertrudis bull on Day 0. At 23 dpc half of each nutritional treatment group was swapped to the alternative post-conception treatment (POST; 24–98 dpc), namely either a high-protein diet (HPost; 102 MJ ME, 1.49 kg CP heifer−1 day−1) or a low-protein diet (LPost; 98 MJ ME, 0.88 kg CP heifer−1 day−1), giving rise to four treatment groups in total: HPeri–HPost (HH), HPeri–LPost (HL), LPeri–HPost (LH) and LPeri–LPost (LL).
Pregnancy was confirmed at 36 dpc and the sex of the fetus determined at 60 dpc by rectal ultrasound. At the end of the first trimester (98 dpc), a subset (n = 46 heifers, singleton pregnancy; HH, 6 male, 6 female; HL, 10 male, 5 female; LH, 5 male, 5 female; LL, 4 male, 5 female) were necropsied (Copping et al. 2014). The remaining 64 heifers were fed the same diet daily until parturition (99 dpc to term; 79 MJ ME, 0.92 kg CP heifer−1 day−1; formulated to provide additional growth of 0.5 kg day−1). Heifers received the pellet portion individually with straw (5% CP) provided ad libitum in pens throughout.
At term, individual feeding ceased (n = 63 heifers with singleton calves; HH, 10 male, 8 female; HL, 14 male, 4 female; LH, 11 male, 4 female; LL, 9 male, 3 female). An additional heifer produced twins and was excluded from the analyses reported herein (LL, 2 males). Heifers were weighed at approximately monthly intervals (Copping et al. 2020).
Fetal necropsy
Heifers (n = 46) were humanely slaughtered in a commercial abattoir at 98 dpc. The gravid uterus was immediately removed and the total weight of the uterus and fetus determined. The fetus was excised, weighed, measured and then dissected. Measures of fetal biparietal diameter (BPD), crown–nose length (CNL), crown–rump length (CRL) and umbilical cord diameter (UD) were obtained using sliding Vernier callipers. A measure of abdominal circumference (AC) was taken at the level of the umbilical cord using a flexible tape measure.
Fetal brain, heart, kidneys, liver, lung and pancreas were collected. Due to time constraints in handling the fetal tissue, only the left kidney was weighed. The heart was dissected into the atrial cap, septum and left and right ventricles, with each weighed individually. All other fetal organs were weighed complete. Liver samples were snap frozen in liquid nitrogen and stored at −80°C until molecular analysis. Placentomes were dissected from the uterus and all placentomes were counted and weighed. Placentome volume was calculated using saline displacement (Kannekens et al. 2006).
RNA extraction and semiquantitative real-time reverse transcription–polymerase chain reaction
RNA was isolated from the liver (~50 mg) using Qiagen QIAzol Lysis Reagent and Qiagen RNeasy purification columns. RNA was quantified using the ratio of the optical density at 260 and 280 nm and used to calculate the correct dilutions of extracted RNA used for cDNA synthesis. The cDNA was synthesised using the Superscript III First Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions with 2 μg total diluted RNA, random hexamers, dNTP, dithiothreitol and Superscript III in a final volume of 20 μL. A no-template control (NTC) containing no RNA transcript and a no-amplification control (NAC) containing no Superscript III were used to check for reagent contamination and genomic DNA contamination respectively, as described previously (Wang et al. 2011; Soo et al. 2012).
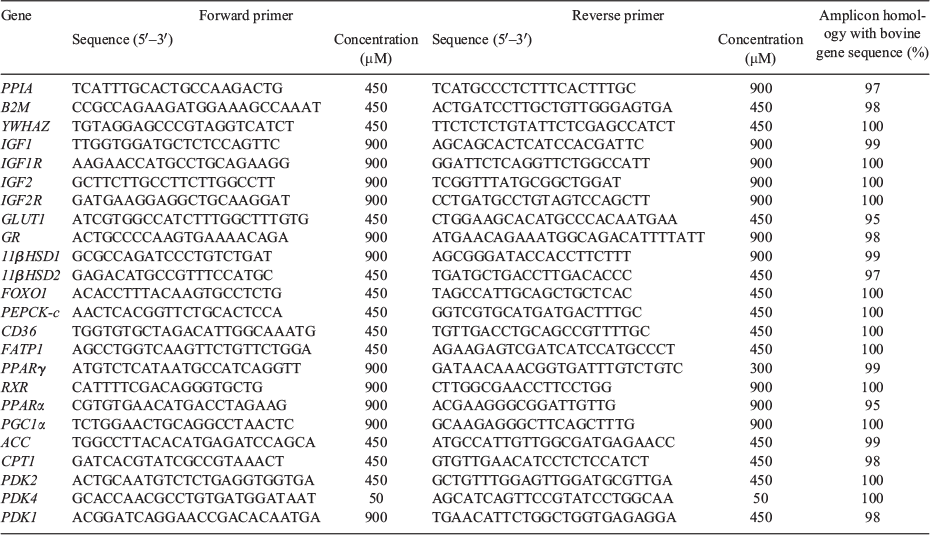
Following Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (Bustin et al. 2009), three reference genes, namely peptidylprolyl isomerase A (PPIA), β2-microglobulin (B2M) and tyrosine 3-monooxygenase (YWHAZ; Passmore et al. 2009), were chosen from a suite of reference (housekeeper) genes analysed using geNorm component of qBase analysis software (Biogazelle; Hellemans et al. 2007). The relative expression of the mRNA transcripts of mitogenic (growth factor) genes (i.e. insulin-like growth factor 1 (IGF1), IGF1 receptor (IGF1R), insulin-like growth factor 2 (IGF2) and IGF2 receptor (IGF2R)), glucose transporter 1 (GLUT1), genes involved in glucocorticoid signalling and hepatic glucose production (i.e. glucocorticoid receptor (GR), 11β-hydroxysteroid dehydrogenase type 1 (HSD11B1) and 2 (HSD11B2), Forkhead Box O1 (FOXO1), and phosphoenolpyruvate carboxykinase-C (PEPCK-C)) and genes involved in fatty acid uptake and metabolism (i.e. cluster of differentiation 36, also known fatty acid translocase (CD36), fatty acid transport protein 1 (FATP1), peroxisome proliferator-activated receptor gamma (PPARγ) and alpha (PPARα), retinoid X receptor (RXR), peroxisome proliferator activated receptor gamma coactivator 1 alpha (PGC1a), acetyl-CoA carboxylase (ACC), carnitine palmitoyltransferase I (CPT1), 3-phosphoinositide-dependent protein kinase-1 (PDK1), -2 (PDK2) and -4 (PDK4), as well as the reference genes, were measured by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) using Fast SYBR Green Master Mix (Applied Biosystems) in a final volume of 6 µL on a ViiA7 Fast Real-time PCR system (Applied Biosystems), as described previously (Zhang et al. 2010; Lie et al. 2014). Each qRT-PCR well contained 3 μL of 2× Fast SYBR Green Master Mix, 2 μL forward and reverse primers mixed with H2O to obtain final primer concentrations given in Table 1 and 1 μL diluted relevant cDNA. Primer sets were originally designed and validated for use in sheep using either ovine or bovine sequences, and thus the product of all sets was sequenced to ensure that it matched the expected sequence (Table 1). Primers were validated to generate a single transcript as confirmed by the presence of an individual double-stranded DNA product of the correct size and sequence. Controls for each primer set containing no cDNA were included on each plate to test for reagent contamination (NTC). Melting curve and dissociation curves were also run to check for non-specific product formation (95°C for 15 s, 60°C for 1 min and 95°C for 15 s). Amplification efficiency reactions were performed on five triplicate serial dilutions of cDNA template for each primer set. Amplification efficiencies were determined from the slope of a plot of Ct (defined as the threshold cycle with the lowest significant increase in fluorescence) against log[cDNA template] (ranging from 1 to 100 ng). Ct values were in the linear amplification range for all genes. Each sample was run in triplicate for target genes and reference genes (CV <10%). The PCR consisted of 40 cycles at 95°C for 1s and 60°C for 20 s. The reactions were quantitated by setting the threshold within the exponential growth phase of the amplification curve and obtaining corresponding Ct values (Ct of all genes <35). The abundance of each transcript relative to the abundance of stable housekeeping genes (Hellemans et al. 2007) was calculated using DataAssist 3.0 analysis software (Applied Biosystems) and expressed as mean normalised expression (Soo et al. 2012).
Neonatal calf measurements
At calving, heifers were monitored visually 24 h day−1. Calf measurements were collected within 15 min of birth and before sucking. The whole-body, trunk and cranial measures that were recorded included calf birth bodyweight (BW), CRL, AC, BPD and CNL (Micke et al. 2010b). Height was measured from the base of the hoof to the top of the wither. Placentas were collected immediately upon expulsion, assessed for completeness and weighed (Sullivan et al. 2009b). If the expulsion time of the placenta exceeded 12 h, the placenta was classified as retained fetal membranes (RFM; Peter 2013). Seven partially eaten placentas and two RFM were excluded from the analyses of all placental measures and calculations. Cotyledons were dissected away from membranes of each placenta, counted and weighed. Cotyledon volume was calculated using saline displacement. Placental efficiency at birth was calculated as the ratio of calf birth BW to placental weight (Leiser et al. 1997).
Statistical analysis
Data were assessed for normality by graphical exploration and square root or Box–Cox transformed (STATA13.1/IC; StataCorp) if required before further analysis. Data are presented as the unadjusted mean ± s.e.m. Multifactorial analysis of variance (ANOVA) was used to explore the effects of maternal diet during PERI and POST, progeny sex and their interaction terms on fetal morphology, placental measures and organ weights at 98 dpc, hepatic gene expression, calf and placental measures at term and indices of disproportionate growth in singleton progeny only. Gestation length was included as a covariate for birth measures to account for the length of exposure to the second and third trimester maternal diet. Interactions were explored with one- or two-way ANOVA and Tukey–Kramer post hoc tests as required. A P value of <0.05 was considered statistically significant and P < 0.10 was considered a tendency.
Results
Fetal measurements at 98 dpc
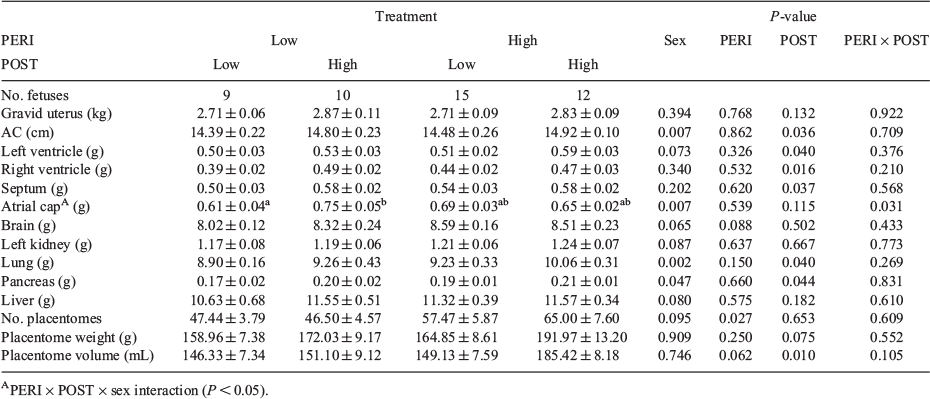
Measures of fetal and placental parameters at 98 dpc are presented in Table 2. Placentome number was decreased (P = 0.03) and placentome volume tended to be reduced by the LPeri versus HPeri diet (P = 0.06), whereas the LPost diet reduced placentome volume (P = 0.01) and tended to reduce weight (P = 0.075) compared with the HPost diet. Placental efficiency (fetal weight/placentome weight) did not differ among groups (P > 0.10).
As reported by Copping et al. (2014), male fetuses were heavier than females overall (P < 0.001) and LPost fetuses were lighter than HPost fetuses. Within males and within females, the LPost diet decreased fetal weight by 8% and 10% respectively (P < 0.05).
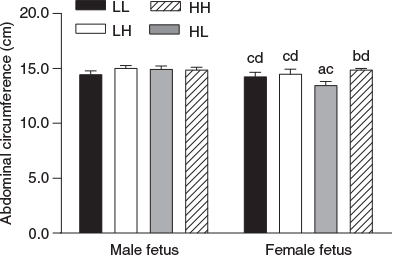
Males had a larger BPD and AC overall at 98 dpc than females (P < 0.05) and BPD tended (P < 0.1) to be decreased in LPeri versus HPeri fetuses (Copping et al. 2014). Abdominal circumference varied with the interaction between PERI and POST diet, and fetal sex (P < 0.05). In females only, AC was reduced in fetuses from heifers in the HL compared with HH group (Fig. 1; P = 0.03).
|
Absolute organ and relative weights for fetuses at 98 dpc are reported in Tables 2 and 3 respectively. Compared with males overall, females tended to have reduced absolute fetal brain weight (8.59 ± 0.14 vs 8.17 ± 0.14 g respectively; P = 0.051) but greater brain weight relative to fetal weight (26.22 ± 0.53 vs 28.22 ± 0.63 g kg−1 respectively; P = 0.005). In addition, compared with males, females had significantly reduced absolute weights of fetal heart (2.44 ± 0.05 vs 2.23 ± 0.06 g), atrial cap (0.72 ± 0.02 vs 0.62 ± 0.02 g), lung (9.82 ± 0.19 vs 8.87 ± 0.27 g) and pancreas (0.21 ± 0.01 vs 0.18 ± 0.01 g; all P < 0.05) and tended to have reduced absolute weights of the liver (11.68 ± 0.26 vs 10.84 ± 0.38 g; P = 0.07) and kidney (1.26 ± 0.04 vs 1.14 ± 0.06 g; P = 0.09). However, organ weights were similar in females and males when assessed relative to fetal BW (all P > 0.10).
Absolute brain weight tended (P = 0.09) to be reduced in LPeri versus HPeri fetuses. The PERI diet did not affect the absolute or relative weights of any other fetal organ (all P > 0.10). In contrast, the POST diet affected the absolute weights of the fetal heart and its structures, along with the weight of the lung and pancreas. Heart weight was reduced in LPost versus HPost fetuses, as reported previously (Hernandez-Medrano et al. 2015), along with septum and left and right ventricle weights (P < 0.05). Furthermore, atrial cap weight varied with an interaction between maternal nutrition during PERI and POST, with atrial cap weight greater in fetuses exposed to LPeri and HPost diets (LH group) than in fetuses exposed to a constant low PERI and POST diet (LL group; P = 0.03). Absolute lung (P = 0.03) and pancreas (P = 0.04) weights were also reduced in LPost fetuses compared with HPost fetuses. Overall, absolute liver weight was unaffected by diet, but was reduced in LPost versus HPost males (Fig. 2a; P = 0.01) when sexes were analysed separately. Absolute weights of the fetal kidney did not differ due to maternal diet treatment (P > 0.10).
Relative fetal brain weight was greater (P = 0.007) in LPost than HPost fetuses and within males (27.22 ± 0.64 vs 24.94 ± 0.75 g kg−1 respectively; P = 0.045), but not females. Relative liver weight varied with an interaction between POST diet and fetal sex (P = 0.02). Within females, but not males, relative liver weight was increased in LPost versus HPost fetuses (Fig. 2b; P < 0.05). Relative heart, lung, kidney and pancreas weights were unaffected by maternal diet treatment (P > 0.10).
Indices of disproportionate growth at 98 dpc
To investigate the effects of maternal diet on proportional growth, BPD : AC, CNL : CRL, CRL : fetal BW and brain : liver ratios were calculated on the 98 dpc fetal measures (Table 4).
Overall, the CRL : fetal BW ratio was less in males than in females (55.88 ± 0.96 vs 62.24 ± 1.17 mm kg−1 respectively; P < 0.001), but there was no difference in the BPD : AC and CNL : CRL ratios between sexes (P > 0.05) and these ratios were not affected by the PERI diet (P > 0.05). The LPost diet increased fetal CNL : CRL (P = 0.039) and CRL : fetal BW (P = 0.003) ratios and tended to increase the BPD : AC ratio compared with the HPost diet (P = 0.08). The brain : liver ratio tended to vary with an interaction between POST diet and fetal sex (P = 0.07). When sexes were analysed separately, the brain : liver ratio was increased only in male LPost versus HPost fetuses (0.78 ± 0.03 vs 0.69 ± 0.02 g g−1 respectively; P = 0.03).
Hepatic gene expression in fetuses at 98 dpc
Relative hepatic gene expression of signalling factors in the 98 dpc fetal liver varied with an interaction between maternal PERI or POST diet and fetal sex for multiple factors (P < 0.10). Subsequently, males and females were analysed separately for all factors. The relative hepatic gene expression of signalling factors that were altered by maternal diet (P < 0.05) are presented in Table 5, with all signalling factors examined presented in Table S1, available as Supplementary Material to this paper.
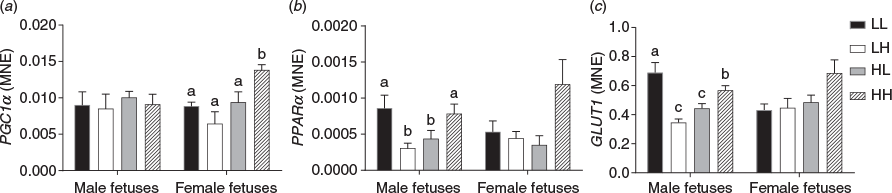
Relative expression of IGF2 was decreased in LPeri female fetus only (P < 0.05), and no change was observed in LPost fetuses overall or within each sex (P > 0.05). Gene expression of the insulin-independent glucose transporter GLUT1 was increased (P < 0.05) in male fetuses exposed to the low diet in both the PERI and POST periods (LL group; Fig. 3; P < 0.05), but decreased (P ≤ 0.05) in LPeri female fetuses. The expression of the transcription factors regulating the expression of PEPCK-C, namely FOXO1 and GR were affected by fetal sex and maternal diet. Overall, FOXO1 and hepatic GR were decreased by the LPeri diet (P < 0.05). When sexes were analysed separately, these effects occurred only in female fetuses (P < 0.05).
Furthermore, consistent with this change, there was a tendency for decreased PEPCK-C expression in LPeri fetuses (P = 0.06). However, the transcription factors regulating the expression of the fatty acid transporters and lipogenesis, RXR and PPARγ, were increased in LPeri female fetuses (P < 0.05), with PPARγ also increased in LPost female fetuses (P < 0.05), whereas PPARα decreased in male fetuses exposed to the low-protein diet in either the PERI or POST periods (LH and HL groups; Fig. 3; P < 0.05). The expression of PDK1 (P < 0.05) was reduced by the LPeri diet overall and in both sexes when analysed separately. Concomitantly, the PPAR coactivator and mitochondrial biogenesis factor, PGC1α (P < 0.05), was also decreased in female fetuses exposed to the low protein diet in either the PERI or POST periods (LH and HL) (Fig. 3; P < 0.05).
Term parameters in neonatal calf and placenta
Placental parameters and placental efficiency at term did not differ according to maternal diet, calf sex or gestation length (Table 6; P > 0.10).
Gestation length, calf height and AC at birth are presented in Table 6. Mean calf birth BW was 32.1 ± 0.6 kg (range 20.0–42.8 kg). Gestation length tended to be decreased by LPost compared with HPost (P = 0.09), and was longer for male than female calves (282.22 ± 0.77 vs 279.47 ± 1.13 days; P = 0.03). Calf height and AC varied with an interaction between PERI and POST diet (P < 0.10), but there were no differences between diet groups (P > 0.10). As reported previously (Copping et al. 2014), calf birth BW and morphology measures of CRL, BPD and CNL were similar between male and female calves (P > 0.10) and were not affected by maternal diet overall or within each sex. The inclusion of maternal BW at calving as a covariate in the statistical model did not alter this outcome.
Indices of disproportionate growth at birth
Proportional growth at birth, as measured by the BPD : AC (0.204 ± 0.004 cm cm−1), CNL : CRL (0.249 ± 0.004 cm cm−1) and CRL : birth BW (2.54 ± 0.04 cm kg−1) ratios and the ponderal index, calculated as weight divided by height cubed (69.9 ± 1.0 kg cm−3), were unaffected by maternal diet or calf sex (P > 0.10).
Discussion
This study explored the effects of maternal protein diets during the periconception and first trimester periods in nulliparous beef heifers on fetal and placental development and hepatic gene expression. It is the first study we believe to report fetoplacental effects of the periconception diet in the bovine with a fetal endpoint and to term. Varying levels of maternal dietary protein during the PERI (from −60 to 23 dpc) and POST (from 24 to 98 dpc) periods resulted in altered fetal size and body proportions at 98 dpc but similar birth BW (Copping et al. 2014) and morphology at term. This was associated with sex-specific asymmetric development at 98 dpc, a characteristic of intrauterine growth restriction (IUGR; Platz and Newman 2008). Further, it resulted in sex-specific programming effects on genes regulating growth, hepatic glucose output and lipid metabolism in the 98 dpc fetus. Maternal dietary protein restriction, at the moderate level used in this study, is commonly observed in commercial cattle operations (Bortolussi et al. 2005). We have previously shown that such restriction alters productivity traits in the progeny (Micke et al. 2010a, 2011) and have reported that both postnatal reproductive development (Copping et al. 2018) and meat quality traits (Alvarenga et al. 2016) were affected in the entire male progeny from the present study. Information presented in this study may provide insights into the underlying molecular pathways that promote susceptibility to increased fat deposition in the growing animal subsequent to protein restriction during early oocyte and/or embryo development.
Fetal organ development
The thrifty phenotype hypothesis (Hales and Barker 2001) suggests that gestational undernutrition induces nutrients and energy to be diverted to ensure the development of critical organs such as the brain at the expense of organs and systems that may be less important for the immediate survival of the fetus (e.g. kidney; Reynolds and Caton 2012). These adaptations have been shown in both human and animal studies to be a contributing factor to the development of metabolic diseases in adult life (McMillen and Robinson 2005) and, in production animals, to affect economically important reproductive (Sullivan et al. 2009a; Mossa et al. 2013; Copping et al. 2018) and carcass (Long et al. 2010; Micke et al. 2010a; Alvarenga et al. 2016) traits.
The periconception period is acknowledged as a particularly sensitive developmental stage (Fleming et al. 2015). During the preimplantation period, primary patterns of development are established within the embryo, including the formation of cell lineages (Alberio 2018) and re-establishment of DNA methylation patterns (Dobbs et al. 2013). In studies in rodents and ruminants, embryos have been shown to respond to nutritional challenges during this period by permanently changing the pattern of development to protect fetal growth and optimise fitness (Fleming et al. 2015; Velazquez 2015). These compensatory changes may, in part, underlie the observed effects of the PERI diet on fetal growth and hepatic gene expression. In the present study, protein restriction during the PERI period had negative effects on embryo survival (Copping et al. 2020) and fetal growth (CRL) as early as 36 dpc (Copping et al. 2014). This is in agreement with previous studies that have shown that nutritional stress in both the pre- and immediate postinsemination period in beef heifers may affect embryo survival and delay embryo development (Perry et al. 2016; Kruse et al. 2017). In the 98 dpc fetus, protein restriction during the PERI period had limited effects on organ development, but was associated with changes in fetal hepatic gene expression. In comparison, the POST diet affected the development of several major organs in the 98 dpc fetus, reflecting the differential compensatory effects on fetoplacental growth depending on the timing of the nutritional insult (Nathanielsz 2006).
In the present study, the LPost diet affected the absolute weight of the heart (Hernandez-Medrano et al. 2015) and its structures in 98 dpc fetuses, along with absolute liver and lung weights. These data are consistent with earlier female bovine fetal studies (Long et al. 2009; Mossa et al. 2013). When each fetal sex was analysed separately, the developing cardiovascular system was affected in the female only, associated with altered cardiovascular development that persisted to the neonatal and adolescent period (Hernandez-Medrano et al. 2015). These findings correspond with those of an earlier study (Mossa et al. 2013).
Absolute liver weight at 98 dpc was reduced by the LPost diet, but only in the male. Relative liver weight was unaffected in the males, but increased in LPost female fetuses. In sheep, maternal nutrient restriction has been shown to not only alter fetal liver growth, but to also be associated with changes in hepatic function (Lie et al. 2014). The increased relative liver weight in the LPost female fetus is dissimilar to the observed effects on fetal hepatic gene expression that occurred in the LPeri cohort: in the LPeri female, hepatic gene expression of IGF2, along with GR, was reduced concomitant with reduced circulating maternal IGF2 (Copping et al. 2020). This has not been reported previously and may be permissive to the sex-specific fetal growth retardation observed at 36 dpc in the female cohort (K. J. Copping, unpubl. data), the GR gene (Nuclear Receptor Subfamily 3 Group C Member 1; NR3C1) being a possible candidate for epigenetic regulation of growth in the fetus (Lillycrop et al. 2007). Hepatic GR plays an important role in embryogenesis and lipid and carbohydrate metabolism. The action of GR in the liver is essential for normal body growth because it is a coactivator in growth hormone signalling on its dependent genes, such as IGF1 and IGF2 (von Horn 2002). Because the liver is a major target organ for glucocorticoids, the role of hepatic GR may be particularly important in regulating sex-specific responses in the progeny (Tronche et al. 2004). Similarly, a concomitant effect on IGF1R gene expression was seen in the LPeri female cohort in this study.
The LPeri female fetuses also exhibited increased hepatic gene expression of RXR and PPARγ, whereas the LL male fetuses exhibited increased expression of GLUT1. The function of PPAR is integral to lipid and carbohydrate homeostasis, with altered function related to dyslipidaemia (Brown and Plutzky 2007). Therefore the effects on the female fetal liver may be permissive to increased adipogenesis after exposure to a maternal low-protein diet during the PERI period. This may also hold true for the male fetuses, in which a different mechanism of increased lipogenesis via activation of GLUT1 was observed; susceptibility to increased postnatal adiposity in the carcass has been noted in 4-month-old sheep following exposure to a low-protein diet during the periconception period (Nicholas et al. 2013). The decreased FOXO1 expression in the LPeri cohort in this study is similar to that reported by Nicholas et al. (2013). FOXO1, together with PGCα and PDK1, regulates the expression of the gluconeogenic rate-limiting gene PEPCK-C. Both PDK1 and PEPCK-C were downregulated by the LPeri diet in male and female fetuses, although PGCα was downregulated by the low protein maternal diet only in female fetuses.
Glucocorticoids regulate the hepatic gluconeogenic rate-limiting genes such as PEPCK-C (Yabaluri and Bashyam 2010). The altered maternal metabolite profiles in these protein-restricted yearling heifers (Copping et al. 2020) may have led to increased fetal glucocorticoid exposure. Therefore, the observed effects on hepatic genes may reflect a physiological fetal survival mechanism following gestational protein restriction. Elevated glucocorticoids are also associated with reductions in the placental IGF system and production of placental hormones (Sferruzzi-Perri et al. 2017), as observed in these heifers, attendant to reduced fetal growth.
Protein restriction also affected the development of the fetal pancreas, lung and brain at 98 dpc Pancreas and lung weights were reduced by the LPost diet. The functional outcomes of these changes in the bovine are unknown, and further histology of both the fetal and adult pancreas and lung is required to facilitate understanding of the relationship between size and function, as explored previously in IUGR sheep (Joyce et al. 2001; Limesand et al. 2006).
Protein restriction during the POST period increased brain weight assessed relative to fetal weight in male but not female fetuses at 98 dpc, indicative of a brain-sparing effect in this cohort. Previous studies in sheep have reported similar effects in IUGR fetuses and attributed this to resources being reallocated to spare brain metabolism to the detriment of the development of other organs, namely trunk and abdominal viscera (McMillen et al. 2001; Osgerby et al. 2002). However, these studies did not differentiate fetuses by sex. Further the brain : liver ratio in this study was higher in LPost male fetuses, providing additional evidence of brain sparing as noted in IUGR models in sheep (Wallace et al. 2002; Field et al. 2015). Together, the observed differences in fetal measures and indices of growth suggest protein restriction resulted in both sex-specific IUGR and asymmetric development in the fetuses by 98 dpc. Such asymmetric IUGR is reported to be related to uteroplacental insufficiency and redistribution of fetal blood flow (Platz and Newman 2008).
Placental parameters
Similar fetal growth restriction in both sheep (McMillen et al. 2001) and cattle (Long et al. 2009; Sullivan et al. 2009b) has been linked to changes in placental development. The present study reported a similar average placental weight (4.9 kg) to that of dairy cattle (5 kg; Laven and Peters 2001) but higher than the 4.1 and 3.9 kg reported in 2- (Miguel-Pacheco et al. 2017) and 3-year-old calving beef heifers (Sullivan et al. 2009b). Placental efficiency in the present study, a measure of the capacity of the placenta to support growth of the fetus (Fowden et al. 2009), was lower (7.0 : 1) than the 8.5 : 1 reported by Sullivan et al. (2009b). This apparent reduction in efficiency may be attributed to the age difference of the heifers, because placental efficiency in beef heifers increases with age (Sullivan et al. 2009b) and the present study represents an adolescent pregnancy with competing requirements between the dam and the fetus for nutrients (Wallace et al. 2004).
The PERI and POST diets were associated with altered placental parameters at 98 dpc but not at term, analogous to the observed effects in the fetus and term neonate. The reduced number of placentomes at 98 dpc in LPeri heifers and the reduction in placentome volume and weight in LPost heifers suggests an adaptation of the placental phenotype in response to maternal protein restriction and consequent metabolic status of the heifers. Sullivan et al. (2009b) reported that restricted maternal dietary protein intake in heifers (between 93 and 180 dpc) was associated with a reduction in cotyledon number at parturition, suggesting that there may be capacity for the bovine placental unit to adjust beyond the first trimester. This is consistent with the reduction in both cotyledon and caruncular weights at 125 dpc noted in placentas from heifers (with female fetuses) that were restricted (50% vs 100%) between 30 and 125 dpc (Zhu et al. 2007). Following realimentation in these heifers, only cotyledon weight remained affected at 250 dpc; however, changes in placental vascularity became apparent by 250 dpc (Vonnahme et al. 2007). Interestingly, trophectoderm volume in heifers that experienced protein restriction during the first trimester was increased at birth (Miguel-Pacheco et al. 2017). This adaptation may alter the functional potential of the placenta and thereby enable enhanced nutrient supply to the fetus during later gestation if maternal nutrition increases (Perry et al. 1999). Doppler measurements of blood flow and uterine artery parameters from 120 dpc suggested that vascular supply to the placenta was similarly altered in the study animals once maternal diet treatment ceased (Hernandez-Medrano et al. 2015), a scenario also reported in cows realimented following nutritional restriction in early gestation, whereby blood flow to the ipsilateral horn was increased following realimentation but not during the period of restriction (Camacho et al. 2014).
As reported, male fetuses were heavier and longer in comparison with female fetuses at 98 dpc, as expected (Eley et al. 1978). Although both male and female fetus BW was similarly reduced by the LPost diet (8% and 10% respectively), different effects on fetal morphology and organ size were evident. Because males have higher growth and metabolism than females (Clarke and Mittwoch 1995), this may have altered sensitivity to the diet restriction. This finding is consistent with previous reports of sex-specific effects of dietary perturbation in early gestation on bovine fetal development (Micke et al. 2015; Taylor et al. 2018). In contrast, studies investigating perturbations occurring during the second trimester in the bovine (Miguel-Pacheco et al. 2017) and in the second and third trimesters in humans (Clifton 2010) report that the female is more responsive. The expected sex-differences in calf birth BW were disrupted at term (Copping et al. 2014), even though birth BW was normalised following maternal protein restriction ceasing on 98 dpc. It is possible that placental adaptations after restriction ceased were sufficient to meet the lower nutritional requirement of the usually smaller female, but not to completely mitigate the early suppression of growth in the male (Rosenfeld 2015). Although the mechanisms behind this effect are not clear, the reported sex-specific differences in uterine artery flow, Doppler fetoplacental perfusion indices and response of these indices following the maternal dietary interventions in these heifers may have contributed to this finding (Hernandez-Medrano et al. 2015). In addition, the sex-specific maternal endocrine adaptations to the maternal diet treatments (Copping et al. 2020) may represent a placental signalling mechanism enabling changes in fetoplacental growth, as noted previously in humans (Hodyl et al. 2010) and cattle (Micke et al. 2015; Taylor et al. 2018).
Collectively, this suggests that the maternal dietary treatments between −60 and 98 dpc, followed by feeding to meet nutritional requirement (but not realimentation), altered the fetal growth trajectory in a sex-specific manner. Further studies, including histology and gene expression studies, are required to examine the role of the placenta in this interaction as explored in other species (Hodyl et al. 2010; O’Connell et al. 2013).
Conclusion
This study demonstrated that protein restriction during the periconception period (from −60 to 23 dpc) and first trimester (from 24 to 98 dpc) decreases early fetal growth, alters placenta parameters and produces asynchronous organ development in the bovine. Similarly, hepatic gene expression is altered in fetal bovine progeny in a sex-specific manner. This may underlie, in part, the sex-specific responses to gestational dietary perturbations reported previously in the adult (Micke et al. 2010a, 2011). We propose that an accelerated growth response in restricted female fetuses, subsequent to the return to 100% of nutrient requirements, reduced the 98 dpc differences and thereby the normal disparity between heifer and bull calf birth BW. Because distinct IUGR was observed at 98 dpc but not at birth, this agrees with previous bovine studies that birth BW is not a satisfactory indicator of IUGR. Thus, quantification of the effects of heifer nutrition programs on long-term productivity of the progeny remains an important area of study.
Conflicts of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
This research was funded by an Australian Research Council linkage grant (Project ID: LP110100649) to V. E. A. Perry, R. J. Rodgers and I. C. McMillen with industry partners S Kidman and Co. and Ridley AgriProducts. K. J. Copping was funded by an Australian Postgraduate award. R. J. Rodgers is funded by the National Health and Medical Research Council of Australia. The authors acknowledge the excellent technical assistance provided by Wendy Bonner and Nicholas Hatzirodos. The authors are grateful for the assistance of Ray Cranney, Mark Irrgang (Dec.), Maddie Jonas and staff at Tungali Feedlot in all animal husbandry procedures. The authors also acknowledge the assistance of Helen Irving-Rodgers, Stacey Dunn, Kimberly Botting, Tim Kuchel, Mel Ceko, Pamela Sim, Beverly Mühlhausler, Robb Muirhead, Erin McGillick, Song Zhang, Wendela Wepenaar, Faye Pooley, Dan Lomas, Rose Young, Elizabeth Funnell and Todd Cranney with the collection of tissues.
References
Adamiak, S. J., Mackie, K., Watt, R. G., Webb, R., and Sinclair, K. D. (2005). Impact of nutrition on oocyte quality: cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol. Reprod. 73, 918–926.| Impact of nutrition on oocyte quality: cumulative effects of body composition and diet leading to hyperinsulinemia in cattle.Crossref | GoogleScholarGoogle Scholar | 15972884PubMed |
Adamiak, S. J., Powell, K., Rooke, J. A., Webb, R., and Sinclair, K. D. (2006). Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro. Reproduction 131, 247–258.
| Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro.Crossref | GoogleScholarGoogle Scholar | 16452718PubMed |
Alberio, R. (2018). Transcriptional and epigenetic control of cell fate decisions in early embryos. Reprod. Fertil. Dev. 30, 73–84.
| Transcriptional and epigenetic control of cell fate decisions in early embryos.Crossref | GoogleScholarGoogle Scholar |
Alvarenga, T. I., Copping, K. J., Han, X., Clayton, E. H., Meyer, R. J., Rodgers, R. J., McMillen, I. C., Perry, V. E., and Geesink, G. (2016). The influence of peri-conception and first trimester dietary restriction of protein in cattle on meat quality traits of entire male progeny. Meat Sci. 121, 141–147.
| The influence of peri-conception and first trimester dietary restriction of protein in cattle on meat quality traits of entire male progeny.Crossref | GoogleScholarGoogle Scholar | 27317848PubMed |
Borowczyk, E., Caton, J. S., Redmer, D. A., Bilski, J. J., Weigl, R. M., Vonnahme, K. A., Borowicz, P. P., Kirsch, J. D., Kraft, K. C., Reynolds, L. P., and Grazul-Bilska, A. T. (2006). Effects of plane of nutrition on in vitro fertilization and early embryonic development in sheep. J. Anim. Sci. 84, 1593–1599.
| Effects of plane of nutrition on in vitro fertilization and early embryonic development in sheep.Crossref | GoogleScholarGoogle Scholar | 16699117PubMed |
Bortolussi, G., McIvor, J. G., Hodgkinson, J. J., Coffey, S. G., and Holmes, C. R. (2005). The northern Australian beef industry, a snapshot. 2. Breeding herd performance and management. Aust. J. Exp. Agric. 45, 1075–1091.
| The northern Australian beef industry, a snapshot. 2. Breeding herd performance and management.Crossref | GoogleScholarGoogle Scholar |
Brown, J. D., and Plutzky, J. (2007). Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 115, 518–533.
| Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets.Crossref | GoogleScholarGoogle Scholar | 17261671PubMed |
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M. W., Shipley, G. L., Vandesompele, J., and Wittwer, C. T. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622.
| The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments.Crossref | GoogleScholarGoogle Scholar | 19246619PubMed |
Camacho, L. E., Lemley, C. O., Prezotto, L. D., Bauer, M. L., Freetly, H. C., Swanson, K. C., and Vonnahme, K. A. (2014). Effects of maternal nutrient restriction followed by realimentation during midgestation on uterine blood flow in beef cows. Theriogenology 81, 1248–1256.
| Effects of maternal nutrient restriction followed by realimentation during midgestation on uterine blood flow in beef cows.Crossref | GoogleScholarGoogle Scholar | 24650930PubMed |
Clarke, C. A., and Mittwoch, U. (1995). Changes in the male to female ratio at different stages of life. Br. J. Obstet. Gynaecol. 102, 677–679.
| Changes in the male to female ratio at different stages of life.Crossref | GoogleScholarGoogle Scholar | 7547755PubMed |
Clifton, V. L. (2010). Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 31, S33–S39.
| Review: sex and the human placenta: mediating differential strategies of fetal growth and survival.Crossref | GoogleScholarGoogle Scholar | 20004469PubMed |
Copping, K. J., Hoare, A., Callaghan, M., McMillen, I. C., Rodgers, R. J., and Perry, V. E. A. (2014). Fetal programming in 2-year-old calving heifers: peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner. Anim. Prod. Sci. 54, 1333–1337.
| Fetal programming in 2-year-old calving heifers: peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner.Crossref | GoogleScholarGoogle Scholar |
Copping, K. J., Ruiz-Diaz, M. D., Rutland, C. S., Mongan, N. P., Callaghan, M. J., McMillen, I. C., Rodgers, R. J., and Perry, V. E. A. (2018). Peri-conception and first trimester diet modifies reproductive development in bulls. Reprod. Fertil. Dev. 30, 703–720.
| Peri-conception and first trimester diet modifies reproductive development in bulls.Crossref | GoogleScholarGoogle Scholar | 29141178PubMed |
Copping, K. J., Hoare, A., McMillen, I. C., Rodgers, R. J., Wallace, C., and Perry, V. E. A. (2020). Maternal periconceptional and first trimester protein restriction in beef heifers: Impacts upon maternal performance and early fetal development. Reprod. Fertil. Dev , .
| Maternal periconceptional and first trimester protein restriction in beef heifers: Impacts upon maternal performance and early fetal development.Crossref | GoogleScholarGoogle Scholar |
DesCôteaux, L., Gnemmi, G., and Colloton, J. (2010) ‘Practical Atlas of Ruminant and Camelid Reproductive Ultrasonography.’ (Wiley-Blackwell: Ames, IA.)
Dobbs, K. B., Rodriguez, M., Sudano, M. J., Ortega, M. S., and Hansen, P. J. (2013). Dynamics of DNA methylation during early development of the preimplantation bovine embryo. PLoS One 8, e66230.
| Dynamics of DNA methylation during early development of the preimplantation bovine embryo.Crossref | GoogleScholarGoogle Scholar | 23799080PubMed |
Edwards, L. J., and McMillen, I. C. (2002). Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo–pituitary adrenal axis in sheep during late gestation. Biol. Reprod. 66, 1562–1569.
| Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo–pituitary adrenal axis in sheep during late gestation.Crossref | GoogleScholarGoogle Scholar | 11967224PubMed |
Eley, R. M., Thatcher, W. W., Bazer, F. W., Wilcox, C. J., Becker, R. B., Head, H. H., and Adkinson, R. W. (1978). Development of the conceptus in the bovine. J. Dairy Sci. 61, 467–473.
| Development of the conceptus in the bovine.Crossref | GoogleScholarGoogle Scholar | 659690PubMed |
Field, M. E., Anthony, R. V., Engle, T. E., Archibeque, S. L., Keisler, D. H., and Han, H. (2015). Duration of maternal undernutrition differentially alters fetal growth and hormone concentrations. Domest. Anim. Endocrinol. 51, 1–7.
| Duration of maternal undernutrition differentially alters fetal growth and hormone concentrations.Crossref | GoogleScholarGoogle Scholar | 25460066PubMed |
Fleming, T. P., Watkins, A. J., Sun, C., Velazquez, M. A., Smyth, N. R., and Eckert, J. J. (2015). Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo’s potential, affecting adult health. Reprod. Fertil. Dev. 27, 684–692.
| Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo’s potential, affecting adult health.Crossref | GoogleScholarGoogle Scholar | 25730413PubMed |
Fowden, A. L., Ward, J. W., Wooding, F. P., Forhead, A. J., and Constancia, M. (2006). Programming placental nutrient transport capacity. J. Physiol. 572, 5–15.
| Programming placental nutrient transport capacity.Crossref | GoogleScholarGoogle Scholar | 16439433PubMed |
Fowden, A. L., Sferruzzi-Perri, A. N., Coan, P. M., Constancia, M., and Burton, G. J. (2009). Placental efficiency and adaptation: endocrine regulation. J. Physiol. 587, 3459–3472.
| Placental efficiency and adaptation: endocrine regulation.Crossref | GoogleScholarGoogle Scholar | 19451204PubMed |
Hales, C. N., and Barker, D. J. (2001). The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20.
| The thrifty phenotype hypothesis.Crossref | GoogleScholarGoogle Scholar | 11809615PubMed |
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F., and Vandesompele, J. (2007). qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19.
| qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data.Crossref | GoogleScholarGoogle Scholar | 17291332PubMed |
Hernandez-Medrano, J. H., Copping, K. J., Hoare, A., Wapanaar, W., Grivell, R., Kuchel, T., Miguel-Pacheco, G., McMillen, I. C., Rodgers, R. J., and Perry, V. E. (2015). Gestational dietary protein is associated with sex specific decrease in blood flow, fetal heart growth and post-natal blood pressure of progeny. PLoS One 10, e0125694.
| Gestational dietary protein is associated with sex specific decrease in blood flow, fetal heart growth and post-natal blood pressure of progeny.Crossref | GoogleScholarGoogle Scholar | 25915506PubMed |
Hodyl, N. A., Wyper, H., Osei-Kumah, A., Scott, N., Murphy, V. E., Gibson, P., Smith, R., and Clifton, V. L. (2010). Sex-specific associations between cortisol and birth weight in pregnancies complicated by asthma are not due to differential glucocorticoid receptor expression. Thorax 65, 677–683.
| Sex-specific associations between cortisol and birth weight in pregnancies complicated by asthma are not due to differential glucocorticoid receptor expression.Crossref | GoogleScholarGoogle Scholar | 20627904PubMed |
Hubbert, W. T., Stalheim, O. H. V., and Booth, G. D. (1972). Changes in organ weights and fluid volumes during growth of the bovine fetus. Growth 36, 217–233.
| 4651636PubMed |
Joyce, B. J., Louey, S., Davey, M. G., Cock, M. L., Hooper, S. B., and Harding, R. (2001). Compromised respiratory function in postnatal lambs after placental insufficiency and intrauterine growth restriction. Pediatr. Res. 50, 641–649.
| Compromised respiratory function in postnatal lambs after placental insufficiency and intrauterine growth restriction.Crossref | GoogleScholarGoogle Scholar | 11641461PubMed |
Kannekens, E. M., Murray, R. D., Howard, C. V., and Currie, J. (2006). A stereological method for estimating the feto-maternal exchange surface area in the bovine placentome at 135 days gestation. Res. Vet. Sci. 81, 127–133.
| A stereological method for estimating the feto-maternal exchange surface area in the bovine placentome at 135 days gestation.Crossref | GoogleScholarGoogle Scholar | 16343568PubMed |
Kruse, S. G., Bridges, G. A., Funnell, B. J., Bird, S. L., Lake, S. L., Arias, R. P., Amundson, O. L., Larimore, E. L., Keisler, D. H., and Perry, G. A. (2017). Influence of post-insemination nutrition on embryonic development in beef heifers. Theriogenology 90, 185–190.
| Influence of post-insemination nutrition on embryonic development in beef heifers.Crossref | GoogleScholarGoogle Scholar | 28166966PubMed |
Larson, D. M., Martin, J. L., Adams, D. C., and Funston, R. N. (2009). Winter grazing system and supplementation during late gestation influence performance of beef cows and steer progeny. J. Anim. Sci. 87, 1147–1155.
| Winter grazing system and supplementation during late gestation influence performance of beef cows and steer progeny.Crossref | GoogleScholarGoogle Scholar | 18997078PubMed |
Laven, R. A., and Peters, A. R. (2001). Gross morphometry of the bovine placentome during gestation. Reprod. Domest. Anim. 36, 289–296.
| Gross morphometry of the bovine placentome during gestation.Crossref | GoogleScholarGoogle Scholar | 11928923PubMed |
Leiser, R., Krebs, C., Ebert, B., and Dantzer, V. (1997). Placental vascular corrosion cast studies: a comparison between ruminants and humans. Microsc. Res. Tech. 38, 76–87.
| Placental vascular corrosion cast studies: a comparison between ruminants and humans.Crossref | GoogleScholarGoogle Scholar | 9260839PubMed |
LeMaster, C. T., Taylor, R. K., Ricks, R. E., and Long, N. M. (2017). The effects of late gestation maternal nutrient restriction with or without protein supplementation on endocrine regulation of newborn and postnatal beef calves. Theriogenology 87, 64–71.
| The effects of late gestation maternal nutrient restriction with or without protein supplementation on endocrine regulation of newborn and postnatal beef calves.Crossref | GoogleScholarGoogle Scholar | 27613252PubMed |
Lie, S., Morrison, J. L., Williams-Wyss, O., Suter, C. M., Humphreys, D. T., Ozanne, S. E., Zhang, S., MacLaughlin, S. M., Kleemann, D. O., Walker, S. K., Roberts, C. T., and McMillen, I. C. (2014). Impact of embryo number and maternal undernutrition around the time of conception on insulin signaling and gluconeogenic factors and microRNAs in the liver of fetal sheep. Am. J. Physiol. Endocrinol. Metab. 306, E1013–E1024.
| Impact of embryo number and maternal undernutrition around the time of conception on insulin signaling and gluconeogenic factors and microRNAs in the liver of fetal sheep.Crossref | GoogleScholarGoogle Scholar | 24496309PubMed |
Lillycrop, K. A., Slater-Jefferies, J. L., Hanson, M. A., Godfrey, K. M., Jackson, A. A., and Burdge, G. C. (2007). Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br. J. Nutr. 97, 1064–1073.
| Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications.Crossref | GoogleScholarGoogle Scholar | 17433129PubMed |
Limesand, S. W., Rozance, P. J., Zerbe, G. O., Hutton, J. C., and Hay, W. W. (2006). Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147, 1488–1497.
| Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction.Crossref | GoogleScholarGoogle Scholar | 16339204PubMed |
Long, N. M., Vonnahme, K. A., Hess, B. W., Nathanielsz, P. W., and Ford, S. P. (2009). Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine. J. Anim. Sci. 87, 1950–1959.
| Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine.Crossref | GoogleScholarGoogle Scholar | 19213703PubMed |
Long, N. M., Prado-Cooper, M. J., Krehbiel, C. R., DeSilva, U., and Wettemann, R. P. (2010). Effects of nutrient restriction of bovine dams during early gestation on postnatal growth, carcass and organ characteristics, and gene expression in adipose tissue and muscle. J. Anim. Sci. 88, 3251–3261.
| Effects of nutrient restriction of bovine dams during early gestation on postnatal growth, carcass and organ characteristics, and gene expression in adipose tissue and muscle.Crossref | GoogleScholarGoogle Scholar | 20525929PubMed |
McMillen, I. C., and Robinson, J. S. (2005). Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633.
| Developmental origins of the metabolic syndrome: prediction, plasticity, and programming.Crossref | GoogleScholarGoogle Scholar | 15788706PubMed |
McMillen, I. C., Adams, M. B., Ross, J. T., Coulter, C. L., Simonetta, G., Owens, J. A., Robinson, J. S., and Edwards, L. J. (2001). Fetal growth restriction: adaptations and consequences. Reproduction 122, 195–204.
| Fetal growth restriction: adaptations and consequences.Crossref | GoogleScholarGoogle Scholar | 11467970PubMed |
Micke, G. C., Sullivan, T. M., Gatford, K. L., Owens, J. A., and Perry, V. E. (2010a). Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Anim. Reprod. Sci. 121, 208–217.
| Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits.Crossref | GoogleScholarGoogle Scholar | 20591585PubMed |
Micke, G. C., Sullivan, T. M., Soares Magalhaes, R. J., Rolls, P. J., Norman, S. T., and Perry, V. E. (2010b). Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight. Anim. Reprod. Sci. 117, 1–10.
| Heifer nutrition during early- and mid-pregnancy alters fetal growth trajectory and birth weight.Crossref | GoogleScholarGoogle Scholar | 19394770PubMed |
Micke, G. C., Sullivan, T. M., McMillen, I. C., Gentili, S., and Perry, V. E. (2011). Heifer nutrient intake during early- and mid-gestation programs adult offspring adiposity and mRNA expression of growth-related genes in adipose depots. Reproduction 141, 697–706.
| Heifer nutrient intake during early- and mid-gestation programs adult offspring adiposity and mRNA expression of growth-related genes in adipose depots.Crossref | GoogleScholarGoogle Scholar | 21310814PubMed |
Micke, G. C., Sullivan, T. M., Kennaway, D. J., Hernandez-Medrano, J., and Perry, V. E. (2015). Maternal endocrine adaptation throughout pregnancy to nutrient manipulation: consequences for sexually dimorphic programming of thyroid hormones and development of their progeny. Theriogenology 83, 604–615.
| Maternal endocrine adaptation throughout pregnancy to nutrient manipulation: consequences for sexually dimorphic programming of thyroid hormones and development of their progeny.Crossref | GoogleScholarGoogle Scholar | 25492373PubMed |
Miguel-Pacheco, G. G., Curtain, L. D., Rutland, C., Knott, L., Norman, S. T., Phillips, N. J., and Perry, V. E. A. (2017). Increased dietary protein in the second trimester of gestation increases live weight gain and carcass composition in weaner calves to 6 months of age. Animal 11, 991–999.
| Increased dietary protein in the second trimester of gestation increases live weight gain and carcass composition in weaner calves to 6 months of age.Crossref | GoogleScholarGoogle Scholar | 27821224PubMed |
Mossa, F., Carter, F., Walsh, S. W., Kenny, D. A., Smith, G. W., Ireland, J. L., Hildebrandt, T. B., Lonergan, P., Ireland, J. J., and Evans, A. C. (2013). Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88, 92.
| Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring.Crossref | GoogleScholarGoogle Scholar | 23426432PubMed |
Mossa, F., Walsh, S. W., Ireland, J. J., and Evans, A. C. O. (2015). Early nutritional programming and progeny performance: is reproductive success already set at birth? Anim. Front. 5, 18–24.
| Early nutritional programming and progeny performance: is reproductive success already set at birth?Crossref | GoogleScholarGoogle Scholar |
Nathanielsz, P. W. (2006). Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J. 47, 73–82.
| Animal models that elucidate basic principles of the developmental origins of adult diseases.Crossref | GoogleScholarGoogle Scholar | 16391433PubMed |
National Health and Medical Research Council (NHMRC) (2004) ‘Australian Code for the Care and Use of Animals for Scientific Purposes.’ 7th edn. (NHMRC: Canberra.)
Nicholas, L. M., Rattanatray, L., MacLaughlin, S. M., Ozanne, S. E., Kleemann, D. O., Walker, S. K., Morrison, J. L., Zhang, S., Muhlhausler, B. S., Martin-Gronert, M. S., and McMillen, I. C. (2013). Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 27, 3786–3796.
| Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring.Crossref | GoogleScholarGoogle Scholar | 23729590PubMed |
O’Connell, B. A., Moritz, K. M., Walker, D. W., and Dickinson, H. (2013). Treatment of pregnant spiny mice at mid gestation with a synthetic glucocorticoid has sex-dependent effects on placental glycogen stores. Placenta 34, 932–940.
| Treatment of pregnant spiny mice at mid gestation with a synthetic glucocorticoid has sex-dependent effects on placental glycogen stores.Crossref | GoogleScholarGoogle Scholar | 23896029PubMed |
Osgerby, J. C., Wathes, D. C., Howard, D., and Gadd, T. S. (2002). The effect of maternal undernutrition on ovine fetal growth. J. Endocrinol. 173, 131–141.
| The effect of maternal undernutrition on ovine fetal growth.Crossref | GoogleScholarGoogle Scholar | 11927392PubMed |
Passmore, M., Nataatmadja, M., and Fraser, J. F. (2009). Selection of reference genes for normalisation of real-time RT-PCR in brain-stem death injury in Ovis aries. BMC Mol. Biol. 10, 72.
| Selection of reference genes for normalisation of real-time RT-PCR in brain-stem death injury in Ovis aries.Crossref | GoogleScholarGoogle Scholar | 19624860PubMed |
Perry, V. E., Norman, S. T., Owen, J. A., Daniel, R. C., and Phillips, N. (1999). Low dietary protein during early pregnancy alters bovine placental development. Anim. Reprod. Sci. 55, 13–21.
| Low dietary protein during early pregnancy alters bovine placental development.Crossref | GoogleScholarGoogle Scholar | 10099675PubMed |
Perry, G. A., Perry, B. L., and Walker, J. A. (2016). Postinsemination diet change on reproductive performance in beef heifers. Prof. Anim. Sci. 32, 316–321.
| Postinsemination diet change on reproductive performance in beef heifers.Crossref | GoogleScholarGoogle Scholar |
Peter, A. T. (2013). Bovine placenta: a review on morphology, components, and defects from terminology and clinical perspectives. Theriogenology 80, 693–705.
| Bovine placenta: a review on morphology, components, and defects from terminology and clinical perspectives.Crossref | GoogleScholarGoogle Scholar | 23849255PubMed |
Platz, E., and Newman, R. (2008). Diagnosis of IUGR: traditional biometry. Semin. Perinatol. 32, 140–147.
| Diagnosis of IUGR: traditional biometry.Crossref | GoogleScholarGoogle Scholar | 18482612PubMed |
Reynolds, L. P., and Caton, J. S. (2012). Role of the pre- and post-natal environment in developmental programming of health and productivity. Mol. Cell. Endocrinol. 354, 54–59.
| Role of the pre- and post-natal environment in developmental programming of health and productivity.Crossref | GoogleScholarGoogle Scholar | 22154989PubMed |
Rosenfeld, C. S. (2015). Sex-specific placental responses in fetal development. Endocrinology 156, 3422–3434.
| Sex-specific placental responses in fetal development.Crossref | GoogleScholarGoogle Scholar | 26241064PubMed |
Sferruzzi-Perri, A. N., Sandovici, I., Constancia, M., and Fowden, A. L. (2017). Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth. J. Physiol. 595, 5057–5093.
| Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth.Crossref | GoogleScholarGoogle Scholar | 28337745PubMed |
Sinclair, K. D., Rutherford, K. M., Wallace, J. M., Brameld, J. M., Stoger, R., Alberio, R., Sweetman, D., Gardner, D. S., Perry, V. E., Adam, C. L., Ashworth, C. J., Robinson, J. E., and Dwyer, C. M. (2016). Epigenetics and developmental programming of welfare and production traits in farm animals. Reprod. Fertil. Dev. 28, 1443–1478.
| Epigenetics and developmental programming of welfare and production traits in farm animals.Crossref | GoogleScholarGoogle Scholar |
Soo, P. S., Hiscock, J., Botting, K. J., Roberts, C. T., Davey, A. K., and Morrison, J. L. (2012). Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod. Toxicol. 33, 374–381.
| Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation.Crossref | GoogleScholarGoogle Scholar | 22326852PubMed |
Sullivan, T. M., Micke, G. C., Greer, R. M., Irving-Rodgers, H. F., Rodgers, R. J., and Perry, V. E. (2009a). Dietary manipulation of Bos indicus × heifers during gestation affects the reproductive development of their heifer calves. Reprod. Fertil. Dev. 21, 773–784.
| Dietary manipulation of Bos indicus × heifers during gestation affects the reproductive development of their heifer calves.Crossref | GoogleScholarGoogle Scholar | 19567220PubMed |
Sullivan, T. M., Micke, G. C., Magalhaes, R. S., Phillips, N. J., and Perry, V. E. (2009b). Dietary protein during gestation affects placental development in heifers. Theriogenology 72, 427–438.
| Dietary protein during gestation affects placental development in heifers.Crossref | GoogleScholarGoogle Scholar | 19540576PubMed |
Symonds, M. E., Pope, M., Sharkey, D., and Budge, H. (2012). Adipose tissue and fetal programming. Diabetologia 55, 1597–1606.
| Adipose tissue and fetal programming.Crossref | GoogleScholarGoogle Scholar | 22402988PubMed |
Taylor, R. K., LeMaster, C. T., Mangrum, K. S., Ricks, R. E., and Long, N. M. (2018). Effects of maternal nutrient restriction during early or mid-gestation without realimentation on maternal physiology and foetal growth and development in beef cattle. Animal 12, 312–321.
| Effects of maternal nutrient restriction during early or mid-gestation without realimentation on maternal physiology and foetal growth and development in beef cattle.Crossref | GoogleScholarGoogle Scholar | 28697817PubMed |
Tronche, F., Opherk, C., Moriggl, R., Kellendonk, C., Reimann, A., Schwake, L., Reichardt, H. M., Stangl, K., Gau, D., Hoeflich, A., Beug, H., Schmid, W., and Schutz, G. (2004). Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev. 18, 492–497.
| Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth.Crossref | GoogleScholarGoogle Scholar | 15037546PubMed |
Velazquez, M. A. (2015). Impact of maternal malnutrition during the periconceptional period on mammalian preimplantation embryo development. Domest. Anim. Endocrinol. 51, 27–45.
| Impact of maternal malnutrition during the periconceptional period on mammalian preimplantation embryo development.Crossref | GoogleScholarGoogle Scholar | 25498236PubMed |
von Horn, H. (2002). GH is a regulator of IGF2 promoter-specific transcription in human liver. J. Endocrinol. 172, 457–465.
| GH is a regulator of IGF2 promoter-specific transcription in human liver.Crossref | GoogleScholarGoogle Scholar | 11874694PubMed |
Vonnahme, K. A., Zhu, M. J., Borowicz, P. P., Geary, T. W., Hess, B. W., Reynolds, L. P., Caton, J. S., Means, W. J., and Ford, S. P. (2007). Effect of early gestational undernutrition on angiogenic factor expression and vascularity in the bovine placentome. J. Anim. Sci. 85, 2464–2472.
| Effect of early gestational undernutrition on angiogenic factor expression and vascularity in the bovine placentome.Crossref | GoogleScholarGoogle Scholar | 17565057PubMed |
Wallace, J. M., Bourke, D. A., Aitken, R. P., Leitch, N., and Hay, W. W. (2002). Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1027–R1036.
| Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep.Crossref | GoogleScholarGoogle Scholar | 11893606PubMed |
Wallace, J. M., Aitken, R. P., Milne, J. S., and Hay, W. W. (2004). Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus. Biol. Reprod. 71, 1055–1062.
| Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus.Crossref | GoogleScholarGoogle Scholar | 15201203PubMed |
Wang, K. C., Zhang, L., McMillen, I. C., Botting, K. J., Duffield, J. A., Zhang, S., Suter, C. M., Brooks, D. A., and Morrison, J. L. (2011). Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J. Physiol. 589, 4709–4722.
| Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb.Crossref | GoogleScholarGoogle Scholar | 21807611PubMed |
Wathes, D. C., and Wooding, F. B. (1980). An electron microscopic study of implantation in the cow. Am. J. Anat. 159, 285–306.
| An electron microscopic study of implantation in the cow.Crossref | GoogleScholarGoogle Scholar | 7211711PubMed |
Yabaluri, N., and Bashyam, M. D. (2010). Hormonal regulation of gluconeogenic gene transcription in the liver. J. Biosci. 35, 473–484.
| Hormonal regulation of gluconeogenic gene transcription in the liver.Crossref | GoogleScholarGoogle Scholar | 20826956PubMed |
Zhang, S., Rattanatray, L., MacLaughlin, S. M., Cropley, J. E., Suter, C. M., Molloy, L., Kleemann, D., Walker, S. K., Muhlhausler, B. S., Morrison, J. L., and McMillen, I. C. (2010). Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J. 24, 2772–2782.
| Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring.Crossref | GoogleScholarGoogle Scholar | 20371620PubMed |
Zhu, M. J., Du, M., Hess, B. W., Means, W. J., Nathanielsz, P. W., and Ford, S. P. (2007). Maternal nutrient restriction upregulates growth signaling pathways in the cotyledonary artery of cow placentomes. Placenta 28, 361–368.
| Maternal nutrient restriction upregulates growth signaling pathways in the cotyledonary artery of cow placentomes.Crossref | GoogleScholarGoogle Scholar | 16822544PubMed |