Doppler ultrasound can be used to monitor umbilical arterial blood flow in lightly sedated pigs at multiple gestational ages
Claire Stenhouse A , Peter Tennant A , W. Colin Duncan B and Cheryl J. Ashworth A CA Developmental Biology Division, The Roslin Institute and Royal (Dick) School of Veterinary Studies, University of Edinburgh, Easter Bush Campus, Edinburgh, EH25 9RG, UK.
B Centre for Reproductive Health, The Queen’s Medical Research Institute, University of Edinburgh, Edinburgh, EH16 4TJ, UK.
C Corresponding author. Email: cheryl.ashworth@roslin.ed.ac.uk
Reproduction, Fertility and Development 30(11) 1402-1411 https://doi.org/10.1071/RD17298
Submitted: 28 July 2017 Accepted: 28 March 2018 Published: 4 May 2018
Journal Compilation © CSIRO 2018 Open Access CC BY
Abstract
Doppler ultrasound was performed under moderate sedation (ketamine and azaperone) for 30 min to monitor umbilical arterial (UA) blood flow in one uterine horn of Large White × Landrace gilts (n = 23) at Gestational Days (GD) 30, 45, 60 and 90. Gilts were scanned before they were killed to examine relationships between litter size, sex ratio and five UA parameters (peak systolic velocity (PSV), end diastolic velocity (EDV), A/B (PSV to EDV) ratio, fetal heart rate (FHR) and resistance index (RI)). In gilts in which scans were obtained from all fetuses in the scanned horn, relationships between UA parameters, and fetal weight and sex were examined. A subset of gilts were sedated, scanned and recovered (SSR) earlier in gestation (GD30 or GD45) to assess the effects of sedation on later fetal development by comparison with control litters (no previous sedation). Temporal changes were observed in all UA parameters (P ≤ 0.001). At GD60 and GD90, FHR decreased with increasing duration of sedation (P ≤ 0.001). Sex ratio and fetal weight affected UA blood flow, whereas litter size and fetal sex did not. SSR at GD30 and GD45 was associated with decreased fetal weight at GD60 (P ≤ 0.001) and GD90 (P = 0.06) respectively, compared with controls. These results suggest maternal sedation during gestation affects fetal development, which should be investigated further. Measuring UA blood flow in growth-restricted porcine fetuses throughout gestation may be feasible.
Additional keywords: gestation, gilt, sedation.
Introduction
Increased litter size has been a focus of recent pig genetic selection programs as an effective means to increase the profitability of the pig industry (Bee 2007). This has increased the within-litter variation in birthweight observed, with many litters containing an intrauterine growth-restricted (IUGR) piglet (Ashworth et al. 2001; Wu et al. 2006; Bee 2007; Vallet et al. 2014; Yuan et al. 2015). The decrease in birthweight observed in the IUGR piglet has severe consequences for postnatal development, with several disadvantageous phenotypes observed, including impaired adaptation to extrauterine life, a permanently reduced growth rate, altered organ growth and development, decreased reproductive performance and increased disease susceptibility (Wu et al. 2006).
It is essential that the umbilical cord and placenta function efficiently throughout gestation to support the growing fetus, with inadequate placental function being associated with adverse pregnancy outcomes, including pre-eclampsia and IUGR (Baschat 2003). It is hypothesised that due to the placental insufficiency associated with IUGR, the fetus redirects available blood flow to its most vital organs, namely the brain and heart, to spare their growth at the expense of other organs (Giussani 2011). In human clinical practice, non-invasive Doppler ultrasound is widely used to identify and monitor umbilical arterial (UA) blood flow in IUGR pregnancies, ensuring that clinical interventions occur at the optimal time (Detti et al. 2002). The waveforms produced by Doppler ultrasound can be used to calculate several parameters, including peak systolic velocity (PSV), end diastolic velocity (EDV), the A/B ratio (or PSV to EDV (S/D) ratio), resistance index (RI) and the pulsatility index (PI; Thompson et al. 1988; Berkley et al. 2012). These parameters fall into two categories: angle dependent and angle independent. Velocity measurements are categorised as angle-dependent measurements, whereby to obtain a truly accurate measurement of the velocity present, the angle of the ultrasound beam needs to be as close to 0° as possible (McDicken 1991; Detti et al. 2002). As the angle increases, the accuracy of determining the velocity decreases. Considering this, angle-independent parameters, such as the A/B ratio, RI and PI, are commonly used in clinical practice (Trudinger et al. 1985; Detti et al. 2002; Berkley et al. 2012). These parameters provide an indication of vascular resistance, which, in turn, provides information regarding arterial blood flow and placental vascular resistance.
In large animals, Doppler ultrasound has been used to investigate uterine, umbilical and fetal vessels in cattle (Bollwein et al. 2002; Panarace et al. 2006; Silva and Ginther 2010), goats (Serin et al. 2010; Elmetwally et al. 2016), horses (Bollwein et al. 2000, 2004) and sheep (Elmetwally et al. 2016). There have also been limited investigations into gestational changes in UA blood flow in the pig (Brüssow et al. 2012; Harris et al. 2013). It has been suggested that an invasive laparoscopic approach is the most effective method to monitor UA blood flow in the pig (Brüssow et al. 2012), although the effect of general anaesthesia is unknown. In addition, the relationship between UA blood flow and fetal growth and development in the pig is poorly understood.
The aim of the present study was to relate several parameters (including litter size, fetal weight and sex and the sex ratio of the litter) to UA blood flow parameters throughout gestation in the pig, in a non-invasive manner, under light sedation. In addition, this protocol was used to monitor gestational changes within the same pregnancy, with an additional objective of investigating the effect of light sedation in early gestation on later fetal development.
Materials and methods
All procedures were performed with approval from The Roslin Institute (University of Edinburgh) Animal Welfare and Ethical Review Board and in accordance with the UK Animals (Scientific Procedures) Act, 1986.
Experimental animals
In all, 23 Large White × Landrace gilts (age 11–14 months; mean weight at death 188.76 kg) were included in this study. All gilts were observed daily for signs of oestrus and were housed six to eight animals per pen, unless stated otherwise. Oestrous cyclicity and ovarian function were controlled in accordance with routine normal practice at The Roslin Institute Large Animal Unit. In a subset of gilts (n = 5), oestrus was synchronised by daily feeding of 20 mg altrenogest (Regumate; Hoechst Roussel Vet) for 18 days followed by injection of pregnant mare’s serum gonadotrophin (PMSG) and human chorionic gonadotrophin (hCG), as described by Lillico et al. (2013). All gilts were inseminated twice daily for the duration of oestrus with semen from one of two sires (Large White). The first day of insemination was assigned as Gestational Day (GD) 0. The average ovulation rate (determined by the number of corpora lutea present) and percentage prenatal survival (determined as the number of live fetuses present divided by the ovulation rate multiplied by 100) was 20.5% and 66.75% respectively.
Doppler ultrasound procedure
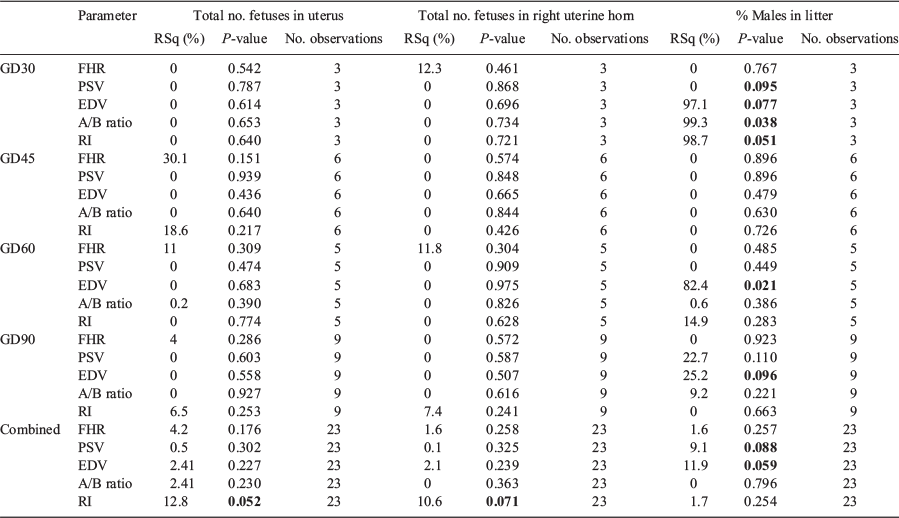
Gilts were sedated by intramuscular injection with 1 mg/kg ketamine (Henry Schein Animal Health) and 2 mg/kg azaperone (Henry Schein Animal Health). Once the animals were sedated (~10–15 min after drug administration), they were positioned in lateral recumbency on their left side. The presumed right uterine horn was scanned by a single operator from the cervix towards the ovary using a Philips iU22 colour Doppler ultrasound machine, with an L9-3 transducer probe (Philips Healthcare). The scanning procedure lasted for approximately 30 min. UA traces were obtained from the fetuses and the following parameters were measured: fetal heart rate (FHR), PSV, EDV, RI and the A/B ratio (PSV : EDV). The number of fetuses and waveform data collected are summarised in Table 1.
Experiment 1: temporal changes in UA blood flow
Pregnant gilts were used in this study at GD30 (range GD29–30; n = 3), GD45 (range GD43–46; n = 6), GD60 (range GD60–61; n = 5) and GD90 (range GD89–92; n = 9). Following completion of the scanning procedure, the gilts were killed with 0.4 mL kg−1 of 20% w/v sodium pentobarbitone (Henry Schein Animal Health), injected intravenously via a cannula inserted in the ear vein.
A subset of pregnant gilts at GD60 (n = 4) and GD90 (n = 3) were sedated, scanned and allowed to regain consciousness (recovered; SSR) at GD30 (range GD29–33; n = 4) and GD45 (range GD43–44; n = 3) respectively. The sedation and scanning procedure was performed as described above. Following this, sedated gilts were hoisted onto a surgical trolley and transferred to a recovery pen. Once the animals had fully recovered, they were transferred to a shed where they were housed individually, in close proximity to other pregnant gilts, for the remainder of the experiment. These animals were killed immediately after the second scanning procedure. To assess temporal changes in UA blood flow, the SSR traces at GD30 and GD45 were combined with those of the killed animals (GD30, n = 7 litters; GD45, n = 9 litters; GD60, n = 5 litters; GD90, n = 9 litters).
Experiment 2: effect of litter size and sex ratio of the litter on UA blood flow
Following confirmation of death, the reproductive tract was revealed by a mid-ventral incision . The tract was lifted from the body cavity and placed on a dissecting tray. Both uterine horns were dissected, from the ovaries towards the cervix, allowing determination of whether traces were obtained from all fetuses in the right uterine horn. All fetuses were identified as ‘live’ or ‘dead’ based on their morphology at the time of dissection. Fetuses were weighed and, at GD45, GD60 and GD90, fetal sex was determined morphologically. DNA was isolated from the GD30 fetuses using the DNeasy Blood and Tissue DNA extraction kit (Qiagen), and polymerase chain reaction (PCR) was performed for the SRY region of the Y chromosome (forward primer, 5′-GAAAGCGGACGATTACAGCC-3′; reverse primer, 5′-TTGCGACGAGGTCGGTATTT-3′). Primers for the homologous zfx/zfy regions of the sex chromosomes served as a positive control (forward primer 5′-ATAATCACATGGAGAGCCACAAGCT-3′; reverse primer 5′-GCACTTCTTTGGTATCTGAGAAAGT-3′; Oliver et al. 2011). For the reaction, 7μL nuclease-free water, 5 μL of 10× PCR Buffer, 1 μL dNTP mix (10 mM), 1 μL FastStart Taq (all solutions from the Roche FastStart Taq kit; Sigma Aldrich) and 5 μL of both forward and reverse primers (5 μM stock) were added to 1 µg sample DNA. A no-template control and postnatal porcine testicular and ovarian DNA were run as controls. The PCR conditions had an initial step of 95°C for 4 min 30 s, which was followed by 30 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 30 s, with a final extension at 72°C for 4 min 30 s and an indefinite hold at 4°C. Samples were processed in 96-well microplates (NUNC; Thermofisher) on a 96-well thermocycler (Biometra). The PCR products were then analysed on a 1.2% (w/v) agarose 1× SybrSafe (Thermofisher) gel with 1× Tris–acetic acid–EDTA buffer. This allowed the results to be related to the number of live fetuses and the sex ratio of the litter at all GD.
Experiment 3: effects of fetal size and sex on UA blood flow
As described in Experiment 2, fetal weight and sex was recorded for all fetuses in all killed litters at all GD investigated. In a subset of the killed GD90 litters (n = 4), UA waveforms were obtained from all fetuses in the right uterine horn, which allowed the parameters obtained to be related to fetal size and sex.
Experiment 4: effect of sedation
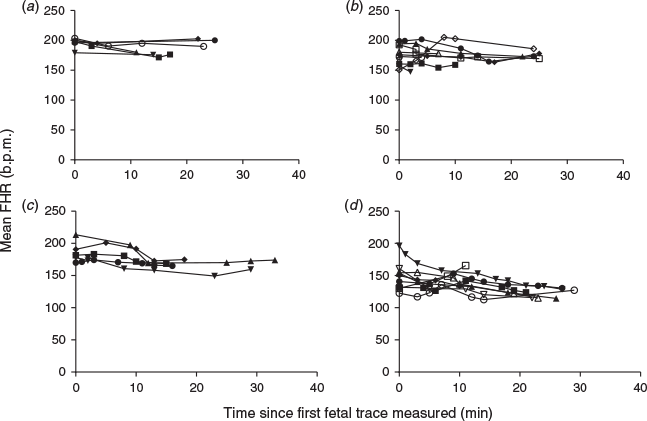
The difference in time since the first fetal trace measurement to each subsequent trace measured was calculated within gilt to determine whether the fetuses were affected by maternal sedation over the scanning period. The mean FHR for each fetus was then related to the time since the first fetal trace measurement. To determine whether the sedation in early gestation for the SSR procedure affected fetal development later in gestation, fetal weight, the number of live fetuses in the uterus and the sex ratio of the litters in the SSR gilts were compared with GD-matched control gilts that had not been subjected to the SSR procedure (n = 8 and 4 control and SSR GD60 litters respectively; n = 6 and 3 control and SSR GD90 litters respectively).
Statistical analysis
All statistical analyses were performed using Minitab 17 or GenStat 13.1 (VSN International). The mean value for each parameter was calculated for each fetus and normality was assessed. Outliers were tested for using a Grubbs outlier test and were excluded systematically, with normality within each group being reassessed following each exclusion. Log-transformed data were used for PSV at GD45, GD60 and GD90, as well as for EDV at GD45 and GD90, to improve the distribution of the data. Non-parametric tests were used whenever the data were found to be not normally distributed after transformation.
Temporal changes in FHR were analysed by one-way analysis of variance (ANOVA) followed by Tukey analysis. The gilt term was blocked to account for the common maternal environment. For the remainder of the parameters, where the data did not have a normal distribution with the four GD combined, a Kruskal–Wallis test was used to investigate temporal changes. Linear regressions were performed within GD for all parameters against the number of live fetuses and percentage of males in the litter (sex ratio). Two-sample t-tests were performed to determine whether fetal sex affected any of the parameters investigated. A restricted maximum likelihood (REML) variance component analysis was performed with gilt fitted as a random effect to identify the effects of fetal weight on FHR, PSV, EDV, A/B ratio and RI. The difference in time from the first fetal trace measurement to each subsequent trace measured was calculated within gilt. The results were grouped by GD and gilt was fitted as a random effect in a REML variance component analysis to account for the variation attributed to gilt within GD. Similarly, two-way ANOVA (GD × treatment (SSR or control)) with a block for gilt was performed to compare the number of live fetuses, the percentage of males in the litter and the s.d. in fetal weight in SSR and control litters. The weight of the SSR and control fetuses were compared within GD (Mann–Whitney at GD60; ANOVA with gilt block at GD90) and fetal weight was then compared within sex (two-way ANOVA (sex × treatment) with gilt block). In all cases, P ≤ 0.05 was considered significant. Data are presented as the mean ± s.e.m.
Results
Experiment 1: temporal changes in FHR, PSV, EDV, RI and A/B ratio
UA waveforms were obtained at all GD studied (Fig. 1). Temporal changes were observed in all parameters investigated, with a decrease in FHR, A/B ratio and RI (all P ≤ 0.001), and an increase in PSV and EDV (all P ≤ 0.001; Fig. 2) with advancing gestation. FHR decreased gradually with advancing gestation, but the largest differences in the other parameters occurred between GD60 and GD90, reflecting the presence of diastolic blood flow at GD90 but not at earlier GD.
Experiment 2: effects of litter sex ratio and litter size on UA blood flow
The number of fetuses in the litter or right uterine horn did not affect any of the parameters investigated within GD (Table 2). A negative relationship between the percentage of males in the litter and EDV at GD30 (P = 0.077) and GD60 (P ≤ 0.05) was observed. In addition, a positive relationship was observed at GD30 between the percentage of males in the litter and both the A/B ratio (P ≤ 0.05) and RI (P ≤ 0.05).
Experiment 3: effects of fetal weight and sex on UA blood flow
In four of the GD90 litters, UA waveforms were obtained from all fetuses in the right uterine horn, allowing relationships between UA parameters and both fetal size and sex to be explored. FHR did not differ significantly between male (n = 16) and female (n = 10) fetuses (135.0 ± 3.0 and 132.9 ± 3.2 b.p.m. respectively; Fig. 3a). Similarly, no differences were observed between male (n = 16) and female (n = 9) fetuses in PSV (69.29 ± 6.54 and 81.45 ± 7.88 cm s−1 respectively) or EDV (15.03 ± 2.24 and 16.54 ± 1.88 cm s−1 respectively; Fig. 3b, c). Sex did not affect the A/B ratio (5.62 ± 0.62 and 5.12 ± 0.45 in male (n = 16) and female (n = 9) fetuses respectively) or RI (0.79 ± 0.02 and 0.79 ± 0.02 respectively; Fig. 3d, e). Fetal weight was negatively related to FHR (P = 0.089), A/B ratio (P ≤ 0.05) and RI (P ≤ 0.05).
Experiment 4: effects of sedation on FHR and later fetal development
A significant decrease in FHR over the sedation period was observed at GD60 and GD90 (P ≤ 0.001), but not at GD30 or GD45 (Fig. 4). The mean decrease in FHR at GD30, GD45, GD60 and GD90 was 8.7 ± 4.6, 5.3 ± 6.2, 18.4 ± 5.7 and 20.6 ± 10.3 b.p.m. respectively. The SSR process at GD30 and GD45 did not affect the number of live fetuses or the sex ratio of the litter at GD60 and GD90 compared with control fetuses whose dams had not been sedated previously. Intriguingly, fetal weight was significantly lower in GD60 SSR fetuses compared with control GD60 fetuses (P ≤ 0.001; Fig. 5a, c). A similar trend was observed at GD90 (P = 0.061; Fig. 5b, d). Analysis of the s.d. in fetal weight within the litter revealed a lower s.d. in SSR litters compared with controls at GD90, but not at GD60 (P ≤ 0.05; Fig. 5e).

|
Discussion
The broad objective of the present study was to determine whether non-invasive Doppler ultrasound could be used to monitor UA blood flow in pregnant gilts under light sedation. To our knowledge, this is the first report of the use of Doppler ultrasound at this early stage of gestation in the pig. This method was successfully used to obtain traces at all GD analysed, and highlighted novel temporal changes in all parameters investigated. The length of the sedation period affected FHR at GD60 and GD90, but not earlier in gestation, suggesting that fetuses in late gestation may be more affected by maternal sedation, albeit with modest numbers. Pregnant gilts were subjected to an SSR procedure to allow measurements on two GD within the same litter. Interestingly, the most striking finding from the present study was that approximately 30 min light sedation in early gestation decreased fetal weight in later gestation, highlighting surprising potential long-term programming effects that warrant further investigation.
Similar temporal changes in UA blood flow parameters have been reported in sheep and goats (Serin et al. 2010; Carr et al. 2012). The two published studies describing pig UA blood flow parameters (Brüssow et al. 2012; Harris et al. 2013) both reported a decrease in FHR as gestation proceeded, but only Harris et al. (2013) observed temporal changes in RI and PI between GD39 and GD81. Changes in FHR observed in both studies follow a similar pattern to those seen in the present study, but not all parameters studied exhibited the same temporal trends in the three studies. The three experiments were performed under different sedation conditions, with Harris et al. (2013) using non-sedated gilts, ‘rubbing’ the udder of the gilts to produce a ‘calming’ effect in the animals, whereas Brüssow et al. (2012) performed the procedure by scanning the exposed uterus directly while the pig was under general anaesthesia. Comparison of the mean FHR values reported in these studies with the values obtained in the present experiment suggests that increasing the level of sedation decreases the mean FHR observed at all stages of gestation studied.
The sex ratio of pig litters is an area of significant interest to the pig industry, with gilts from female-biased litters having an increased number of teats, a higher fertility rate and an increased conception rate on their first breeding attempt than gilts from male-biased litters (Drickamer et al. 1997; Górecki 2003). It has also been proposed that male piglets are disadvantaged, with male piglets being crushed by the sow more than females, more males dying from disease-related causes than females and males having impaired thermoregulation compared with females (Baxter et al. 2012). In the present study, the percentage of males in the litter affected EDV, the A/B ratio and RI, highlighting that the sex ratio of the litter could affect UA blood flow. Recent studies have revealed that sexual dimorphism exists in human placentas (for reviews, see Adibi et al. 2017; Kalisch-Smith et al. 2017). In addition, it has been shown that male babies have increased perinatal mortality and morbidity when human pregnancy is complicated by pre-eclampsia and IUGR (Challis et al. 2013; Muralimanoharan et al. 2013). Similarly, fetal sex has been shown to affect the expression of placental genes and the inflammatory response in humans, which is thought to be attributed to sexual dimorphism in placental function (Ghidini and Salafia 2005; Challis et al. 2013). It has also been shown in the pig that placental and endometrial gene expression and vascularity are associated with both fetal size and sex (C. Stenhouse, C. O. Hogg and C. J. Ashworth, unpubl. obs.). Widnes et al. (2017) reported that in humans, UA PI is increased in females compared with males, although these authors did not suggest that fetal sex affected any of the other parameters investigated. Therefore, if differences are observed in placental and endometrial tissue supplying fetuses of different sex, this may indicate that the uterine environment of a male-biased litter would be very different to that of a female-biased litter, which may explain the relationships between the sex ratio of the litter and UA blood flow parameters measured in the present study.
Surprisingly, light sedation in early gestation decreased fetal weight in later gestation. This, alongside the decrease in FHR over the sedation period, suggests that sedation during gestation could have significant effects on fetal development. The mechanisms behind this decrease in fetal weight warrant much further investigation. It has previously been suggested that azaperone has a vasodilatory effect, and can therefore result in a decrease in blood pressure and body temperature (Van Woerkens et al. 1990; Hall and Clarke 1991; Brodbelt and Taylor 1999; Hodgkinson 2007). Although care was taken to ensure that the gilts were kept warm throughout the process, temperature and blood pressure were not monitored in the present study. In addition, there is some evidence suggesting that azaperone can promote activation of the hypothalamic–pituitary–adrenal (HPA) axis. Daş et al. (2016) investigated the effects of azaperone- and azaperone plus ketamine-induced general anaesthesia on plasma cortisol levels in pigs. Administration of azaperone led to a prolonged and significant increase in plasma cortisol within 45 min of drug administration for the remaining 255 min of the experiment, and this effect was increased further with the addition of ketamine (Daş et al. 2016). This may indicate that cortisol mediates the observed physiological effects of azaperone, suggesting that azaperone may promote an increased stress response in pigs.
Considering this information, it could be hypothesised that sedation with azaperone and ketamine promoted a decrease in body temperature and blood pressure, activating the maternal HPA axis and increasing cortisol production by the adrenal cortex. Although cortisol is metabolised by 11β-hydroxysteroid dehydrogenase 2 into inactive corticosterone at the placenta from GD24 in the pig (Klemcke 2003), not all the available cortisol will be converted into inactive corticosterone, leading to an increase in the levels of maternal cortisol crossing the placenta to affect the fetus. This would ultimately act as a stressful environment for the fetus, leading to the observed decreased fetal weight in late gestation, and potentially having effects postnatally. Interestingly, it has previously been suggested that there is an inverse relationship between fetal plasma cortisol and fetal size (Klemcke and Christenson 1997), indicating that lighter fetuses may have a higher baseline stress response. To test the proposed mechanism of action in the future, it would be useful to monitor maternal rectal and surface temperature, blood pressure and salivary cortisol at regular intervals throughout the Doppler process.
In conclusion, we have demonstrated that Doppler ultrasound can be used to non-invasively monitor UA blood flow in the pregnant pig from GD30. Our results have indicated, for the first time, that fetal weight and the sex ratio of the litter could affect UA blood flow in the pig. Intriguingly, sedation with azaperone and ketamine had a significant effect on fetal development; the mechanisms and long-term effects of this warrant much further investigation and could have clinical relevance. It is hoped that further optimisation of Doppler ultrasound under light sedation would allow evaluation of UA blood flow parameters in relation to fetal growth and development, especially in IUGR fetuses.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank the staff of The Roslin Institute Large Animal Unit for skilled assistance, Eddie Clutton for helpful discussions and advice and Darren Shaw for statistical advice. The Roslin Institute receives Institute Strategic Grant funding from the Biotechnology and Biological Sciences Research Council (BBSRC) (BBS/E/D/30002276). C. Stenhouse is in receipt of a studentship from the University of Edinburgh.
References
Adibi, J., Burton, G. J., Clifton, V., Collins, S., Frias, A. E., Gierman, L., Grigsby, P., Jones, H., Lee, C., Maloyan, A., Markert, U. R., Morales-Prieto, D. M., Murthi, P., Myatt, L., Pollheimer, J., Roberts, V., Robinson, W., Salafia, C., Schabel, M., Shah, D., Sled, J., Vaillancourt, C., Weber, M., and O’Tierney-Ginn, P. F. (2017). IFPA meeting 2016 workshop report II: placental imaging, placenta and development of other organs, sexual dimorphism in placental function and trophoblast cell lines. Placenta 60, S10–S14.| IFPA meeting 2016 workshop report II: placental imaging, placenta and development of other organs, sexual dimorphism in placental function and trophoblast cell lines.Crossref | GoogleScholarGoogle Scholar |
Ashworth, C. J., Finch, A. M., Page, K. R., Nwagwu, M. O., and McArdle, H. J. (2001). Causes and consequences of fetal growth retardation in pigs. Reprod. Suppl. 58, 233–246.
| 1:STN:280:DC%2BD383kt1Smtw%3D%3D&md5=185a6ac3b16b1b6ac3406fa33f295252CAS |
Baschat, A. A. (2003). Integrated fetal testing in growth restriction: combining multivessel Doppler and biophysical parameters. Ultrasound Obstet. Gynecol. 21, 1–8.
| Integrated fetal testing in growth restriction: combining multivessel Doppler and biophysical parameters.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s%2FotFantw%3D%3D&md5=59164e6694ba72ea165ece6296d27a26CAS |
Baxter, E. M., Jarvis, S., Palarea-Albaladejo, J., and Edwards, S. A. (2012). The weaker sex? The propensity for male-biased piglet mortality. PLoS One 7, e30318.
| The weaker sex? The propensity for male-biased piglet mortality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslKmt7c%3D&md5=33b7718199606ed68ecd79861f1e5152CAS |
Bee, G. (2007). Birth weight of litters as a source of variation in postnatal growth, and carcass and meat quality. Adv. Pork Prod. 18, 191–196.
Berkley, E., Chauhan, S. P., and Abuhamad, A. (2012). Doppler assessment of the fetus with intrauterine growth restriction. Am. J. Obstet. Gynecol. 206, 300–308.
| Doppler assessment of the fetus with intrauterine growth restriction.Crossref | GoogleScholarGoogle Scholar |
Bollwein, H., Meyer, H. H. D., Maierl, J., Weber, F., Baumgartner, U., and Stolla, R. (2000). Transrectal Doppler sonography of uterine blood flow in cows during the estrous cycle. Theriogenology 53, 1541–1552.
| Transrectal Doppler sonography of uterine blood flow in cows during the estrous cycle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvislCquw%3D%3D&md5=03aca0d7b60718ac99b3412d2a503ed3CAS |
Bollwein, H., Baumgartner, U., and Stolla, R. (2002). Transrectal Doppler sonography of uterine blood flow in cows during pregnancy. Theriogenology 57, 2053–2061.
| Transrectal Doppler sonography of uterine blood flow in cows during pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38zisVSqsA%3D%3D&md5=aed0fad1412b6ebf9a337c33e1b64557CAS |
Bollwein, H., Weber, F., Woschée, I., and Stolla, R. (2004). Transrectal Doppler sonography of uterine and umbilical blood flow during pregnancy in mares. Theriogenology 61, 499–509.
| Transrectal Doppler sonography of uterine and umbilical blood flow during pregnancy in mares.Crossref | GoogleScholarGoogle Scholar |
Brodbelt, D. C., and Taylor, P. M. (1999). Comparison of two combinations of sedatives before anaesthetising pigs with halothane and nitrous oxide. Vet. Rec. 145, 283–287.
| Comparison of two combinations of sedatives before anaesthetising pigs with halothane and nitrous oxide.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FksVWguw%3D%3D&md5=62ba628239c1ee3b1c41ea36ab683eabCAS |
Brüssow, K. P., Kurth, J., Vernunft, A., Becker, F., Tuchscherer, A., and Kanitz, W. (2012). Laparoscopy guided Doppler ultrasound measurement of fetal blood flow indices during early to mid-gestation in pigs. J. Reprod. Dev. 58, 243–247.
| Laparoscopy guided Doppler ultrasound measurement of fetal blood flow indices during early to mid-gestation in pigs.Crossref | GoogleScholarGoogle Scholar |
Carr, D. J., Aitken, R. P., Milne, J. S., David, A. L., and Wallace, J. M. (2012). Fetoplacental biometry and umbilical artery Doppler velocimetry in the overnourished adolescent model of fetal growth restriction. Am. J. Obstet. Gynecol. 207, 141.e6–15.
| Fetoplacental biometry and umbilical artery Doppler velocimetry in the overnourished adolescent model of fetal growth restriction.Crossref | GoogleScholarGoogle Scholar |
Challis, J., Newnham, J., Petraglia, F., Yeganegi, M., and Bocking, A. (2013). Fetal sex and preterm birth. Placenta 34, 95–99.
| Fetal sex and preterm birth.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3s3ktV2qsg%3D%3D&md5=a3f38d0b1a6b1a0773c0b5a90eabcd6bCAS |
Daş, G., Vernunft, A., Görs, S., Kanitz, E., Weitzel, J. M., Brüssow, K. P., and Metges, C. C. (2016). Effects of general anesthesia with ketamine in combination with the neuroleptic sedatives xylazine or azaperone on plasma metabolites and hormones in pigs. J. Anim. Sci. 94, 3229–3239.
| Effects of general anesthesia with ketamine in combination with the neuroleptic sedatives xylazine or azaperone on plasma metabolites and hormones in pigs.Crossref | GoogleScholarGoogle Scholar |
Detti, L., Akiyama, M., and Mari, G. (2002). Doppler blood flow in obstetrics. Curr. Opin. Obstet. Gynecol. 14, 587–593.
| Doppler blood flow in obstetrics.Crossref | GoogleScholarGoogle Scholar |
Drickamer, L. C., Arthur, R. D., and Rosenthal, T. L. (1997). Conception failure in swine: importance of the sex ratio of a female’s birth litter and tests of other factors. J. Anim. Sci. 75, 2192–2196.
| Conception failure in swine: importance of the sex ratio of a female’s birth litter and tests of other factors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXltl2itbk%3D&md5=055ff4c81ca48c61c7ec150dc076b41dCAS |
Elmetwally, M., Rohn, K., and Meinecke-Tillmann, S. (2016). Noninvasive color Doppler sonography of uterine blood flow throughout pregnancy in sheep and goats. Theriogenology 85, 1070–1079.
| Noninvasive color Doppler sonography of uterine blood flow throughout pregnancy in sheep and goats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC28ngtFOmsg%3D%3D&md5=a0723ea9cebb5a2a41486d26a77afd19CAS |
Ghidini, A., and Salafia, C. M. (2005). Gender differences of placental dysfunction in severe prematurity. BJOG 112, 140–144.
| Gender differences of placental dysfunction in severe prematurity.Crossref | GoogleScholarGoogle Scholar |
Giussani, D. A. (2011). The vulnerable developing brain. Proc. Natl Acad. Sci. USA 108, 2641–2642.
| The vulnerable developing brain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisVeks70%3D&md5=d07f27c3aaf2ae552f802a716698a790CAS |
Górecki, M. T. (2003). Sex ratio in litters of domestic pigs (Sus scrofa f. domestica Linnaeus, 1758). Biol. Lett. 40, 111–118.
Hall, L. W., and Clarke, K. W. (1991). Chapter 14: Anaesthesia of the pig. In ‘Veterinary Anaesthesia.’ 9th Edn. (Eds L. W. Hall and K. W. Clarke.) pp. 275–289. (Baillière Tindall: London.)
Harris, E. K., Berg, E. P., Berg, E. L., and Vonnahme, K. A. (2013). Effect of maternal activity during gestation on maternal behavior, fetal growth, umbilical blood flow, and farrowing characteristics in pigs. J. Anim. Sci. 91, 734–744.
| Effect of maternal activity during gestation on maternal behavior, fetal growth, umbilical blood flow, and farrowing characteristics in pigs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvVKnu7s%3D&md5=992860e10d54d1a6456cfd69018dc70cCAS |
Hodgkinson, O. (2007). Practical sedation and anaesthesia in pigs. In Pract. 29, 34–39.
| Practical sedation and anaesthesia in pigs.Crossref | GoogleScholarGoogle Scholar |
Kalisch-Smith, J. I., Simmons, D. G., Dickinson, H., and Moritz, K. M. (2017). Review: sexual dimorphism in the formation, function and adaptation of the placenta. Placenta 54, 10–16.
| Review: sexual dimorphism in the formation, function and adaptation of the placenta.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC1c%2FkvFCnug%3D%3D&md5=c0911e8f6f392623ce7596cf69f4f348CAS |
Klemcke, H. G. (2003). 11-Hydroxysteroid dehydrogenase and glucocorticoid receptor messenger RNA expression in porcine placentae: effects of stage of gestation, breed, and uterine environment. Biol. Reprod. 69, 1945–1950.
| 11-Hydroxysteroid dehydrogenase and glucocorticoid receptor messenger RNA expression in porcine placentae: effects of stage of gestation, breed, and uterine environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsVCnsL0%3D&md5=30d17ab7592adc90461ca57c3a33ce57CAS |
Klemcke, H. G., and Christenson, R. K. (1997). Porcine fetal and maternal adrenocorticotropic hormone and corticosteroid concentrations during gestation and their relation to fetal size. Biol. Reprod. 57, 99–106.
| Porcine fetal and maternal adrenocorticotropic hormone and corticosteroid concentrations during gestation and their relation to fetal size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXktFCmtLw%3D&md5=46bc5fc97a11c64e64289a4030845ba1CAS |
Lillico, S. G., Proudfoot, C., Carlson, D. F., Stverakova, D., Neil, C., Blain, C., King, T. J., Ritchie, W. A., Tan, W., Mileham, A. J., McLaren, D. G., Fahrenkrug, S. C., and Whitelaw, C. B. A. (2013). Live pigs produced from genome edited zygotes. Sci. Rep. 3, 2847.
| Live pigs produced from genome edited zygotes.Crossref | GoogleScholarGoogle Scholar |
McDicken, W. N. (1991). ‘Diagnostic Ultrasonics – Principles and Use of Instruments.’ 3rd edn. (Churchill Livingstone: Edinburgh.)
Muralimanoharan, S., Maloyan, A., and Myatt, L. (2013). Evidence of sexual dimorphism in the placental function with severe preeclampsia. Placenta 34, 1183–1189.
| Evidence of sexual dimorphism in the placental function with severe preeclampsia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1OrtLjN&md5=80bda9339bf76176e45bce1f85e6c8baCAS |
Oliver, G., Novak, S. A., Patterson, J. L. A., Pasternak, J. A. A., and Paradis, F. A. (2011). Restricted feed intake in lactating primiparous sows. II. Effects on subsequent litter sex ratio and embryonic gene expression. Reprod. Fertil. Dev. 23, 899–911.
| Restricted feed intake in lactating primiparous sows. II. Effects on subsequent litter sex ratio and embryonic gene expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVOrtLfF&md5=544408962895bf6716779f650b3c1451CAS |
Panarace, M., Garnil, C., Marfil, M., Jauregui, G., Lagioia, J., Luther, E., and Medina, M. (2006). Transrectal Doppler sonography for evaluation of uterine blood flow throughout pregnancy in 13 cows. Theriogenology 66, 2113–2119.
| Transrectal Doppler sonography for evaluation of uterine blood flow throughout pregnancy in 13 cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nmsVequg%3D%3D&md5=0cee8563a7bbb62b8b58c96b1e5bbc14CAS |
Serin, G., Gökdal, Ö., Tarimcilar, T., and Atay, O. (2010). Umbilical artery Doppler sonography in Saanen goat fetuses during singleton and multiple pregnancies. Theriogenology 74, 1082–1087.
| Umbilical artery Doppler sonography in Saanen goat fetuses during singleton and multiple pregnancies.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cjpvFyiug%3D%3D&md5=91247289b450d16b72af2fda808ca138CAS |
Silva, L. A., and Ginther, O. J. (2010). Local effect of the conceptus on uterine vascular perfusion during early pregnancy in heifers. Reproduction 139, 453–463.
| Local effect of the conceptus on uterine vascular perfusion during early pregnancy in heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitFKitLc%3D&md5=2afd4fea11bc19bd81ad7ab059e75f77CAS |
Thompson, R. S., Trudinger, B. J., and Cook, C. M. (1988). Doppler ultrasound waveform indices: A/B ratio, pulsatility index and Pourcelot ratio. Br J Obstet Gynaecol 95, 581–588.
| Doppler ultrasound waveform indices: A/B ratio, pulsatility index and Pourcelot ratio.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c3mvFClsw%3D%3D&md5=8e659836f46d0dee7bfdfbbdb6f1dc0dCAS |
Trudinger, B. J., Giles, W. B., Cook, C. M., Bombardieri, J., and Collins, L. (1985). Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. Br J Obstet Gynaecol 92, 23–30.
| Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M%2FpslOjtw%3D%3D&md5=34f3959b17a0b371a9d8bd09f3aa8768CAS |
Vallet, J. L., McNeel, A. K., Miles, J. R., and Freking, B. A. (2014). Placental accommodations for transport and metabolism during intra-uterine crowding in pigs. J. Anim. Sci. Technol. 5, 55.
| Placental accommodations for transport and metabolism during intra-uterine crowding in pigs.Crossref | GoogleScholarGoogle Scholar |
Widnes, C., Flo, K., and Acharya, G. (2017). Exploring sexual dimorphism in placental circulation at 22–24 weeks of gestation: a cross-sectional observational study. Placenta 49, 16–22.
| Exploring sexual dimorphism in placental circulation at 22–24 weeks of gestation: a cross-sectional observational study.Crossref | GoogleScholarGoogle Scholar |
van Woerkens, L. J., Duncker, D. J., Huigen, R. J., Van Der Giessen, W. J., and Verdouw, P. D. (1990). Redistribution of cardiac output caused by opening of arteriovenous anastomoses by a combination of azaperone and metomidate. Br. J. Anaesth. 65, 393–399.
| Redistribution of cardiac output caused by opening of arteriovenous anastomoses by a combination of azaperone and metomidate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXlsFKhsQ%3D%3D&md5=bcc9c53c72f3861e6ca0747a77787d36CAS |
Wu, G., Bazer, F. W., Wallace, J. M., and Spencer, T. E. (2006). Board-invited review: intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84, 2316–2337.
| Board-invited review: intrauterine growth retardation: implications for the animal sciences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XovFGktLs%3D&md5=645c12369f244ac13e7b89419111577bCAS |
Yuan, T. L., Zhu, Y., Shi, M., Li, T., Li, N., Wu, G., Bazer, F. W., Zang, J., Wang, F., and Wang, J. (2015). Within-litter variation in birth weight: impact of nutritional status in the sow. J. Zhejiang Univ. Sci. B 16, 417–435.
| Within-litter variation in birth weight: impact of nutritional status in the sow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhtFCgsb7P&md5=481f0197f3502dd779cab186d4bc106fCAS |