Morphometric and gene expression analyses of stromal expansion during development of the bovine fetal ovary
M. D. Hartanti A , K. Hummitzsch A , H. F. Irving-Rodgers A B , W. M. Bonner A , K. J. Copping A , R. A. Anderson C , I. C. McMillen D , V. E. A. Perry E and R. J. Rodgers A F
A F
A Discipline of Obstetrics and Gynaecology, School of Medicine, Robinson Research Institute, The University of Adelaide, Adelaide, SA 5005, Australia.
B School of Medical Science, Griffith University, Gold Coast Campus, Qld 4222, Australia.
C Medical Research Council Centre for Reproductive Health, University of Edinburgh, Edinburgh, EH16 4TJ, UK.
D The Chancellery, University of Newcastle, Callaghan, NSW 2308, Australia.
E School of Veterinary and Medical Science, University of Nottingham, Sutton Bonington, LE12 5RD, UK.
F Corresponding author. Email: ray.rodgers@adelaide.edu.au
Reproduction, Fertility and Development 31(3) 482-495 https://doi.org/10.1071/RD18218
Submitted: 9 June 2018 Accepted: 18 August 2018 Published: 3 December 2018
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
During ovarian development stroma from the mesonephros penetrates and expands into the ovarian primordium and thus appears to be involved, at least physically, in the formation of ovigerous cords, follicles and surface epithelium. Cortical stromal development during gestation in bovine fetal ovaries (n = 27) was characterised by immunohistochemistry and by mRNA analyses. Stroma was identified by immunostaining of stromal matrix collagen type I and proliferating cells were identified by Ki67 expression. The cortical and medullar volume expanded across gestation, with the rate of cortical expansion slowing over time. During gestation, the proportion of stroma in the cortex and total volume in the cortex significantly increased (P < 0.05). The proliferation index and numerical density of proliferating cells in the stroma significantly decreased (P < 0.05), whereas the numerical density of cells in the stroma did not change (P > 0.05). The expression levels of 12 genes out of 18 examined, including osteoglycin (OGN) and lumican (LUM), were significantly increased later in development (P < 0.05) and the expression of many genes was positively correlated with other genes and with gestational age. Thus, the rate of cortical stromal expansion peaked in early gestation due to cell proliferation, whilst late in development expression of extracellular matrix genes increased.
Additional keywords: extracellular matrix, proliferation, stroma.
Introduction
Connective tissue or stroma plays an important role in the formation of the ovary and during folliculogenesis as shown in mouse (Weng et al. 2006), cow (Wandji et al. 1996) and human (Heeren et al. 2015). Ovarian stroma contains fibroblasts and extracellular matrix. The matrix contains reticular fibres (Bandeira et al. 2015), fibrillar collagens (Iwahashi et al. 2000; Hummitzsch et al. 2013, 2015), decorin (Hummitzsch et al. 2013, 2015), fibronectin (Hummitzsch et al. 2013, 2015), versican (McArthur et al. 2000; Hummitzsch et al. 2013, 2015), fribillins (Prodoehl et al. 2009; Hatzirodos et al. 2011; Hummitzsch et al. 2013, 2015; Bastian et al. 2016), latent transforming growth factor β (TGFβ)-binding proteins (Prodoehl et al. 2009; Hatzirodos et al. 2011; Bastian et al. 2016) and hyaluronan (Kobayashi et al. 1999; Irving-Rodgers and Rodgers 2007). Stromal extracellular matrix components have been linked to the growth and maturation of follicles in sheep (Huet et al. 2001) by maintaining the shape of granulosa cells as well as their survival and proliferation in vitro.
The ovarian stroma is initially derived from the underlying mesonephros and infiltrates the genital ridge composed of the somatic gonadal ridge epithelial-like (GREL) cells and primordial germ cells–oogonia (Hummitzsch et al. 2013). When stroma first penetrates the ovaries, it is vascularised with capillaries. As it penetrates towards the surface of the ovary primordium the stroma branches and thus corrals the germ cells and somatic GREL cells into forming the ovigerous cords. This region containing the ovigerous cords and the branches of stroma becomes the cortex of the ovary, while the medullary area is substantially mesonephric stroma containing the rete derived from mesonephric ducts. Later, commencing at the medullary–cortical interface, primordial follicles are formed and some become activated and develop further into primary and preantral follicles (Sarraj and Drummond 2012; Smith et al. 2014). As the stroma reaches to below the ovarian surface, it expands laterally below the surface sequestering a population of GREL cells underlain by a basal lamina at the interface (Hummitzsch et al. 2013); the hallmark of an epithelium. At the final stage of ovarian development, the stroma just below the surface develops into the avascular, collagen-rich tunica albuginea.
Many changes in gene expression occur during fetal ovarian development, such as expression of the pluripotency marker POU class 5 homeobox 1 (POU5F1 or Oct4), deleted in azoospermia-like (DAZL), forkhead box L2 (FOXL2), as well as members of the TGF family including TGFB1, 2 and 3, activins, inhibins and bone morphogenetic proteins (BMPs; reviewed in Sarraj and Drummond 2012). The ovarian stroma is potentially influenced by several factors, including members of the TGFβ pathway (Roy and Kole 1998; Bastian et al. 2016) and androgens and oestrogens (Abbott et al. 2006; Loverro et al. 2010). Abnormal development of ovarian stroma could potentially lead to an altered stromal volume in adulthood, as observed in polycystic ovary syndrome (PCOS; Hughesdon 1982; Fulghesu et al. 2001; Abbott et al. 2006; Li and Huang 2008). Despite the importance of stroma, it is probably the least studied compartment of the ovary. Using the bovine ovary, which is similar to the human ovary (Adams and Pierson 1995; Kagawa et al. 2009), including fetal ovarian development (Hatzirodos et al. 2011), stereometric and gene expression analyses were undertaken to evaluate stromal development and the expression of several genes respectively, in relation to major histological changes during the development of the ovary.
Materials and methods
Abattoir ovary collection
Bovine fetal ovarian pairs (n = 27) from different stages of development were collected from pregnant Bos taurus cows from a local abattoir (Thomas Foods International) and crown–rump length (CRL) was measured to estimate the gestational age (Russe 1983). All samples were transported to the laboratory on ice in Hank’s balanced-salt solution containing Mg2+ and Ca2+ (HBSS+/+; Sigma-Aldrich Pty Ltd, Australia). To determine the sex of fetuses with CRL <10 cm in order to exclude males in this study, a tail sample was taken, the DNA was extracted and a polymerase chain reaction (PCR) for sex-determining region Y (SRY) was performed as previously described (Hummitzsch et al. 2013). For histology and immunohistochemistry, one ovary from each pair was weighed and processed for further analysis, whereas for RNA analysis, the corresponding ovary was snap-frozen on dry ice and stored at −80°C for subsequent RNA extraction.
Ovaries of known gestational age
An additional group of fetal ovaries was derived from another study in which the age of the fetus was known. The experimental design of the cattle trial from which these fetal ovaries were obtained has been described previously (Copping et al. 2014). Briefly, the influence of diet on development in utero was assessed on Santa Gertrudis (Bos taurus × Bos indicus) heifers divided into four groups according to different dietary protocols based on a two-by-two factorial crossover design. The heifers were randomly assigned to either a high (H = 14% crude protein, (CP)) or low (L = 7% CP) protein diet 60 days before conception to 23 days postconception (dpc; periconception treatment). This diet was individually fed in stalls and straw (5% crude protein) was available ad libitum. The ration was as isocaloric as possible in the ruminant and supplemented with a commercial vitamin and mineral preparation (Ridley Agriproducts). The heifers were synchronously artificially inseminated to a single bull. At 23 dpc, half of each nutritional treatment group was swapped to the alternative postconception treatment, high or low. At the end of the first trimester (98 dpc), heifers were culled and the fetuses and placentae were collected. The fetuses were sexed and the fetal gonads were excised and weighed. One gonad from each fetus was processed for histology and two ovaries from each of the four groups were analysed by morphometry.
Histology
Fetal ovaries from the abattoir (n = 27) and the ovaries of known gestational age (n = 8) were fixed in 4% paraformaldehyde (Merck Pty Ltd) in 0.1 M phosphate buffer (pH 7.4) and embedded in paraffin using a Leica EG 1140H (Leica Microsystems). Serial sections of 6 µm were prepared using a CM1850 V2.2 Leica microtome (Leica Microsystems), mounted on Superfrost glass slides (HD Scientific Supplies) and stored at room temperature until used for haematoxylin–eosin staining and immunohistochemistry.
Sample grouping
Abattoir samples were sorted into five groups based on histological morphology, as follows: Stage I, ovigerous cord formation (the period when the ovigerous cords are formed (n = 7)); Stage II, ovigerous cord breakdown (the period when most of the ovigerous cords have broken down (n = 4)); Stage III, follicle formation (the period when most of primordial and primary follicles have formed but the cords are still open to the ovarian surface (n = 3)); Stage IV, ovarian surface epithelium formation (the time point when the ovarian surface epithelium is completely formed (n = 8)) and Stage V, tunica albuginea formation (the time point when tunica albuginea is formed (n = 5)). Additionally, the age of each fetus was estimated from the CRL (y = −0.0103x2 + 3.4332x + 36.08, where y is age in days and x is CRL in cm and calculated from the method of Russe (1983)).
Immunohistochemistry
An indirect immunofluorescence method was used for dual localisation of Ki67 and collagen type I (Irving-Rodgers et al. 2002). Paraffin-embedded sections from different ovaries (n = 35 animals) were dewaxed then subjected to a pressure-cooker antigen retrieval method for 20 min (2100 retriever; Prestige Medical Ltd) in 10 mM Tris–ethylenediamine tetraacetic acid (EDTA) buffer (pH 9.0). The primary antibodies used were mouse anti-human Ki67 (1 : 800; M7240/MIB-1; DAKO Australia Pty Ltd) to identify proliferating cells in combination with rabbit anti-human collagen type I (1 : 400; 20 μg mL−1; ab34710; Abcam) to mark the stroma. Secondary antibodies were donkey anti-mouse IgG conjugated to Cy3 (1 : 100; 715 166 151) and Biotin–(SP)-conjugated AffiniPure donkey anti-rabbit IgG (1 : 100; 711 066 152) followed by dichlorotriazinylamino fluorescein (DTAF)-conjugated streptavidin (1 : 100; 016 010 084). All secondary antibodies and conjugated streptavidin were from Jackson ImmunoResearch Laboratories Inc. Cell nuclei were counterstained with 4’,6’-diamidino-2-phenylindole dihydrochloride (DAPI) solution (Molecular Probes). The bovine adult ovary was used as a positive control whereas nonimmune mouse and rabbit sera (Sigma-Aldrich) were used as negative controls. All sections were photographed with an Olympus BX51 microscope with an epifluorescence attachment and a Spot RT digital camera (Diagnostic Instruments) at a magnification of 40×.
Morphometric analyses
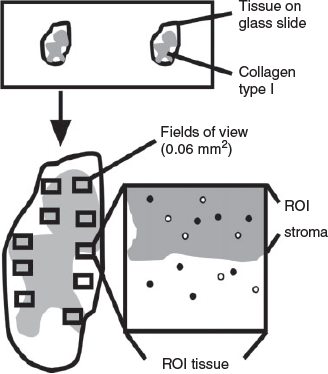
The largest cross-section from each ovary, determined by haematoxylin–eosin staining for every 10th section, was examined using NPD.view2 software (Hamamatsu Photon). Using the ImageJ software (Schindelin et al. 2012), images of the cortex at 40× magnification were taken randomly with a single image dimension set at 1600 × 1200 pixels (0.06 mm2). Each image represented a field of view that was used for morphometric analysis in this study. The total number of fields of view to be examined for each analysis was first determined by examining the coefficient of variation (CV) of 3–40 fields of view. For measuring the stromal area, the following steps were performed (Fig. 1). First, the total cortical area was identified, saved as a region of interest (tissue area) and its area measured. Next, the stromal area in the cortex was identified based on positive collagen type I staining (Fig. 2). To calculate the proportion of stroma in the cortex (volume density) and total stromal volume, the following calculations were applied and all calculations used equations that are listed in Table 1. The ovarian volume (Vovary) was estimated using the ovarian weight, assuming a density of 1 g cm−3. Then, the proportion of stroma in the cortex (Vv[stroma]) and the total stromal volume (Vstroma) were calculated using the equations shown in Table 1. The number of proliferating stromal cells (Ki67 positive) and all stromal cells (DAPI positive) in a field of view were counted using the multipoint and find maxima tool with adjusted noise tolerance (ImageJ). Nuclei, which were not detected by the threshold, were added manually using the multipoint tool. Results of proliferating cells are presented as a proliferation index (PI) and as a numerical density (Ndp/Nd) in the stromal area using the equations shown in Table 1.
|
RNA extraction and cDNA synthesis
RNA was extracted from the whole fetal ovary using 1 mL Trizol (Thermo Fisher Scientific) with 0.5 g of ceramic beads in homogenisation tubes using the Mo Bio Powerlyser 24 (Mo Bio Laboratories Inc.) and 200 µL chloroform (RNase-free) according to the manufacturer’s instructions. The RNA concentration was determined using a Nanodrop spectrophotometer (NanoDrop 1000 3.7.1; Nanodrop Technologies) based on the 260λ (wavelength) absorbance. All samples which had a 260 : 280λ absorbance ratio >1.8 were used and subsequently treated with DNase I (Promega/Life Technologies Australia Pty Ltd). Complementary DNA was then synthesised from 200 ng of DNase-treated RNA using 250 ng µL−1 random hexamers (Geneworks) and 200 U Superscript Reverse Transcriptase III (Thermo Fisher Scientific) as previously described (Matti et al. 2010). For a negative control, diethyl pyrocarbonate (DEPC)-treated water instead of the Superscript Reverse Transcriptase III was added.
Quantitative real-time PCR
To conduct quantitative real-time PCR (qPCR), primers were designed against the published reference RNA sequences (Table 2) using Primer3 plus (Rozen and Skaletsky 2000) and Net primer (PREMIER Biosoft) software. To test the combination of primers, the cDNA was diluted to five different concentrations from 1 : 4 to 1 : 1000 to generate a standard curve of cycle threshold (Ct) versus concentrations. Primer combinations that showed a single sharp peak and achieved an amplification efficiency of 0.9–1.1 and an R2 value ≥ 0.98 were used for further analysis.

|
Quantitative PCR was carried out using a Rotor-Gene 6000 series 1.7 thermal cycler (Qiagen GmbH) in duplicate at 95°C for 15 s then 60°C for 60 s for 40 cycles. Amplification of cDNA dilutions were prepared in 10 μL reactions containing 2 μL of the 1 : 20 cDNA dilution, 5 μL Power SYBR Green PCR Master Mix (Applied Biosystems), 0.2 μL each of forward and reserve primers (Geneworks; Table 2) for the target genes and 2.6 μL of DEPC-treated water. Ct values were determined using the Rotor-Gene 6000 software (Q series; Qiagen GmbH) at a threshold of 0.05 normalised fluorescence units. Gene expression was determined by the mean of 2−ΔCt, where ΔCt represents the target gene Ct – glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Ct. GAPDH was used as a housekeeping gene because it is stably expressed in bovine adult ovarian tissues (Berisha et al. 2002) and showed stable expression in our samples.
Statistical analyses
All statistical analyses were carried out using Microsoft Office Excel 2010 and GraphPad Prism Version 6.00 (GraphPad Software Inc.). All data that were not normally distributed or showed significantly different standard deviations between groups were first log-transformed. The morphometric and 2−ΔCt data for each fetal ovarian sample were compared using ANOVA with Tukey’s post-hoc test. A value of P < 0.05 was considered to be significant. For testing the association between expression levels of each gene throughout development, correlation coefficients were determined using the Spearman correlation coefficient. After correlation values between genes were identified, a network graph was plotted using the qgraph R package (Epskamp et al. 2012) and illustrated using an adjacent matrix plot.
Results
Classification of ovaries
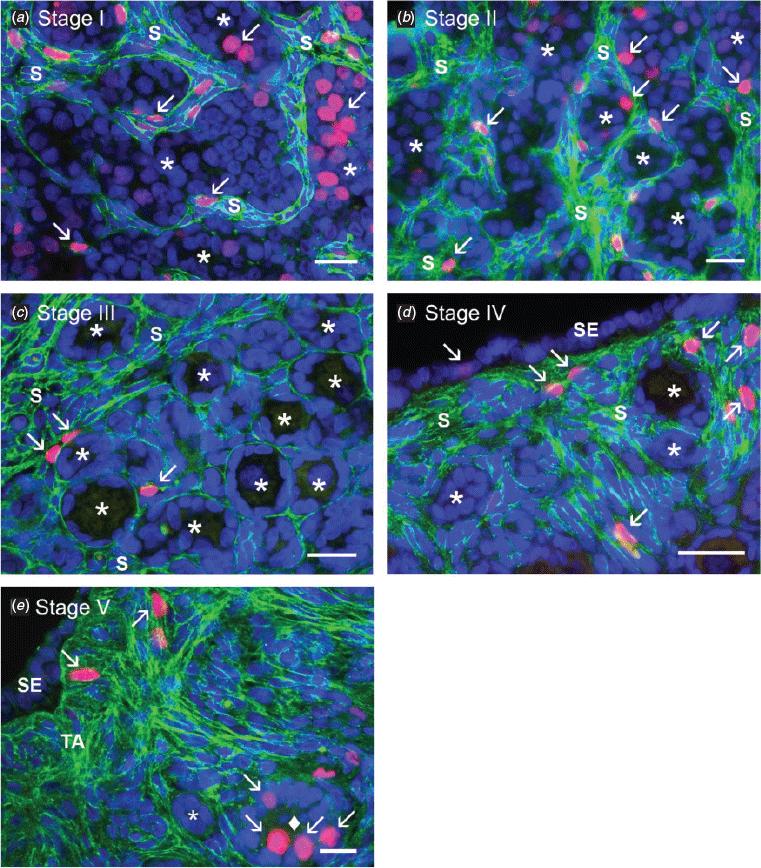
As we have observed previously (Hummitzsch et al. 2013), there was variation in the developmental stage of ovaries from fetuses of the same crown–rump length, particularly at early stages. We therefore devised a classification system based upon five identifiable stages of development of the cortex commencing with ovigerous cord formation (Stage I), ovigerous cord breakdown (Stage II), follicle formation (Stage III), surface epithelium formation (Stage IV) and tunica albuginea formation (Stage V) as illustrated in Fig. 2 and subsequently analysed data from the abattoir collection using this classification system.
Determination of total number of fields of view
Using the ovaries from our abattoir collections we first determined the optimum number of total fields of view required for the morphometric measurements of the proportion of stroma in the cortex and the proportion of proliferating stromal cells in the ovarian cortex. A CV analysis was conducted from 3 up to 40 fields of view photographed at 40× magnification accounting for 0.06 mm2 (Fig. S1, available as Supplementary Material to this paper). For fetuses with a CRL <30 cm, the CV of the volume density of stroma (Fig. S1a–c) and the proportion of proliferating stromal cells in the ovarian cortex (Fig. S1g–i) was relatively variable until nine fields of view and then remained similar until 20 fields of view. However, for fetuses with CRL of 39 cm the CV of the proportion of stroma in the cortex (Fig. S1d) and the proportion of proliferating stromal cells in the ovarian cortex (Fig. S1j) were similar for almost all numbers of fields of view. For fetuses with CRL >50 cm, the parameters were highly variable from 3 to 20 fields of view (Fig. S1e, f, k, l) and then stabilised from 20 to 40 fields of view. Thus, for morphometric quantitation of fetuses with a CRL of <50 cm we assessed 10 fields of view and for fetuses with a CRL of >50 cm we assessed 20 fields of view.
Ovary changes in Stages I to V
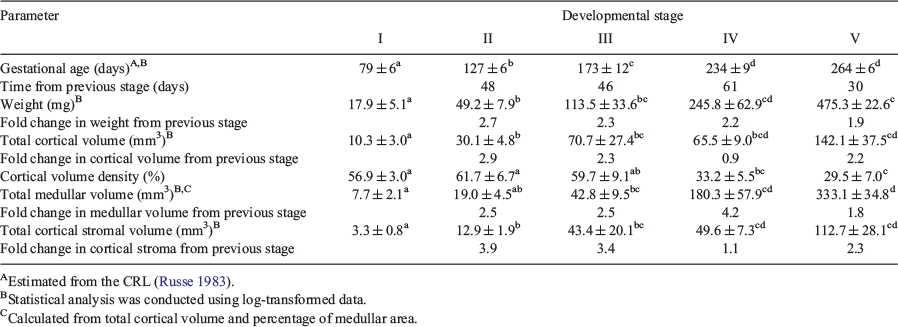
Details of the ovaries and fetuses of each stage from our abattoir collection are presented in Table 3. With advancing stages the fetuses were older and the ovaries became significantly heavier and they had more cortex and medulla, but proportionally less cortex relative to medulla in the last two stages of development (Table 3). We calculated the time to transit from one stage to the next and fold changes between each stage in weight, cortical volume and medullary volume (Table 3). The transition time between Stages I and II, II and III, III and IV and IV and V were 48, 46, 61 and 30 days respectively (Table 3); thus, the duration of development before Stage III was 94 (48 + 46) days and after Stage III was 91 (61 + 30) days. From Stages I to III versus Stages III to V the fold changes in weight were 6.2 and 4.2, the fold changes in cortical volume were 6.7 and 2.0 and the fold changes in medullary volume were 6.3 and 7.6 respectively (calculated from Table 3). Thus, after Stage III when follicles formed, the expansion of the cortex slowed but the medulla continued to expand at its previous rate, leading to proportionally more medulla. From 79 to 264 days of gestation the ovarian volume increased 26.5 fold (17.9 to 475.3 mm3), the cortex increased 13.8 fold in volume (10.3 to 142.1 mm3) and the medulla increased 34.1 fold in volume (3.3 to 112.7 mm3).
Morphometric characteristics of fetal ovarian stroma
During ovigerous cord formation at Stage I (Fig. 2a), the stroma containing many Ki67-positive cells formed branches between ovigerous cords (Fig. 3c, d). At this stage the ovigerous cords contained oogonia, undergoing mitosis as shown by colocalisation with the proliferation marker Ki67 (Fig. 2a). The proportion of stroma in the ovarian cortex increased during ovigerous cord breakdown at Stage II (Fig. 3a, b), although it was not statistically significant. Ki67-positive cells were observed in the stromal area and in the partitioned ovigerous cords (Fig. 2b). In the stromal area, the total number of Ki67-positive cells was lower than during Stage I (Fig. 3c, d); however, the difference was not statistically significant. During follicle formation at Stage III (Fig. 2c), the proportion of stroma in the ovarian cortex had increased further (Fig. 3a, b). The stroma now surrounded the primordial follicles, which were first formed at the inner cortex adjacent to the medulla. Proliferating cells were observed in the stroma but not in primordial follicles (Fig. 2c); however, the total number of Ki67-positive cells in stroma were lower than during Stage II (Fig. 3c, d), although the difference was not statistically significant. During the formation of surface epithelium at Stage IV (Fig. 2d), the ovary had more stroma underneath the ovarian surface. At this stage Ki67-positive cells localised in the stroma as well as the ovarian surface. Tunica albuginea formation at Stage V was characterised by bundles of thick collagen type I fibres underneath the ovarian surface epithelium (Fig. 2e). At this stage stromal cells and some granulosa cells in growing follicles were positive for Ki67.
We quantitatively measured the proportion of stroma in the cortex and the total volume of the stroma in the cortex (Fig. 3) and both were significantly increased during ovarian development (P < 0.05; Fig. 3a, b). Interestingly, the cell proliferation index of the stroma in the ovarian cortex significantly declined during ovarian development (Fig. 3c). The numerical density of proliferating cells also significantly declined in the cortical stroma throughout gestation (Fig. 3d). However, the numerical density of cortical stromal cells was stable during ovarian development (Fig. 3e), suggesting that the increase in stromal volume during gestation was not affected by an increase in extracellular space in the cortical stroma.
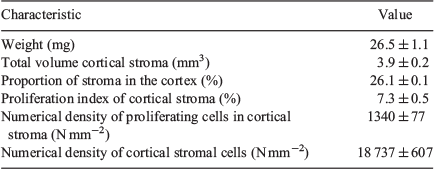
To assess if our morphometric analyses used for the abattoir collection were adequate, we analysed fetal ovarian samples from the trial collected on Day 98 of gestation. Using the CRL, age was estimated by the method of Russe (1983) to be 95.3 ± 1.2 days, which is in very good agreement with the known age of 98 days. During this time of gestation, the stroma occupied 26.1 ± 0.1 % of the cortex and was 3.9 ± 0.2 mm3 in total volume. The proliferation index of the cortical stroma was 7.3 ± 0.5 %, the numerical density of the proliferating stromal cells in the cortex was 1340 ± 77 cells mm−2 and the numerical density of all the stromal cells was 18 737 ± 607 cells mm−2 (Table 4).
|
Gene expression
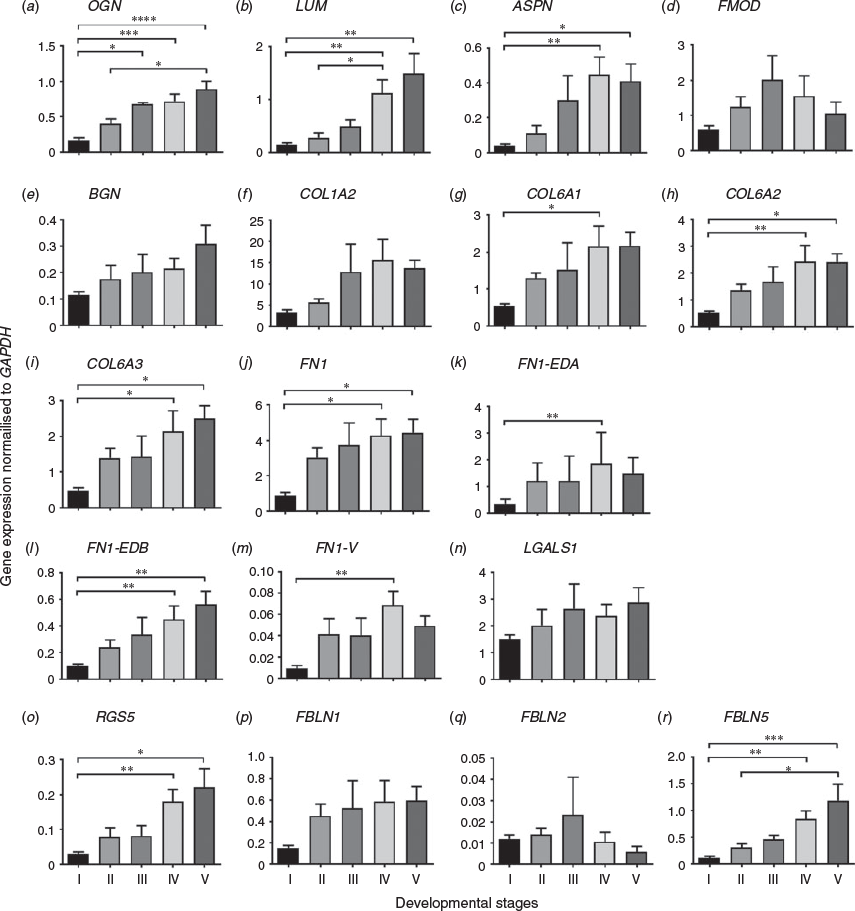
We analysed the mRNA expression of genes that are specifically expressed in the stroma of many adult tissues, including the ovary. The mRNA expression of osteoglycin (OGN), lumican (LUM), asporin (ASPN), collagen type VI A1 (COL6A1), COL6A2, COL6A3, fibronectin (FN1), regulator of G-protein signalling 5 (RGS5) and fibulin 5 (FBLN5) in fetal ovaries significantly increased in Stages IV and V of development (Fig. 4a–c, g–j, o, r) relative to earlier stages. We also analysed the mRNA expression of another three FN1 splice variants (FN1 extra domain A (FN1-EDA), FN1 extra domain B (FN1-EDB) and FN1 variable (FN1-V)), which have three different additional domains. Our results showed that FN1-EDA, FN1-EDB and FN1-V were also significantly increased late in development (Fig. 4k–m). The expression of fibromodulin (FMOD), biglycan (BGN), COL1A2, FBLN1, FBLN2 and Lectin galactoside-binding soluble 1 (galectin 1) (LGALS1) did not show any significant differences across stages of ovarian development (Fig. 4d–f, n, p, q).
Correlation analyses
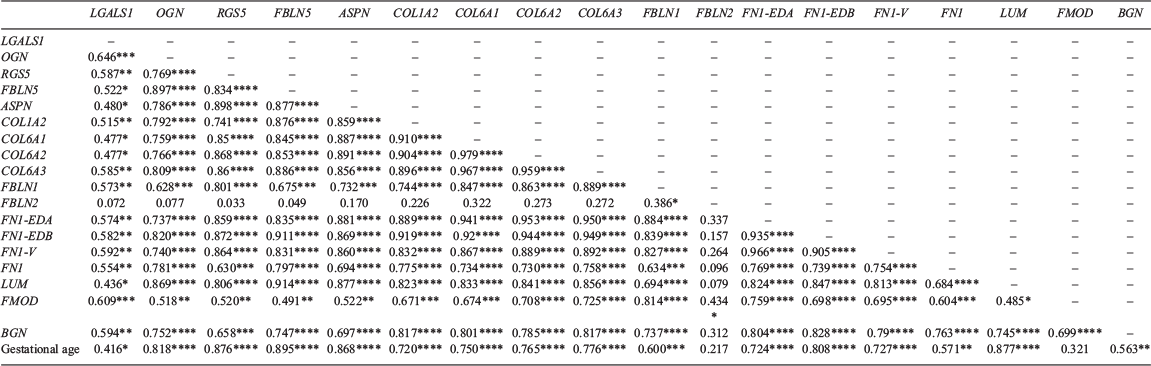
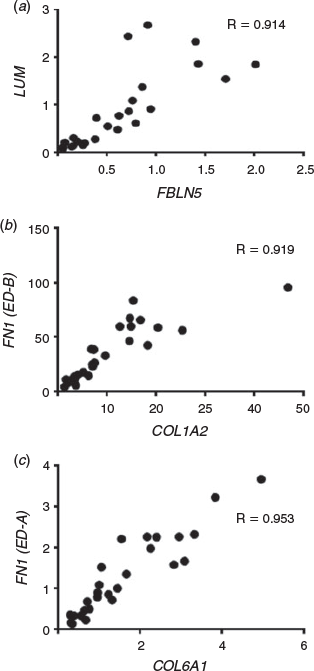
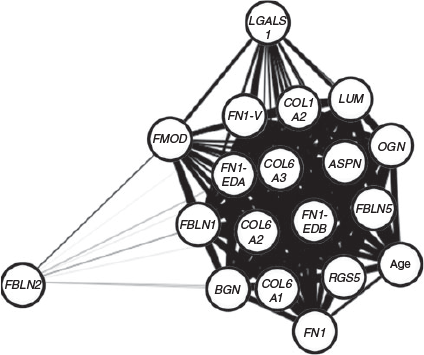
To analyse the correlations between the 18 genes of interest and additionally with gestational age (Table 5), we generated the Spearman correlation matrix from the Ct values of all genes. After correlation values between genes were identified, a network graph was plotted using the qgraph R package (Epskamp et al. 2012) to plot an adjacent matrix (Fig. 5) and some examples of these are correlations as shown in Fig. 6. OGN, LUM, ASPN, BGN, COL6A1, COL6A2, COLA3, COL1A2, FN1, FN1-EDA, FN1-EDB, FN1-V, RGS5, FBLN1 and FBLN5 were all strongly positively associated with each other (r >0.6). In addition, FMOD had strong positive correlations (r > 0.6) with BGN, COL6A1, COL6A2, COLA3, COL1A2, FN1, FN1-EDA, FN1-EDB, FN1-V and LGALS1. FBLN2 and LGALS1 showed weaker positive relationships to other genes (r <0.6). Gestational age had strong positive correlations (r >0.6) with FBLN5, LUM, RGS5, ASPN, OGN, FN1-EDB, COL6A3, COL6A2, COLA1, FN1-V, FN1-EDA, COL1A2 and FBLN1, but had weaker positive correlations (r <0.6) with FN1, BGN, LGALS1 and FMOD (Table 5). Interestingly, FBLN2 had a weak negative correlation with gestational age (Table 5).
|
|
|
Discussion
In this study we conducted morphometric analyses of the developing bovine fetal ovary, focusing on cortical stroma. We were able to identify the stroma by immunostaining of the stromal extracellular matrix collagen type I. We optimised our morphometric sampling regime and also examined ovaries of a known stage of gestation. Across gestation we measured the total volume and relative proportions of cortex and medulla, the proportion and total volume of stroma in the cortex and the numerical and proliferation index of cells in the stroma of the cortex. We also examined the expression of 18 genes that had previously been reported as relevant to stroma in other organs. We believe this is one of the first critical studies of the development of ovarian stroma.
Collagen type I is an extracellular matrix of stroma that has been localised in the stroma of fetal bovine (Hummitzsch et al. 2013) and rat ovaries (Paranko 1987), as well as adult bovine (Figueiredo et al. 1995), mouse (Berkholtz et al. 2006) and human (Lind et al. 2006) ovaries. Collagen type I was observed at all stages of fetal bovine ovary development by immunostaining but the intensity of immunostaining increased qualitatively throughout development. This suggests that greater deposition of collagen type I occurs during fetal development, leading to a stiffer stromal matrix.
An earlier morphometric study examining the bovine fetal ovary has been conducted but over a shorter period of time than our study. From their results we calculated that the ovary volume increased 4.2 fold in volume (35 to 148 mm3), the cortex increased 2.8 fold in volume (21.9 to 62.4 mm3) and the medulla increased 6.5 fold (13.1 to 85.6 mm3) from 3 to 7 months of gestation (Santos et al. 2013). Our measurements are in agreement with theirs where, during Stages I to III (taking 94 days) versus Stages III to V (taking 91 days) the changes in ovarian weight were 6.2 and 4.2 fold, in medullary volume were 6.3 and 7.6 fold and in cortical volume were 6.7 and 2.0 fold respectively. Our values are higher than theirs as our study was over a longer time frame. Our results also show that throughout gestation the medulla expanded substantially more (34.1 fold) than the cortex (13.8 fold) did and that the rate of expansion of the cortex declined past Stage III when follicles were formed. What drives the continued expansion of the medulla is not known, nor why this would continue.
The numerical density (cells per area or volume) of cells in the cortical stroma, however, did not change across gestation. Assuming that the sizes of the stromal cells did not change, and there was no observable evidence that they did, then the proportion of extracellular space in the cortical stroma was constant across gestation. This is different from what is known to happen in the tunica albuginea, which has a significantly lower cell numerical density than cortical stroma and has proportionally more extracellular space rich in extracellular matrix containing at least collagens, versican, fibronectin, decorin (Hummitzsch et al. 2013), latent transforming growth factor β-binding protein 2 and fibrillin 1 (Prodoehl et al. 2009). Thus, as also observed in the human fetal ovary from 20 to 25 weeks of gestational age (Sforza et al. 1993), there was an increase in stroma expansion across gestation but this rate of expansion slowed after Stage III. These rates of expansion are mirrored by the proliferation of stromal cells, but not the proportion of extracellular space, suggesting that stromal cell proliferation is largely responsible for stromal expansion during ovarian development.
Since stroma has been shown to expand significantly during ovarian development, genes that encode extracellular matrix proteins that might be involved in the expansion of the ovarian stroma were examined. Small leucine-rich proteoglycans (SLRPs) have a role in regulating collagen type I fibrillogenesis, which has been shown to be expressed during ovarian development in the rat (Paranko 1987) and cow (Hummitzsch et al. 2013). There are three classes of SLRP, Class I, Class II and Class III, which consist of extracellular matrix proteins, including asporin (ASPN) and biglycan (BGN), lumican (LUM) and fibromodulin (FMOD) and osteoglycin (OGN; Ameye and Young 2002). ASPN and BGN have been shown to be involved in collagen type I fibrillogenesis in human embryonic kidney cells and adult mouse ovary respectively (Oksjoki et al. 1999; Kalamajski et al. 2009). Another study showed that LUM and FMOD bind to collagen type I in an antagonistic manner during tendon development, which might be related to the formation of progressively thicker collagen fibrils (Kalamajski and Oldberg 2009). Additionally, OGN is involved in regulating collagen type I fibrillogenesis in mouse embryo fibroblasts (Ge et al. 2004). Since collagen type I also increases as the stroma expands during ovarian development, our findings indicate that ASPN, LUM and OGN might be involved in the deposition and assembly of extracellular matrix in stroma in bovine fetal ovary.
The establishment and the remodelling of the ovarian vascular system is required for development of the ovary (Robinson et al. 2009). Initially the penetration and expansion of the mesonephric stroma into the developing ovary brings with it capillaries contained therein (Hummitzsch et al. 2013; Smith et al. 2014). It has been shown that RGS5 and FBLN5, which encode regulator of G-protein signalling 5 and fibulin 5 protein respectively, are involved in vascular remodelling (Berger et al. 2005; Spencer et al. 2005). RGS5 protein is a pericyte marker observed in the vasculature of mouse ovarian follicles (Berger et al. 2005), rat cerebral capillaries (Kirsch et al. 2001) and mouse embryonic pericytes (Bondjers et al. 2003). Pericytes have been observed in 23–24 week human fetal ovary as a part of a vascular network in the stroma (Niculescu et al. 2011). Additionally, a study using FBLN5 knockout mice suggests the important role of FBLN5 in neointima formation and vascular remodelling after an induced vascular injury (Spencer et al. 2005). Since the interaction between endothelial cells, pericytes and smooth muscle cells is critical for the development of the vasculature (Niculescu et al. 2011), the increased expression of RGS5 and FBLN5 suggests that these genes may play a role in expansion of the vasculature in the cortical stroma.
COL6A1, COL6A2 and COL6A3 encode the extracellular matrix component collagen type VI, which is predicted to help in anchoring tissues and cells to the connective tissue extracellular matrix (Cescon et al. 2015). In human adult ovary, collagen type VI is observed in the thecal layer, especially in the theca externa, and has a role in the interactions between the thecal cell and extracellular matrix during folliculogenesis (Iwahashi et al. 2000). A study using a yeast two-hybrid system showed an interaction between collagen type VI and collagen type IV (Kuo et al. 1997), which is localised in follicular basal lamina during ovarian development (Hummitzsch et al. 2013). Additionally, collagen type VI also interacts with other extracellular matrix components, such as collagen type I (Bonaldo et al. 1990) and fibronectin (Sabatelli et al. 2001). A study in the bovine fetal ovary showed that collagen type I and fibronectin were specifically expressed in the ovarian stroma (Hummitzsch et al. 2013), suggesting that collagen type VI might have an important role in anchoring vasculature in the stroma during the late stage of ovarian development.
FN1 encodes fibronectin, which modulates cell–cell and cell–matrix interactions (Goldberg et al. 2006). Fibronectin is composed of three structurally homologous types of repeated domains: Type I, II and III. Three different alternative splicing regions are located in Type III fibronectin: extra domain A (ED-A), B (ED-B) and the variable (V) region, encoded by FN1-EDA, FN1-EDB and FN1-V, respectively, (the variable region can have three to five alternative splicing events). The ED-A, ED-B and V regions are located between the 11th and 12th, between the 7th and 8th and between the 14th and 15th Type III repeats respectively (De Candia and Rodgers 1999). Collectively, these alternative splicing events potentially produce multiple different isoforms in humans, cows, mice and rats: ED-A+, ED-A–, ED-B+, ED-B–, V+ and V–. These have been shown to be expressed in bovine antral follicles of 0.5–9 mm diameter, in corpora lutea and in fetal bovine liver, lung and kidney but fetal ovaries were not examined in that study (De Candia and Rodgers 1999). The ED-A+ isoform has been identified in ovarian follicles and is predicted to be associated with the replication of granulosa cells (Colman-Lerner et al. 1999), whereas the ED-B+ and V+ isoforms have been shown to be involved in angiogenesis (Castellani et al. 1994; De Candia and Rodgers 1999). It is possible that the isoforms of FN1 might be involved in follicle formation and angiogenesis during ovarian development.
Many of the genes examined in the present study were highly positively correlated with gestational age and also with each other. This is not surprising as many of these genes were extracellular matrix genes associated with stroma, which also expanded during fetal development. Comparisons with earlier studies of bovine fetal ovaries (Hatzirodos et al. 2011) showed that fibrillin 3, another extracellular matrix gene that is familiarly linked to PCOS, is highly expressed only in the first trimester and declines in expression thereafter. This is interesting as fibrillin 3 is thus expressed in the fetal cortical stroma when it is proportionally expanding the most during fetal development. Why this particular gene should exhibit this behaviour and not other stromal extracellular matrix genes is not known, but it does suggest that fibrillin 3 may have a unique role during ovarian cortical stroma expansion.
In summary we have shown quantitatively that the rate of expansion of cortical stroma is greatest early in development when the stroma penetrates the ovarian primordium from the mesonephros. The expansion of the cortical stroma occurs due to cell proliferation and not a change in cell size or a change in the amount of extracellular space. The mRNA expression levels of many extracellular matrix genes increased in the later stages of ovarian development and were highly correlated with each other, suggesting that they might be co-regulated. In conclusion, the behaviour of stroma changes during ovarian development and this might have consequences for its roles.
Conflicts of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
This research was funded by the Australia Awards Scholarship from the Australian Government, The University of Adelaide, the National Health and Medical Research Council (NHMRC) of Australia, the NHMRC Centre for Research Excellence in the Evaluation, Management and Health Care Needs of Polycystic Ovary Syndrome, the Society for Reproductive Biology and the Robinson Research Institute. We would like to acknowledge the Australian Research Council, Griffith University, S. Kidman and Co. and Ridley Agriproducts Pty Ltd for also funding this research. We would like to thank Thomas Food International, Murray Bridge, South Australia for providing the bovine tissues for this research.
References
Abbott, D. H., Padmanabhan, V., and Dumesic, D. A. (2006). Contributions of androgen and estrogen to fetal programming of ovarian dysfunction. Reprod. Biol. Endocrinol. 4, 17.| Contributions of androgen and estrogen to fetal programming of ovarian dysfunction.Crossref | GoogleScholarGoogle Scholar |
Adams, G. P., and Pierson, R. A. (1995). Bovine model for study of ovarian follicular dynamics in humans. Theriogenology 43, 113–120.
| Bovine model for study of ovarian follicular dynamics in humans.Crossref | GoogleScholarGoogle Scholar |
Ameye, L., and Young, M. F. (2002). Mouse deficient in small leucine-rich proteoglycans novel in vivo models for osteoporosis, osteoarthritis, Ehlers–Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 12, 107R–116R.
| Mouse deficient in small leucine-rich proteoglycans novel in vivo models for osteoporosis, osteoarthritis, Ehlers–Danlos syndrome, muscular dystrophy, and corneal diseases.Crossref | GoogleScholarGoogle Scholar |
Bandeira, F. T., Carvalho, A. A., Castro, S. V., Lima, L. F., Viana, D. A., Evangelista, J., Pereira, M., Campello, C. C., Figueiredo, J. R., and Rodrigues, A. (2015). Two methods of vitrification followed by in vitro culture of the ovine ovary: evaluation of the follicular development and ovarian extracellular matrix. Reprod. Domest. Anim. 50, 177–185.
| Two methods of vitrification followed by in vitro culture of the ovine ovary: evaluation of the follicular development and ovarian extracellular matrix.Crossref | GoogleScholarGoogle Scholar |
Bastian, N. A., Bayne, R. A., Hummitzsch, K., Hatzirodos, N., Bonner, W. M., Hartanti, M. D., Irving-Rodgers, H. F., Anderson, R. A., and Rodgers, R. J. (2016). Regulation of fibrillins and modulators of TGFβ in fetal bovine and human ovaries. Reproduction 152, 127–137.
| Regulation of fibrillins and modulators of TGFβ in fetal bovine and human ovaries.Crossref | GoogleScholarGoogle Scholar |
Berger, M., Bergers, G., Arnold, B., Hammerling, G. J., and Ganss, R. (2005). Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 105, 1094–1101.
| Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization.Crossref | GoogleScholarGoogle Scholar |
Berisha, B., Pfaffl, M. W., and Schams, D. (2002). Expression of estrogen and progesterone receptors in the bovine ovary during estrous cycle and pregnancy. Endocrine 17, 207–214.
| Expression of estrogen and progesterone receptors in the bovine ovary during estrous cycle and pregnancy.Crossref | GoogleScholarGoogle Scholar |
Berkholtz, C. B., Lai, B. E., Woodruff, T. K., and Shea, L. D. (2006). Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem. Cell Biol. 126, 583–592.
| Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis.Crossref | GoogleScholarGoogle Scholar |
Bonaldo, P., Russo, V., Bucciotti, F., Doliana, R., and Colombatti, A. (1990). Structural and functional features of the alpha3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry 29, 1245–1254.
| Structural and functional features of the alpha3 chain indicate a bridging role for chicken collagen VI in connective tissues.Crossref | GoogleScholarGoogle Scholar |
Bondjers, C., Kalén, M., Hellström, M., Scheidl, S. J., Abramsson, A., Renner, O., Lindahl, P., Cho, H., Kehrl, J., and Betsholtz, C. (2003). Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am. J. Pathol. 162, 721–729.
| Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells.Crossref | GoogleScholarGoogle Scholar |
Castellani, P., Viale, G., Dorcaratto, A., Nicolo, G., Kaczmarek, J., Querze, G., and Zardi, L. (1994). The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int. J. Cancer 59, 612–618.
| The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis.Crossref | GoogleScholarGoogle Scholar |
Cescon, M., Gattazzo, F., Chen, P., and Bonaldo, P. (2015). Collagen VI at a glance. J. Cell Sci. 128, 3525–3531.
| Collagen VI at a glance.Crossref | GoogleScholarGoogle Scholar |
Colman-Lerner, A., Fischman, M. L., Lanuza, G. M., Bissel, D. M., Kornblihtt, A. R., and Baranao, J. L. (1999). Evidence for a role of the alternatively spliced ED-I sequence of fibronectin during ovarian follicular development. Endocrinology 140, 2541–2548.
| Evidence for a role of the alternatively spliced ED-I sequence of fibronectin during ovarian follicular development.Crossref | GoogleScholarGoogle Scholar |
Copping, K. J., Hoare, A., Callaghan, M., McMillen, I. C., Rodgers, R. J., and Perry, V. E. A. (2014). Fetal programming in 2-year-old calving heifers: peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner. Anim. Prod. Sci. 54, 1333–1337.
De Candia, L. M., and Rodgers, R. J. (1999). Characterization of the expression of the alternative splicing of the ED-A, ED-B and V regions of fibronectin mRNA in bovine ovarian follicles and corpora lutea. Reprod. Fertil. Dev. 11, 367–377.
| Characterization of the expression of the alternative splicing of the ED-A, ED-B and V regions of fibronectin mRNA in bovine ovarian follicles and corpora lutea.Crossref | GoogleScholarGoogle Scholar |
Epskamp, S., Cramer, A. O. J., Waldorp, L. J., Schmittmann, V. D., and Borsboom, D. (2012). qgraph: network visualizations of relationships in psychometric data. J. Stat. Softw. 48, 1–18.
| qgraph: network visualizations of relationships in psychometric data.Crossref | GoogleScholarGoogle Scholar |
Figueiredo, J. R., Hulshof, S. C. J., Thiry, M., Van den Hurk, R., Bevers, M. M., Nusgens, B., and Beckers, J. F. (1995). Extracellular matrix proteins and basement membrane: their identification in bovine ovaries and significance for the attachment of cultured preantral follicles. Theriogenology 43, 845–858.
| Extracellular matrix proteins and basement membrane: their identification in bovine ovaries and significance for the attachment of cultured preantral follicles.Crossref | GoogleScholarGoogle Scholar |
Fulghesu, A. M., Clampelli, M., Belosi, C., Apa, R., Pavone, V., and Lanzone, A. (2001). A new ultrasound criterion for the diagnosis of polycystic ovary syndrome: the ovarian stroma/total area ratio. Fertil. Steril. 76, 326–331.
| A new ultrasound criterion for the diagnosis of polycystic ovary syndrome: the ovarian stroma/total area ratio.Crossref | GoogleScholarGoogle Scholar |
Ge, G., Seo, N. S., Liang, X., Hopkins, D. R., Hook, M., and Greenspan, D. S. (2004). Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J. Biol. Chem. 279, 41626–41633.
| Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis.Crossref | GoogleScholarGoogle Scholar |
Goldberg, M., Septier, D., Oldberg, A., Young, M. F., and Ameye, L. G. (2006). Fibromodulin-deficient mice display impaired collagen fibrillogenesis in predentin as well as altered dentin mineralization and enamel formation. J. Histochem. Cytochem. 54, 525–537.
| Fibromodulin-deficient mice display impaired collagen fibrillogenesis in predentin as well as altered dentin mineralization and enamel formation.Crossref | GoogleScholarGoogle Scholar |
Hatzirodos, N., Bayne, R. A., Irving-Rodgers, H. F., Hummitzsch, K., Sabatier, L., Lee, S., Bonner, W., Gibson, M. A., Rainey, W. E., Carr, B. R., Mason, H. D., Reinhardt, D. P., Anderson, R. A., and Rodgers, R. J. (2011). Linkage of regulators of TGF-beta activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 25, 2256–2265.
| Linkage of regulators of TGF-beta activity in the fetal ovary to polycystic ovary syndrome.Crossref | GoogleScholarGoogle Scholar |
Heeren, A. M., van Iperen, L., Klootwijk, D. B., de Melo Bernardo, A., Roost, M. S., Gomes Fernandes, M. M., Louwe, L. A., Hilders, C. G., Helmerhorst, F. M., van der Westerlaken, L. A., and Chuva de Sousa Lopes, S. M. (2015). Development of the follicular basement membrane during human gametogenesis and early folliculogenesis. BMC Dev. Biol. 15, 4.
| Development of the follicular basement membrane during human gametogenesis and early folliculogenesis.Crossref | GoogleScholarGoogle Scholar |
Huet, C., Pisselet, C., Mandon-Pépin, B., Monget, P., and Monniaux, D. (2001). Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function. J. Endocrinol. 169, 347–360.
| Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function.Crossref | GoogleScholarGoogle Scholar |
Hughesdon, P. E. (1982). Morphology and morphogenesis of the Stein–Leventhal ovary and of so-called “hyperthecosis”. Obstet. Gynecol. Surv. 37, 59–77.
| Morphology and morphogenesis of the Stein–Leventhal ovary and of so-called “hyperthecosis”.Crossref | GoogleScholarGoogle Scholar |
Hummitzsch, K., Irving-Rodgers, H. F., Hatzirodos, N., Bonner, W., Sabatier, L., Reinhardt, D. P., Sado, Y., Ninomiya, Y., Wilhelm, D., and Rodgers, R. J. (2013). A new model of development of the mammalian ovary and follicles. PLoS One 8, e55578.
| A new model of development of the mammalian ovary and follicles.Crossref | GoogleScholarGoogle Scholar |
Hummitzsch, K., Anderson, R. A., Wilhelm, D., Wu, J., Telfer, E. E., Russell, D. L., Robertson, S. A., and Rodgers, R. J. (2015). Stem cells, progenitor cells, and lineage decisions in the ovary. Endocr. Rev. 36, 65–91.
| Stem cells, progenitor cells, and lineage decisions in the ovary.Crossref | GoogleScholarGoogle Scholar |
Irving-Rodgers, H. F., and Rodgers, R. J. (2007). Extracellular matrix in ovarian follicular and luteal development. In ‘Novel Concepts in Ovarian Endocrinology’. (Ed. A. Gonzalez-Bulnes.) pp. 83–112. (Transworld Research Network: Kerala, India.)
Irving-Rodgers, H. F., Bathgate, R. A. D., Ivell, R., Domagalski, R., and Rodgers, R. J. (2002). Dynamic changes in the expression of relaxin-like factor (Insl3), cholesterol side-chain cleavage cytochrome p450, and 3beta-hydroxysteroid dehydrogenase in bovine ovarian follicles during growth and atresia. Biol. Reprod. 66, 934–943.
| Dynamic changes in the expression of relaxin-like factor (Insl3), cholesterol side-chain cleavage cytochrome p450, and 3beta-hydroxysteroid dehydrogenase in bovine ovarian follicles during growth and atresia.Crossref | GoogleScholarGoogle Scholar |
Iwahashi, M., Muragaki, Y., Ooshima, A., and Nakano, R. (2000). Type VI collagen expression during growth of human ovarian follicles. Fertil. Steril. 74, 343–347.
| Type VI collagen expression during growth of human ovarian follicles.Crossref | GoogleScholarGoogle Scholar |
Kagawa, N., Silber, S., and Kuwayama, M. (2009). Successful vitrification of bovine and human ovarian tussue. Reprod. Biomed. Online 18, 568–577.
| Successful vitrification of bovine and human ovarian tussue.Crossref | GoogleScholarGoogle Scholar |
Kalamajski, S., and Oldberg, A. (2009). Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding. J. Biol. Chem. 284, 534–539.
| Homologous sequence in lumican and fibromodulin leucine-rich repeat 5–7 competes for collagen binding.Crossref | GoogleScholarGoogle Scholar |
Kalamajski, S., Aspberg, A., Lindblom, K., Heinegard, D., and Oldberg, A. (2009). Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization. Biochem. J. 423, 53–59.
| Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization.Crossref | GoogleScholarGoogle Scholar |
Kirsch, T., Wellner, M., Luft, F. C., Haller, H., and Lippoldt, A. (2001). Altered gene expression in cerebral capillaries of stroke-prone spontaneously hypertensive rats. Brain Res. 910, 106–115.
| Altered gene expression in cerebral capillaries of stroke-prone spontaneously hypertensive rats.Crossref | GoogleScholarGoogle Scholar |
Kobayashi, H., Sun, W. G., and Terao, T. (1999). Immunolocalization of hyaluronic acid and inter-alpha-trypsin inhibitor in mice. Cell Tissue Res. 296, 587–597.
| Immunolocalization of hyaluronic acid and inter-alpha-trypsin inhibitor in mice.Crossref | GoogleScholarGoogle Scholar |
Kuo, H.-J., Maslen, C. L., Keene, D. R., and Glanville, R. W. (1997). Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem. 272, 26522–26529.
| Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen.Crossref | GoogleScholarGoogle Scholar |
Li, Z., and Huang, H. (2008). Epigenetic abnormality: a possible mechanism underlying the fetal origin of polycystic ovary syndrome. Med. Hypotheses 70, 638–642.
| Epigenetic abnormality: a possible mechanism underlying the fetal origin of polycystic ovary syndrome.Crossref | GoogleScholarGoogle Scholar |
Lind, A. K., Weijdegard, B., Dahm-Kahler, P., Molne, J., Sundfeldt, K., and Brannstrom, M. (2006). Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstet. Gynecol. Scand. 85, 1476–1484.
| Collagens in the human ovary and their changes in the perifollicular stroma during ovulation.Crossref | GoogleScholarGoogle Scholar |
Loverro, G., De Pergola, G., Di Naro, E., Tartagni, M., Lavopa, C., and Caringella, A. M. (2010). Predictive value of ovarian stroma measurement for cardiovascular risk in polycyctic ovary syndrome: a case control study. J. Ovarian Res. 3, 25.
| Predictive value of ovarian stroma measurement for cardiovascular risk in polycyctic ovary syndrome: a case control study.Crossref | GoogleScholarGoogle Scholar |
Matti, N., Irving-Rodgers, H. F., Hatzirodos, N., Sullivan, T. R., and Rodgers, R. J. (2010). Differential expression of focimatrix and steroidogenic enzymes before size deviation during waves of follicular development in bovine ovarian follicles. Mol. Cell. Endocrinol. 321, 207–214.
| Differential expression of focimatrix and steroidogenic enzymes before size deviation during waves of follicular development in bovine ovarian follicles.Crossref | GoogleScholarGoogle Scholar |
McArthur, M. E., Irving-Rodgers, H. F., Byers, S., and Rodgers, R. J. (2000). Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol. Reprod. 63, 913–924.
| Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles.Crossref | GoogleScholarGoogle Scholar |
Niculescu, M., Novac, L., Mateescu, G. O., Mihail, S. R., Neamtu, S., and Papachristu, A. (2011). Original study the vasculogenesis of the fetal ovary – morphological and immunohistochemical study. Analele Universitatii “Dunarea De Jos” Galati Medicina 17, 5–9.
Oksjoki, S., Sallinen, S., Vuorio, E., and Anttila, L. (1999). Cyclic expression of mRNA transcripts for connective tissue components in the mouse ovary. Mol. Hum. Reprod. 5, 803–808.
| Cyclic expression of mRNA transcripts for connective tissue components in the mouse ovary.Crossref | GoogleScholarGoogle Scholar |
Paranko, J. (1987). Expression of type I and III collagen during morphogenesis of fetal rat testis and ovary. Anat. Rec. 219, 91–101.
| Expression of type I and III collagen during morphogenesis of fetal rat testis and ovary.Crossref | GoogleScholarGoogle Scholar |
Prodoehl, M. J., Irving-Rodgers, H. F., Bonner, W. M., Sullivan, T. M., Micke, G. C., Gibson, M. A., Perry, V. E., and Rodgers, R. J. (2009). Fibrillins and latent TGFbeta binding proteins in bovine ovaries of offspring following high or low protein diets during pregnancy of dams. Mol. Cell. Endocrinol. 307, 133–141.
| Fibrillins and latent TGFbeta binding proteins in bovine ovaries of offspring following high or low protein diets during pregnancy of dams.Crossref | GoogleScholarGoogle Scholar |
Robinson, R. S., Woad, K. J., Hammond, A. J., Laird, M., Hunter, M. G., and Mann, G. E. (2009). Angiogenesis and vascular function in the ovary. Reproduction 138, 869–881.
| Angiogenesis and vascular function in the ovary.Crossref | GoogleScholarGoogle Scholar |
Roy, S. K., and Kole, A. R. (1998). Ovarian transforming growth factor-b (TGF-b) receptors: in vitro effects of follicle stimulating hormone, epidermal growth factor and TGF-b on receptor expression in human preantral follicles. Mol. Hum. Reprod. 4, 207–214.
| Ovarian transforming growth factor-b (TGF-b) receptors: in vitro effects of follicle stimulating hormone, epidermal growth factor and TGF-b on receptor expression in human preantral follicles.Crossref | GoogleScholarGoogle Scholar |
Rozen, S., and Skaletsky, H. J. (2000). Primer3 on the WWW for general users and for biologist programmers. In ‘Bioinformatics methods and protocols: methods in molecular biology’. (Eds S. Krawetz and S. Misener.) pp. 365–386. (Humana Press: Totowa, NJ, USA.)
Russe, I. (1983). Oogenesis in cattle and sheep. Bibl. Anat 24, 77–92.
Sabatelli, P., Bonaldob, P., Lattanzia, G., Braghettab, P., Bergaminb, N., Capannic, C., Mattiolid, E., Columbarod, M., Ognibenec, A., Pepee, G., Bertinif, E., Merlinid, L., Maraldia, N. M., and Squarzonia, S. (2001). Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biol. 20, 475–486.
| Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts.Crossref | GoogleScholarGoogle Scholar |
Santos, S. S., Ferreira, M. A., Pinto, J. A., Sampaio, R. V., Carvalho, A. C., Silva, T. V., Costa, N. N., Cordeiro, M. S., Miranda, M. S., Ribeiro, H. F., and Ohashi, O. M. (2013). Characterization of folliculogenesis and the occurrence of apoptosis in the development of the bovine fetal ovary. Theriogenology 79, 344–350.
| Characterization of folliculogenesis and the occurrence of apoptosis in the development of the bovine fetal ovary.Crossref | GoogleScholarGoogle Scholar |
Sarraj, M. A., and Drummond, A. E. (2012). Mammalian foetal ovarian development: consequences for health and disease. Reproduction 143, 151–163.
| Mammalian foetal ovarian development: consequences for health and disease.Crossref | GoogleScholarGoogle Scholar |
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., and Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682.
| Fiji: an open-source platform for biological-image analysis.Crossref | GoogleScholarGoogle Scholar |
Sforza, C., Ferrario, V. F., De Pol, A., Marzona, L., Forni, M., and Forabosco, A. (1993). Morphometric study of the human ovary during compartmentalization. Anat. Rec. 236, 626–634.
| Morphometric study of the human ovary during compartmentalization.Crossref | GoogleScholarGoogle Scholar |
Smith, P., Wilhelm, D., and Rodgers, R. J. (2014). Development of mammalian ovary. J. Endocrinol. 221, R145–R161.
| Development of mammalian ovary.Crossref | GoogleScholarGoogle Scholar |
Spencer, J. A., Hacker, S. L., Davis, E. C., Mecham, R. P., Knutsen, R. H., Li, D. Y., Gerard, R. D., Richardson, J. A., Olson, E. N., and Yanagisawa, H. (2005). Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl. Acad. Sci. USA 102, 2946–2951.
| Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration.Crossref | GoogleScholarGoogle Scholar |
Wandji, S. A., Sršeň, V., Voss, A. K., Eppig, J. J., and Fortune, J. E. (1996). Initiation in vitro of growth of bovine primordial follicles 1. Biol. Reprod. 55, 942–948.
| Initiation in vitro of growth of bovine primordial follicles 1.Crossref | GoogleScholarGoogle Scholar |
Weng, Q., Wang, H., Medan, M. S., Jin, W., Xia, G., Watanabe, G., and Taya, K. (2006). Expression of inhibin-activin subunits in the ovaries of fetal and neonatal mice. J. Reprod. Dev. 52, 607–616.
| Expression of inhibin-activin subunits in the ovaries of fetal and neonatal mice.Crossref | GoogleScholarGoogle Scholar |