Peri-conception and first trimester diet modifies reproductive development in bulls
K. J. Copping A F , M. D. Ruiz-Diaz B F , C. S. Rutland B , N. P. Mongan B , M. J. Callaghan C , I. C. McMillen D , R. J. Rodgers A and V. E. A. Perry B EA Robinson Research Institute, The University of Adelaide, Adelaide, SA 5005, Australia.
B School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, Leicestershire, LE12 5RD, UK.
C Ridley Agriproducts, Suite 4 Level 1, 49 Sherwood Road, Toowong, Qld 4066, Australia.
D The Chancellery, University of Newcastle, Callaghan, NSW 2308, Australia.
E Corresponding author. Email: viv.perry@nottingham.ac.uk
F Denotes joint first authorship.
Reproduction, Fertility and Development 30(5) 703-720 https://doi.org/10.1071/RD17102
Submitted: 17 March 2017 Accepted: 19 September 2017 Published: 16 November 2017
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Nutritional perturbation during gestation alters male reproductive development in rodents and sheep. In cattle both the developmental trajectory of the feto–placental unit and its response to dietary perturbations is dissimilar to that of these species. This study examined the effects of dietary protein perturbation during the peri-conception and first trimester periods upon reproductive development in bulls. Nulliparous heifers (n = 360) were individually fed a high- or low-protein diet (HPeri and LPeri) from 60 days before conception. From 24 until 98 days post conception, half of each treatment group changed to the alternative post-conception high- or low-protein diet (HPost and LPost) yielding four treatment groups in a 2 × 2 factorial design. A subset of male fetuses (n = 25) was excised at 98 days post conception and fetal testis development was assessed. Reproductive development of singleton male progeny (n = 40) was assessed until slaughter at 598 days of age, when adult testicular cytology was evaluated. Low peri-conception diet delayed reproductive development: sperm quality was lowered during pubertal development with a concomitant delay in reaching puberty. These effects were subsequent to lower FSH concentrations at 330 and 438 days of age. In the fetus, the low peri-conception diet increased the proportion of seminiferous tubules and decreased blood vessel area in the testis, whereas low first trimester diet increased blood vessel number in the adult testis. We conclude that maternal dietary protein perturbation during conception and early gestation may alter male testis development and delay puberty in bulls.
Additional keywords: fetal programming, morphology, puberty, testis.
Introduction
Fetal developmental programming of physiological systems is a well-established concept (McMillen and Robinson 2005). Maternal peri- and post-conception nutrition influences fetal development, which, in turn, can affect postnatal growth, gonad development, gamete quality and hormonal status of the offspring (Sullivan et al. 2009a, 2010; Micke et al. 2010, 2011; Dupont et al. 2012; Mossa et al. 2013). Seasonal variation in the nutritional value and the quantity of pasture available to pregnant ruminants can occur in grass-fed production systems (Burns et al. 2010). Such variation in prenatal nutrition has been shown to affect both testicular development and circulating gonadotrophin levels in the prepubertal bull (Sullivan et al. 2010). However, the implications for adult reproductive performance in cattle progeny are not known; this paucity of research on the direct effects of in utero nutrition on male progeny postnatal reproductive function and fertility is widely acknowledged (Dupont et al. 2012; Chavatte-Palmer et al. 2014; Mossa et al. 2015; Sinclair et al. 2016). Comparable studies in rams have reported effects upon age at puberty (Da Silva et al. 2001), testicular weight (Bielli et al. 2002), testicular volume (Da Silva et al. 2001), Sertoli cell numbers, the diameter of seminiferous tubules (Kotsampasi et al. 2009), prepubertal testosterone (Da Silva et al. 2001) and pituitary response to gonadotrophin-releasing hormone (GnRH; Kotsampasi et al. 2009). Age of puberty in cattle (as in sheep) is considered a driver of efficiency; shortening the generation interval, increasing genetic gain and thereby overall lifetime productivity (Barth and Ominski 2000; Yilmaz et al. 2006).
Many studies have shown that folliculogenesis (Fair 2010) and early embryo development are sensitive to perturbations in the maternal environment (Edwards and McMillen 2002; Ashworth et al. 2009; Mossa et al. 2013). The response to such perturbations is orchestrated via the developing placenta (Sullivan et al. 2009b). As the growth trajectory of the bovine placenta differs from the ovine and rodent models, and is, in fact, more similar to the human (Wooding and Flint 1994), the resultant response of the feto–placental unit also differs (Hernandez-Medrano et al. 2015). Correspondingly, unlike altricial or small ruminant models, bovine embryo development occurs at similar developmental time points to the human; organogenesis is complete by 42 days post conception (dpc) (Hopper 2014), with the genital ridges, forming at 27 to 30 dpc (Ross et al. 2009). Sertoli cells begin to proliferate between 40 and 50 dpc and play a crucial role in gonad development during fetal life and in postnatal spermatogenesis (Griswold and McLean 2006). Disruptions to the proliferation of fetal Sertoli cells may occur through modifications in the development of the hypothalamic–pituitary–gonadal axis in early fetal life (Klonisch et al. 2004; O’Shaughnessy and Fowler 2011) and associated changes in the concentration of hormones including follicle-stimulating hormone (FSH), triiodothyronine (T3), thyroxine (T4) and growth hormone (GH; Dupont et al. 2012). Consequently, this may affect development of other testicular cells, leading to altered testicular function in postnatal life (Sharpe et al. 2003; Dupont et al. 2012).
Spermatogenesis is a complex process of cellular replication and differentiation (Barth and Oko 1989; Wrobel 2000). A suite of molecular pathways is regulated by an interdependent complement of hormones including testosterone, FSH, inhibin and activin, which rise and fall in a specified sequence during prepuberty and peri-puberty to result in functional spermatozoa in the adult bull (Evans et al. 1996; Matsuzaki et al. 2000; Kaneko et al. 2001). This sequence is known to be disrupted by nutritional intervention during the preweaning period (Brito et al. 2007b, 2007c) possibly mediated by metabolic hormones (i.e. insulin-like growth factor 1 (IGF1); Brito et al. 2007a, 2007c; Barth et al. 2008) with consequent effects upon the development of spermatogenesis. Previously reported effects of prenatal nutrition also include changes in concentrations of many of the aforementioned hormones (Da Silva et al. 2001; Micke et al. 2010; Sullivan et al. 2010).
The aim of the present study was to examine the effects of dietary protein intake in heifers during the peri-conception period and the first trimester on the reproductive development of their male progeny. We hypothesised that the peri-conception and first trimester low-protein diet would delay puberty with deleterious effects upon testicular development and sperm production and, furthermore, this would be associated with alterations to the hormonal milieu in the developing bull.
Materials and methods
Ethics approvals
All procedures were performed with the prior approval of University of South Australia IMVS Animal Ethics Committee, Australia (Approval number: 18/11), The University of Adelaide, Australia (Approval number: S2012–249), The University of New England, Australia (Approval number AEC14–037) and the University of Nottingham, UK (Approval number 1117 140320).
Experimental design and animal management
The purpose of this experiment was to evaluate the impact of maternal dietary protein during the peri-conception (PERI; −60 to 23 dpc (implantation being 18 to 22 dpc; Wathes and Wooding 1980; Spencer and Hansen 2015)) and first trimester (POST; 24 to 98 dpc) periods in nulliparous beef heifers upon fetal and postnatal reproductive development in the male progeny.
The study was a 2 × 2 factorial design. The animals and fetuses studied were singleton male progeny of 2-year-old heifers that have previously been described (Copping et al. 2014). Briefly, 360 nulliparous weaned Santa Gertrudis (Bos taurus × Bos indicus) heifers were selected on the basis of weight (289.4 ± 23.4 kg) from S. Kidman and Co herds located at ‘Glengyle’ and ‘Morney Plains’, south-western Queensland, Australia. Heifers were transported to ‘Tungali’, Sedan, South Australia (34°29′S, 139°18′E) where they underwent 60 days of acclimatisation before commencement of the study. Heifers that did not acclimatise to the individual feeding were removed from the study.
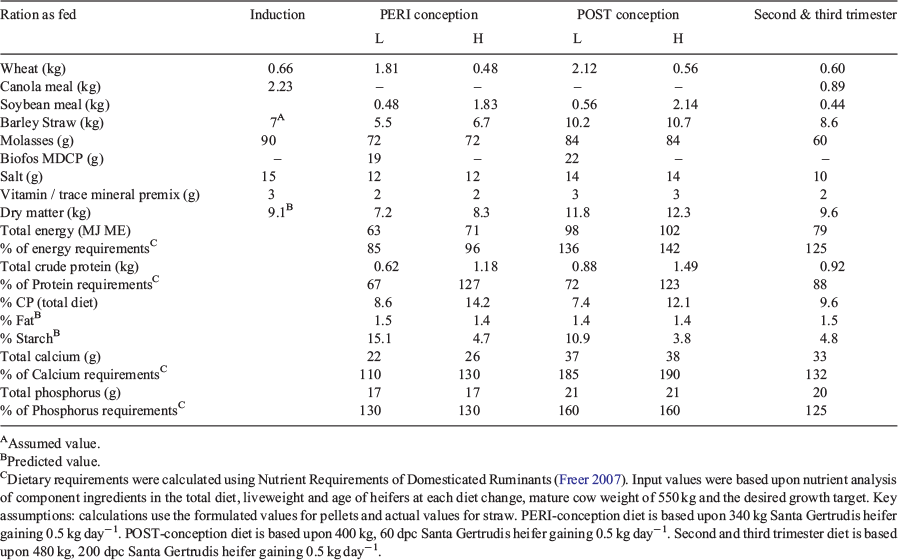
At ~12 months of age, 60 days before artificial insemination, heifers were stratified by bodyweight and randomly assigned to two equal peri-conception (PERI) treatment groups, high and low protein (HPeri and LPeri). Each heifer was fed a high (71 MJ ME and 1.18 kg crude protein per head per day) or low (63 MJ ME and 0.62 kg crude protein per head per day) protein diet (Table 1) consisting of a pelleted diet supplemented with a commercial vitamin and mineral preparation (Minmix; Ridley Agriproducts) that was individually fed in stalls. Straw (5% crude protein) was available ad libitum in pens.
Heifers underwent a progesterone-based oestrus synchronisation program as previously described (Hernandez-Medrano et al. 2015) and were artificially inseminated on Day 0 with frozen semen from one Santa Gertrudis bull. At 23 dpc, half of each nutritional treatment group was swapped to the alternative post-conception (POST) treatment, high (HPost: 102 MJ ME and 1.49 kg crude protein per head per day) or low (LPost: 98 MJ ME and 0.88 kg crude protein per head per day; Table 1), giving rise to four groups: HPeri-HPost (HH), HPeri-LPost (HL), LPeri-HPost (LH), LPeri-LPost (LL). Pregnancy was confirmed in 124 heifers at 36 dpc and fetal sex was determined at 60 dpc by transrectal ultrasonography. At 98 dpc a sub-set of heifers (n = 46; singleton pregnancy) was humanely slaughtered at a commercial abattoir and fetuses of both sexes (n = 46; singletons) collected as described (Copping et al. 2014), with the 25 singleton male fetuses reported herein (n: HH = 6, HL = 10, LH = 5, LL = 4). The fetal cohort was randomly selected based on maternal weight and sex of the fetus; however, the HL group had a disproportionate number of male fetuses so a larger number of these animals was available at this point. Fetal gonads were dissected, weighed and collected for histological processing.
From the end of the first trimester of gestation (98 dpc), all heifers were fed the same diet, which was formulated to provide additional growth of 0.5 kg per head per day until parturition (79 MJ ME and 0.92 kg crude protein per head per day; Table 1). Heifers received the pellet portion of their diet individually on a daily basis with straw (5% crude protein) provided ad libitum in pens until the animals reached parturition.
Sixty-four heifers completed the study and gave birth to 18 live singleton female and 43 live singleton bull calves. Progeny remained with their mothers as one group grazing on improved and native pastures until weaning at 183.3 ± 0.8 days of age. After weaning, progeny were segregated according to sex and grazed improved and native pasture until 507.3 ± 0.8 days of age. Non-castrated male progeny were transported from Sedan, South Australia to the ‘Tulimba’ Research Feedlot, Kingstown, NSW (30°28′S, 151°11′E) before slaughter at a commercial abattoir on 598.3 ± 0.8 days of age with a final liveweight ± s.d. of 652.3 ± 11.4 (HH), 677.0 ± 10.0 (HL), 678.6 ± 19.1 (LH) and 647.4 ± 15.5 (LL) kg. At slaughter, gonads were dissected, weighed and collected for histological processing. Two progeny were removed from the study after birth, due to poor mothering. An additional animal was a cryptorchid and was excluded from the analysis leaving 40 singleton male progeny that completed the study reported herein (n: HH = 10, HL = 14, LH = 8, LL = 8).
Tissue fixation and processing
The complete left testis in the fetus and a 1 cm3 piece from each testis (same for every sample) in the adult were dissected and fixed overnight in 4% paraformaldehyde diluted in 0.1 M phosphate-buffered saline (PBS; 0.14 M NaCl, 0.03 M NaH2PO4, 0.05 M Na2HPO4) in a ratio of 1 : 5 (tissue volume : fixative solution volume). Samples were washed three times for 24 h each in PBS. Tissues were processed on an automated tissue processor in the following solutions, 30 min in the case of fetal testis and 1 h for the adult testis per solution: 70% ethanol, 80% ethanol, 95% ethanol, three times in 100% ethanol, two times in 100% xylene and two times in paraffin wax at 60°C under vacuum. Following processing, the testes were orientated and embedded in paraffin wax.
All samples, both adult and fetus, were sectioned at a thickness of 10 µm using a microtome (Leica 5M 2255). The sections were dried onto polysilinated slides (Thermo Scientific) on a hot plate at 42°C for 1 h and then for 24 h at room temperature before histological staining.
Cell counts and proportions
The development of the testis was assessed by the measurement of the following structures: testicular cell number (Sertoli, germ and interstitial cells), seminiferous tubules and blood vessels. These were distinguished within the testis by staining with two techniques: immunohistochemistry using a Novolink Polymer Detection immunostaining kit (Leica Microsystems) with Mis-C20 primary antibody (1 : 1000 dilution; Santa Cruz Biotechnology) and Picrosirius staining (Polysciences, Inc.). Mis-C20 was combined with haematoxylin staining in order to differentiate the Sertoli cells (Mis-C20 stained), germ cells (morphologically apparent by their distinctive cytoplasm) and the morphologically distinct interstitial cells/Leydig cells. In the early stages of testicular development, fetal Sertoli cells are localised in the periphery of the sex cords (Vergouwen et al. 1991; Jégou 1992; Abd-Elmaksoud 2005) surrounding the germ cells, which are situated near the centre of the testicular cord (Vergouwen et al. 1991; Jégou 1992; Abd-Elmaksoud 2005). Interstitial/Leydig cells distribute across the interstitium between the seminiferous cords (Vergouwen et al. 1991; Abd-Elmaksoud 2005). Picrosirius staining was used to assist identification of blood vessel from seminiferous tubules. Following tissue staining, sections were photomicrographed using a DM5000B microscope (Leica Microsystems Inc.) with a Leica CTR5000 light box and Leica DFC420 colour capture camera. The magnification of the eyepiece and lens is stated below for each count using systematic random sampling and stereology methods previously described (Mayhew 1991, 2011). In brief, sections were selected using systematic random sampling (ensuring a minimum of 200 µm between samples to avoid double cell counting). Photomicrographs were captured from each section in a systematic random manner before stereological counting and measurements being undertaken (n = 5 sections per sample for cell counts and proportions and n = 3 sections per sample for seminiferous tubules and blood vessel measurement). This technique ensured unbiased measurements throughout the tissue. In the fetal testis every testicular cell was identified and manually counted on a total of 420 photomicrographs. Seminiferous tubule numbers and dimensions (n = 315 photomicrographs, 20× magnification) and capsular and parenchymal blood vessels (n = 5670, photomicrographs at 10× and 40× magnification respectively) were measured manually using an image analysis program (Image-Pro Plus, Version 6.3; Media Cybernetics; n: HH = 5, HL = 7, LH = 5, LL = 4). In the adult testis seminiferous tubules and blood vessels were measured using the same image analysis program (n = 400 micrographs, 10× magnification and n = 800 photomicrographs, 20× magnification respectively; n: HH = 10, HL = 14, LH = 8, LL = 8). Following calibration of the imaging software, tubules and vessels were manually circumscribed and the average number of tubules and blood vessels per tissue area (referred to throughout as ‘number’ of blood vessels or seminiferous tubules) was calculated. In addition, the blood vessel and seminiferous tubule areas occupied per tissue section were calculated; this is referred to as blood vessel or tubule ‘area’ throughout. Importantly all samples were fixed, processed and sectioned in the same manner so that groups could be directly compared.
Animal measures
Liveweight and scrotal circumference
Heifers were visually monitored 24 h a day throughout calving. Progeny birthweight was recorded within 15 min of birth and before first suckling. Liveweight was recorded monthly from birth. Scrotal circumference was assessed monthly from 214.3 ± 0.8 days of age (after weaning) using the Australian Cattle Veterinarians recommended procedure (Beggs et al. 2013) with a Reliabull scrotal measuring tape (Lane Manufacturing Inc.).
Blood sampling
Progeny blood samples were collected approximately monthly from weaning until slaughter at 20 months of age. Prior to the commencement of other procedures, samples of whole blood were collected by venipuncture directly into Vacutainer tubes containing lithium-–heparin (Becton, Dickinson and Co.). Tubes were rotated by hand for 5 to 10 s and stored on ice before centrifugation (Eppendorf 5702R; Eppendorf Zentrifugen GMBH) at 3000g for 10 min at 4°C within 90 min of collection. Plasma was harvested then stored frozen at −80°C until analysis.
Assays
Plasma concentrations of FSH, leptin, IGF1, testosterone, anti-Müllerian hormone (AMH), inhibin and activin A were assayed as detailed.
Plasma FSH was measured in duplicate by a double-antibody radioimmunoassay (Atkinson and Adams 1988) using NIAMDD-oFSH-RP-1 (biopotency 75× NIH-FSH-S1) and NIADDK-anti-oFSH-1 serum. The intra-assay coefficients of variation were 5.7%, 2.7% and 4.4% for control plasma with means of 1.27 ng mL−1, 2.25 ng mL−1 and 3.15 ng mL−1 respectively. The limit of detection was 0.15 ng mL−1. As the sample levels were 3–4 times higher than the limit of detection they were read in the linear part of the standard curve.
Plasma was assayed for leptin in duplicate by a double-antibody radioimmunoassay (RIA; Blache et al. 2000) with samples processed in a single assay. The assay included six replicates of three control samples containing 0.29, 0.71 and 1.68 ng mL−1, which were used to estimate the intra-assay coefficients of variation of 5.4%, 4.4% and 6.6%. The limit of detection was 0.05 ng mL−1.
Plasma testosterone was assayed in duplicate using the reagents of the Immunochem double antibody testosterone RIA kit (MP Biomedical Australia) following the manufacturer’s protocol and validated using a serial dilution of two bovine samples. The intra-assay coefficients of variation for quality control samples containing 0.26 ng mL−1 and 2.3 ng mL−1 were 6.5% and 2.9% respectively. The lowest and highest limits of detection were 0.07 ng mL−1 and 6.5 ng mL−1 respectively.
Plasma was assayed for IGF1 in duplicate by double-antibody radioimmunoassay with human recombinant IGF1 (ARM4050; Amersham-Pharmacia Biotech) and anti-human IGF1 antiserum (AFP4892898; National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases) following acid–ethanol extraction and cryoprecipitation (Breier et al. 1991). The assay was previously validated for bovine samples (Chagas et al. 2007). Samples were processed in a single assay. The intra-assay coefficients of variation for control samples containing 51.6 ng mL−1 and 253.6 ng mL−1 were 6.2% and 5.9% respectively. The limit of detection was 0.1 ng mL−1.
AMH levels were determined using a bovine AMH enzyme-linked immunosorbent assay (ELISA) kit (Ansh Laboratories) following the manufacturer’s protocol. Samples were diluted 15 times using the sample diluents provided in the kit. The intra-assay coefficients of variation for quality control samples containing 290.8 pg mL−1 and 875.0 pg mL−1 were 2.6% and 3.7% respectively. The limit of detection was 28.4 pg mL−1.
Bovine inhibin levels were measured at the Hudson Institute of Medical Research using a radioimmunoassay employing a rabbit antiserum raised against the α-subunit of bovine inhibin (McLachlan R.I. et al. 1986), which detects both inhibin A and B proteins and free inhibin α-subunit (including pro-α-C) in multiple species. Iodinated human recombinant 31-kDa inhibin was used as tracer and 31-kDa human recombinant inhibin was used as standard. Goat anti-rabbit IgG (GAR#12; Hudson Institute) was used as second antibody. The assay has been validated for measurement of inhibin in bovine serum samples and values (in ng mL−1) are expressed relative to the purified human inhibin standard. The intra-assay coefficient of variation was 6.2% and the lowest and highest limits of detection were 0.26 ng mL−1 and 8.73 ng mL−1 respectively (based on effective dose (ED) 90 and ED10 values).
Total bovine serum activin A concentrations were measured at the Hudson Institute of Medical Research employing a two-site enzyme immunoassay specific for activin A (Knight et al. 1996) modified and validated for measurement of bovine serum samples. Human recombinant activin A, which is identical in sequence to bovine activin A, purified as described previously from material provided by Biotech Australia Pty Ltd (Robertson et al. 1992), was used as the standard. Values (in pg mL−1) are expressed relative to the purified activin A standard. The mean intra- and inter-assay coefficients of variation for three plates were 5.4% and 7.3% respectively. The lowest and highest limits of detection were 8.84 pg mL−1 and 1984 pg mL−1 (2 s.d.) respectively.
Semen collection
Semen collection commenced in spring at approximately monthly intervals from 10 months of age until slaughter at 20 months of age. After preliminary stimulation of the ampulla via rectal massage, semen was collected using a standard electroejaculation technique (Lane Pulsator IV; Lane Manufacturing Inc.) as previously described (McAuliffe et al. 2010; Beggs et al. 2013). If an animal did not produce a satisfactory sample within several minutes following electrostimulation, the animal was released and a single further attempt was made after a 10-min interval (Callaghan et al. 2016).
Assessment of semen traits was undertaken immediately following collection using established methodology and standards (Entwistle and Fordyce 2003; Fordyce et al. 2006) by the same technician blinded to treatment. Briefly, ejaculate density was scored immediately following collection using a 1 (clear to cloudy) to 5 (creamy) scale. A drop of semen was placed on a pre-warmed glass slide (37°C) with a plastic transfer pipette (1 mL) and assessments made of motility (%) and mass motility (or wave motion) using a phase-contrast microscope. Motility was estimated as the percentage of spermatozoa that were progressively motile under their own propulsion (viewed at 400× magnification). Mass motility was assessed under 40× magnification on a 1 (no swirl) to 5 (fast distinct swirl with continuous dark waves) scale (Burns et al. 2013; Corbet et al. 2013). Animals that did not produce an ejaculate were assigned a value of zero for density, motility and mass motility (Corbet et al. 2013). Semen (0.1 mL) was diluted with phosphate-buffered formal saline (4.9 mL) for sperm concentration assessment, with spermatozoa counted in a haemocytometer (Perry et al. 1990).
Semen (1 to 2 drops) was placed into phosphate-buffered formal saline (1.0 mL) for assessment of sperm morphology. The morphology of 100 individual spermatozoa in each sample considered to contain sufficient spermatozoa for examination (Burns et al. 2013) was assessed using 1000× magnification under differential interference contrast microscopy by an Australian Cattle Veterinarians accredited sperm morphologist blinded to treatment at a commercial third-party pathology laboratory. Morphology traits were individually recorded based on the sperm abnormality format as described (Fordyce et al. 2006). The sperm abnormality categories included midpiece abnormalities, knobbed acrosomes, proximal cytoplasmic droplets, abnormal tails and loose heads, pyriform heads, vacuoles and teratoid spermatozoa and swollen acrosomes (Fordyce et al. 2006). Total remaining normal spermatozoa were noted as percentage normal spermatozoa per ejaculate at each time point (Entwistle and Fordyce 2003).
Determination of pubertal age and sexual maturity
The threshold used for age at puberty was defined as the first time an ejaculate contained a semen concentration of ≥50 × 106 spermatozoa mL−1 with ≥10% motile spermatozoa (Wolf et al. 1965). Sexual maturity was characterised as the first time an ejaculate contained ≥70% morphologically normal spermatozoa with semen concentration ≥50 × 106 spermatozoa mL−1 (Brito et al. 2004).
Statistical analysis
Data were checked for normality and transformed before analysis if required. Data for maternal liveweight, maternal average daily gain, fetal weight, fetal testis weight, testicular cell development, gestation length paired testis weight, age of puberty, age of maturity, inhibin, activin A and AMH were analysed using two-way ANOVA (STATA 13.1; Stata Corp College Station) to determine the effects of maternal diet during PERI and POST periods and their interaction term. Significant interactions were explored with Tukey–Kramer post hoc test as required.
To investigate the interactions between maternal diet (PERI and POST) and time, hormone concentrations (leptin, FSH, IGF1, testosterone), sperm traits and scrotal circumference, linear mixed-effects models were performed, adjusting for repeated measures over time for each of the 40 calves. An autoregressive (1) covariance structure was used as it provided the best fitting model compared with other structures. For sperm morphological abnormalities, generalised estimating equation (GEE) models with a Poisson distribution were performed, adjusting for repeated measures over time for each of the 40 calves. Post hoc comparisons were made for each model: differences of least-squares means for the linear mixed-effects models and incidence rate ratios (IRR) for the Poisson GEE models. The statistical software used was SAS 9.3 (SAS Institute Inc.). There were no significant interactions between maternal diet during PERI and POST periods for the variables investigated unless expressly stated in the results. Thus, for clarity, the results have been presented as the main effects of PERI and POST maternal diet. Statistical significance is reported at P < 0.05 and tendency at P < 0.10.
Results
Maternal liveweight
At both the commencement and end of the PERI maternal diet period (−60 to 23 dpc) and the POST maternal diet period (24 to 98 dpc), the liveweights of the heifers were similar (Tables 2 and 3; all P > 0.10). There was no interaction of the PERI and POST diet (P = 0.475) on liveweight at the end of the POST maternal diet period. Average daily weight gain (ADG) during the PERI diet period was lower (P < 0.001) for the LPeri (LL + LH) heifers compared with the HPeri (HH + HL) heifers. ADG during the POST maternal diet period did not differ between LPost (LL + HL) and HPost (HH + LH) groups (P = 0.164), nor was there a diet interaction (P = 0.482). Heifers that had received the LPeri diet had higher ADG during the POST diet period compared with those that received the HPeri diet (0.36 ± 0.04 vs 0.17 ± 0.04 kg per head per day; P = 0.002).

|

|
From the end of the POST diet period to late gestation (99 dpc to 256 dpc), during which time all dams received the same diet, maternal liveweights did not differ due to PERI or POST diet, nor was there a diet interaction (all P > 0.10; LPeri 506.4 ± 7.3 vs HPeri 507.2 ± 6.3 kg and LPost 507.3 ± 6.1 vs HPost 506.3 ± 7.6 kg). ADG also did not differ (all P > 0.10; LPeri 0.63 ± 0.02 vs HPeri 0.64 ± 0.02 and LPost 0.65 ± 0.02 vs HPost 0.61 ± 0.02 kg per head per day). Immediately after calving, a similar pattern was observed whereby maternal liveweights did not differ due to PERI or POST maternal diet, nor was there a diet interaction (all P > 0.10; LPeri 456.6 ± 8.3 vs HPeri 464.2 ± 7.7 and LPost 468.6 ± 7.1 vs HPost 452.4 ± 8.5 kg).
Fetal and animal measures
Fetal and gonad weight at 98 dpc
As previously reported (Copping et al. 2014), male fetuses from LPost dams were lighter at 98 dpc compared with males from HPost dams (see Table S1, available as Supplementary Material to this paper; P < 0.05). There was no effect of PERI diet or the diet interaction term on male fetal weight at 98 dpc. Maternal diet did not influence absolute gonad weight or relative gonad weight at 98 dpc (Table S1).
Birthweight and post-weaning growth
At birth there was no effect of maternal diet upon birthweight or gestation length (Table S2; P > 0.05). Increased gestation length was associated with increased birthweight (r = 0.475; P < 0.001). From weaning until slaughter (600 days), liveweight increased with age (P < 0.0001) but did not vary due to maternal diet (data not shown; P > 0.10).
Scrotal circumference
Scrotal circumference in all treatment groups increased with age (see Fig. S1, available as Supplementary Material to this paper; P < 0.0001). There was no overall effect of either maternal diet or gestation length upon progeny scrotal circumference measurements between 214 and 554 days of age (Fig. S1; all P > 0.10).
Semen traits
Semen quality parameters
There were maternal nutrition and time effects on a range of semen quality parameters (Fig. 1). There were effects of time (P < 0.0001) and PERI maternal diet (P = 0.0433) on mass motility, such that bulls from LPeri dams had lower semen mass motility scores compared with bulls from HPeri dams (Fig. 1a). There were interactions between POST maternal diet and time for mass motility (P = 0.0181), such that bulls from LPost dams had increased mass motility compared with bulls from HPost dams at 554 days of age (Fig. 1b; P = 0.0433) and tended to be higher at 351 (P = 0.08) days of age. There were effects of time (P < 0.001) and PERI maternal diet on semen density (Fig. 1c) and sperm motility (Fig. 1e). Overall, bulls from LPeri dams had lower sperm density (Fig. 1c; P = 0.04) and motility (Fig. 1e; P = 0.0217) compared with bulls from HPeri dams. There was an interaction between PERI maternal diet and time for the motility parameter (Fig. 1e; P = 0.0124). Bulls from LPeri dams produced ejaculates with reduced motility at 351 (P = 0.03), 395 (P = 0.024) and 438 (P = 0.0024) days of age and tended to have reduced motility at 465 (P = 0.08) days of age compared with bulls from HPeri dams. Overall, there were effects of time (P < 0.001) on semen concentration and concentration tended to be lower in bulls from LPeri dams (Fig. 1g; P = 0.058) but there was no interaction of maternal diet and age. The POST maternal diet did not influence density (Fig. 1d), motility (Fig. 1f) or concentration parameters (Fig. 1h), nor was there any difference in any semen quality parameters due to the diet interaction (all P > 0.05).
Sperm morphology
There were effects of time (P < 0.0001) and PERI maternal diet (P = 0.0208) on percentage normal spermatozoa (Fig. 2). Overall, the percentage of normal spermatozoa was lower in bulls from LPeri dams (Fig. 2a) compared with HPeri. The reduction in percentage normal spermatozoa within LPeri bulls was consequent to increased levels of sperm abnormalities (Fig. 3).

|

|
Specifically, a higher overall incidence of abnormal midpieces and knobbed acrosome defects (Fig. 3a, c; P < 0.05) were observed in ejaculates from LPeri bulls. There were interactions between PERI maternal diet and time (Fig. 3c; P = 0.0043) for knobbed acrosomes with a higher incidence of this defect in ejaculates from LPeri bulls at 438 (IRR = 4.27; P = 0.0061), 465 (IRR = 3.60; P = 0.0156) and 520 (IRR = 5.39; P = 0.005) days of age. There were also interactions between PERI maternal diet and time for abnormal tail and loose head defects (Fig. 3e; P = 0.0024) such that bulls from LPeri dams produced ejaculates with a higher incidence of abnormal tails and loose heads compared with bulls from HPeri dams at 465 (IRR = 1.87; P = 0.0394), 520 (IRR = 2.62; P = 0.0039) and 554 days of age (IRR = 2.41; P = 0.0248). There was an interaction between PERI maternal diet and age for vacuole and teratoid defects (Fig. 3g; P = 0.0434); however, there were no differences at any individual age. There was also an interaction between POST maternal diet and time for proximal droplet defects (Fig. 3j; P = 0.0032); however, once again there were no differences at any individual age. Overall, POST maternal diet increased the incidence of swollen acrosome defects, which was higher overall in ejaculates from bulls with LPost dams than bulls from HPost dams (P = 0.0352). POST diet did not influence the incidence of any other sperm defect, nor was there any difference in any sperm defect due to the diet interaction (all P > 0.05). There were effects of age overall (Fig. 3; all P < 0.0001) for all defects reported. (Data not shown for swollen acrosome and pyriform head defects).
Puberty
Puberty was first reached by a bull at 329 days of age with the final bull reaching the threshold by 521 days of age. Puberty was achieved later in LPeri bulls compared with HPeri bulls (Table 4; P = 0.049). There was no difference in puberty due to POST maternal diet or the diet interaction (Table 4; P > 0.05).
Sexual maturity
Maturity as assessed using the minimum threshold of 70% normal spermatozoa was not achieved by 17.5% (n = 7: LPeri = 4; HPeri = 3; LPost = 4; HPost = 3) of the bulls in this study. The first bull reached the threshold at 330 days of age. Of those bulls that achieved maturity (n = 33), there was a tendency for bulls from LPeri dams to reach maturity later than bulls from HPeri dams (466.9 ± 19.0 vs 425.9 ± 12.2 days of age; P = 0.079). There were no differences due to POST maternal diet (LPost 435.3 ± 15.6 vs HPost 448.0 ± 15.2 days of age; P > 0.10) or the diet interaction (P > 0.05).
Paired testes weight
The absolute and relative weights of the paired testes were similar between maternal diet groups at slaughter at 598.3 ± 0.8 days of age (Table S2; P > 0.05). Total paired testis weight at slaughter was highly correlated with the final scrotal circumference (Table S2) measured at 554.3 ± 0.8 days (r = 0.82; P < 0.05) irrespective of maternal diet.
Hormone concentrations
Circulating inhibin and activin A concentrations measured at 3 and 4 months of age were not influenced by either PERI or POST maternal diet (Table 5; P > 0.10) or the diet interaction (P > 0.05). However, circulating AMH concentrations at 10 months of age were higher in bulls from LPeri dams (Table 5; P = 0.04) compared with HPeri bulls and tended to be higher in bulls from LPost dams compared with HPost bulls (Table 5; P = 0.09).
There were overall effects of time (P < 0.001) on plasma FSH (Fig. 4), IGF1 (Fig. 5) and leptin levels (Fig. 5). Time also tended to influence plasma testosterone concentration (Fig. 4; P = 0.09). There were interactions between PERI maternal diet and time for FSH (Fig. 4a; P = 0.0435) such that LPeri bulls had lower circulating FSH at 330 (P = 0.0317) and 438 (P = 0.0147) days of age and tended to have lower levels at 273 (P = 0.06) and 302 (P = 0.09) days of age. There were also interactions between POST maternal diet and time for IGF1 (Fig. 5b; P = 0.0127) such that LPost bulls had higher circulating IGF1 at 465 days of age (P = 0.004) compared with HPost bulls. There were no main effects overall of PERI or POST maternal diet or their interaction term on FSH, testosterone, IGF1 or leptin concentrations (P > 0.10).
Testis development
The proportions of testicular cells (Sertoli, germ, interstitial/Leydig cells) in 98 dpc fetuses were not altered either by the PERI or POST maternal diet or their interaction term (Table S3; Fig. S2; P > 0.05). Seminiferous tubule and blood vessel parameters were altered by dietary treatment (Tables 6 and 7). A higher proportion of seminiferous tubules within the testis (Table 6; P = 0.04) due to a greater number of tubules within the tissue (Table 6; P = 0.04) were observed in the LPeri diet fetal gonad compared with the HPeri gonad. There were no observed effects of maternal diet in the adult progeny in seminiferous tubule parameters (Table 6; P > 0.05).
The LPost fetal gonad displayed decreased numbers of blood vessels within the capsule of the testis (Table 7; P = 0.02) whilst the tissue area of blood vessels within the parenchyma of the testis (Table 7; P = 0.03) was decreased in the LPeri fetal gonad compared with the HPeri. In the adult testis, the number of blood vessels was increased by the LPost maternal diet (Table 6; P = 0.03).
Discussion
This study is the first to our knowledge to investigate the effects of maternal dietary protein during the peri-conception period and early gestation upon bovine male reproductive development. We examined this during fetal development and postnatally through to adulthood. The key findings were that the LPeri dietary treatment in nulliparous heifers altered reproductive development of their male progeny in the early postpubertal period as reflected by differences in reproductive hormones, testicular cytology and sperm production with a subsequent delay in reaching puberty. Increasing protein intake in the peri-conception period may therefore be viable for bull producers as the ability to use yearling bulls reduces production costs and shortens the genetic interval (Barth and Ominski 2000).
Decreased protein intake during early gestation reduced early fetal growth (Copping et al. 2014). This in utero effect was, however, not discernible in later gross measures such as birthweight or postnatal growth as previously reported in lambs (Kotsampasi et al. 2009) or calves (Micke et al. 2015) but effects upon postnatal reproductive development were evident: the LPeri diet decreased blood vessel area in the fetal testis. Moreover, seminiferous tubule number and percentage was increased, although this effect was not evident in the adult. In the developing bull, the LPeri maternal diet lowered sperm quality with this effect occurring after lower FSH concentrations in this group at both 330 and 438 days of age compared with the HPeri group.
Nutrition
Variations in natural feed resources in extensive farming systems are common in many countries. In the northern Australian rangelands, protein, rather than energy, is often the major limiting nutrient (Norman 1963) with protein supplementation of replacement heifers a common practice (Bortolussi et al. 2005; Burns et al. 2010). The dietary protein levels used in the present study therefore reflected pasture conditions in Australian rangelands without (low) and with (high) protein supplement. There was a 1.9- to 2.1-fold difference in crude protein (CP) content and a 1.1-fold difference in energy content between the high and low diets. The ration was as isocaloric as possible for ruminants fed the forage component of the diet under group housing. Dietary fat content was similar and although starch content differed, levels in both low and high diets were moderately low. Protein intake was restricted during both the peri-conception period and first trimester in the low group whilst both groups received similar energy intake. As the variation in CP content between the high and low diets was much greater than that in energy, we therefore consider the differences observed in the present study are likely attributable to the effects of protein rather than energy intake.
Testis histology
The lack of effect upon Sertoli, germ and interstitial cells is in contrast to studies that reported a reduction in the number of Sertoli cells in newborn lambs undernourished in utero during the second trimester of gestation (Bielli et al. 2002; Kotsampasi et al. 2009) but concurs with studies that excised the testis at a fetal endpoint (Da Silva et al. 2003; Andrade et al. 2013).
The observed decrease in vasculature in the LPeri and LPost 98-dpc fetal testis is a novel finding and may reflect a mechanism whereby maternal protein restriction reduces male reproductive function as previously reported (Zambrano et al. 2005). Although the observed reduction in parenchymal blood vessel area and in the number of capsular blood vessels in the LPeri and LPost testis respectively was transient (suggesting a compensatory ability of either the fetal or pubertal testis), blood supply affects the physiological function of every organ. The testes are, however, particularly sensitive to alterations in vasculature as minor episodes of ischaemia lead to functional disturbances (Wrobel et al. 1981; Polguj et al. 2015). Furthermore, the capsule vasculature in ruminants, essential to metabolite and heat exchange (Godinho et al. 1973), was observed to be compromised in the LPost cohort. We have previously reported the long-term effects of this protein restriction model upon hypertension in the female cohort (Hernandez-Medrano et al. 2015). We propose that this transient vascular perturbation during this critical gestational phase (O’Shaughnessy and Fowler 2011) may lead to testicular oxidative stress as previously reported in a rat model following gestational protein restriction (Rodríguez-González et al. 2014). Interestingly this model of gestational protein restriction in the postnatal rat also led to long-term effects upon semen quality and morphology as we similarly report below.
Concomitantly, in the 98-dpc fetus, the LPeri diet caused an increment in the number of seminiferous tubules and the proportion of seminiferous tubules per testis but did not affect tubule area. In combination, these results may indicate that the differentiation and proliferation of testicular cells and the development of the seminiferous tubules is not linked to the development of the blood vessels during the first trimester.
In the adult bulls the number and proportion of seminiferous tubules were unaffected by the dietary regimes, further suggesting that compensation may occur during developmental stages after our dietary intervention either in late gestation or postnatally. A prior study observed reduced seminiferous tubule diameters in bull calves at 5 months of age after supplementation of their mothers’ diets with protein (0 to 180 dpc; Sullivan et al. 2010). This suggests that compensatory mechanisms occur during the pubertal period.
Postnatal development
In this study an in utero LPeri diet increased the age at which bulls reach puberty predicated by the motility, morphology and concentration of spermatozoa produced in the ejaculate (Barth and Oko 1989; Perry et al. 1990; Holroyd et al. 2002). The higher levels of spermatozoa with non-progressive motility, the overall increased numbers of morphologically abnormal spermatozoa and the tendency for lower concentrations suggest that both epididymal function and spermatogenesis were delayed or disrupted by the LPeri maternal diet. As expected in pubertal bulls, the initial high level of proximal droplets in ejaculates decreased over time (Lunstra and Echternkamp 1982; Barth and Oko 1989; Perry et al. 1991; Evans et al. 1995) but was not altered by in utero diet. Midpiece defects and abnormal heads and tails were increased in the LPeri bulls; both defects are reported to be associated with disturbance of epididymal function (Barth and Bowman 1994). Knobbed acrosomes were similarly increased in the LPeri bulls at 438, 465 and 520 days of age indicating disturbed spermiogenesis during this peri-pubertal period (Barth and Bowman 1994; Beggs et al. 2013). In the present study, the bulls reached puberty at a similar age to that previously reported for Bos indicus × Bos taurus crossbred bulls (Chase et al. 2001; Brito et al. 2004) and intermediate to that reported for Bos taurus (Lunstra et al. 1978; Evans et al. 1995) and Bos indicus breeds (Fields et al. 1982; Aponte et al. 2005). The earlier age of puberty observed in the HPeri bull cohort is a desirable production outcome (Barth and Ominski 2000).
There was no effect of maternal dietary treatment upon scrotal circumference or paired testis weight at 600 days. These findings are in agreement with those in rams (6 weeks and 20 months of age) where Rae et al. (2002) reported no effect of maternal undernutrition on scrotal circumference. The absence of in utero dietary effect upon scrotal circumference concurs with the observed lack of effect upon fetal testis weight and Sertoli cell count at 98 dpc. Consequently, the effects of maternal protein restriction on sperm parameters and age of puberty were considered to be not directly the result of altered Sertoli cell numbers in the developing postnatal animal (Sharpe et al. 2003).
The effects on sperm parameters were, however, subsequent to lower FSH concentrations in the LPeri cohort; FSH is an integral part of the hormonal cascade regulating sperm production (Perry et al. 1991), epididymal function (Grover et al. 2005) and spermatogenesis in the mature bull (Barth and Bowman 1994; Matsuzaki et al. 2000; O’Shaughnessy 2014). In ruminants, no comparative studies have documented the associations between prenatal nutrition and sperm abnormalities in the progeny. The results are, however, consistent with those in the adult male rat (90 days of age) where Toledo et al. (2011) reported impairment of sperm counts, sperm motility and higher levels of spermatozoa with morphological abnormalities following in utero protein restriction (0–21 dpc).
The relationship between reduced FSH and delayed postnatal activation of the reproductive axis observed in the pubertal and postpubertal LPeri cohort concurs with previous research: FSH levels, along with LH, rise transiently between 1 and 4 months of age in the prepubertal bull (Rawlings et al. 1978; Evans et al. 1996; Moura and Erickson 1997; Kaneko et al. 2001; Bagu et al. 2006), a rise reported to be associated with the initiation of rapid testis growth (Moura and Erickson 1997). FSH levels then fall, remaining low during peri-puberty and puberty (Moura and Erickson 1997; Kaneko et al. 2001; Brito et al. 2007c, 2007d). In the adult, FSH levels increase as bulls age in association with improvement in sperm quality and quantity (Matsuzaki et al. 2000). Thus, the observed lower basal FSH in the LPeri bulls during the pubertal and postpubertal period (330 and 438 days of age) may potentially indicate a hormonal regulation pathway contributing to the delayed elevation of sperm traits discussed above.
Collectively, the lack of effect of maternal diet on testosterone (Rawlings et al. 2008), prepubertal inhibin (Kaneko et al. 2001) and prepubertal activin A, (Mather et al. 1992), all known to be involved in regulation of postnatal FSH secretion in the developing bull, would suggest that the differences in FSH levels associated with the PERI diet were modulated via other pathways. Alternatively, the monthly blood sampling regimen may have been inadequate to detect the effects of maternal diet on testosterone, inhibin or activin A, particularly considering the pulsatile and diurnal nature of testosterone secretion.
The later age of puberty in the LPeri bulls was also associated with higher AMH levels at 10 months. This may suggest a delay in the downregulation of AMH expression that occurs at puberty (Rey and Josso 1996; Rey et al. 2003) coincident with Sertoli cell maturation (Sharpe et al. 2003). As circulating AMH levels decline sharply in the pubertal bull (Rota et al. 2002), it is possible the differences measured at one time point may reflect differences in maturity as opposed to resulting from the dietary perturbation. However, as birthweight and postnatal liveweights were similar, the observed effects on age of puberty are unlikely to have been mediated by the persisting influences of prenatal nutrition on postnatal growth (Micke et al. 2010). This is further supported by the lack of maternal dietary effect upon progeny IGF1 and leptin profiles; the relationship between energy homeostasis and puberty being well recognised (Blache et al. 2003; Barb and Kraeling 2004; Zieba et al. 2005; Brito et al. 2007a, 2007c, 2007d; Barth et al. 2008). Collectively these observations indicate that postnatal diet and liveweight were not involved in the observed changes in postnatal reproductive development, in contrast to findings reported in prenatally growth-restricted rams (Da Silva et al. 2001).
Early maternal undernutrition has been reported to disrupt a range of endocrine pathways with long-term effects on progeny health (McMillen and Robinson 2005; Gardner et al. 2006; McMillen et al. 2008). Furthermore, previous studies support the concept that early maternal undernutrition impacts hypothalamic and/or pituitary function at later postnatal stages, causing alterations to the endocrine system. These include changes in gonadotrophin profiles (Rae et al. 2002), reduced testosterone concentrations and delayed seasonal increase in testosterone (Da Silva et al. 2001) as well as altered hypothalamic–pituitary responsiveness to postnatal GnRH challenge in sheep (Kotsampasi et al. 2009) and prepubertal bulls (Sullivan et al. 2010). In the present study, a GnRH challenge was not undertaken and bulls were allowed to progress through puberty without any exogenous hormonal influence; hence, it is not possible to report on pituitary responsiveness in this study. Further studies are required to explore the role of maternal nutrition on the development and function of the hypothalamic–pituitary–gonadal axis in both the fetal and adult male bovine.
Conclusion
In summary, we have uniquely shown that in the developing bull the LPeri maternal diet delayed the onset of puberty and sexual maturity with negative effects on semen parameters in the early postpubertal period. These effects were subsequent to lower FSH concentrations in the LPeri diet group. The histology of the fetal and adult testis suggests that the early perturbation of the cytology of the testis has been compensated for during later development as no corresponding effects were observed in the adult testis. Whether the effects of this perturbation influenced testicular function through puberty before excision of the testis at 20 months, however, is unknown. The circulating hormone data suggest that the peri-conception diet may have altered the development of the hypothalamic–pituitary–gonadal axis or the receptivity to circulating hormones during the peri-pubertal period.
Whilst this study provides evidence that low maternal dietary protein has a negative impact on reproductive development in the pubertal and postpubertal offspring, some of the mechanisms that mediate this effect remain to be elucidated. Further research in cattle is warranted to enable exploration of causal relationships between gestational nutrition and consequent postnatal male reproductive development of progeny.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are grateful for the excellent support provided by Ray Cranney, Wendy Bonner, Katja Hummitzsch, Helen Irving-Rodgers, Nic Hatzirodos, Paul Jonas, Mark Irrgang (deceased) and staff at Tungali Feedlot, along with the statistical support provided by the Data Management and Analysis Centre, The University of Adelaide. We also acknowledge Dr Dominique Blache and Margaret Blackberry (University of Western Australia) and Aziza Alibhai (University of Nottingham) for their contributions to laboratory analysis in this work. This research was funded by an Australian Research Council linkage grant (Project ID: LP110100649) with S. Kidman and Co. and Ridley AgriProducts to V. E. A. Perry, R. J. Rodgers and I. C. McMillen. K. J. Copping was funded by an Australian Postgraduate award and M. D. Ruiz-Diaz by a University of Nottingham Vice Chancellor’s award to C. S. Rutland, V. E. A. Perry and N. P. Mongan. R. J. Rodgers is funded by the National Health and Medical Research Council of Australia.
References
Abd-Elmaksoud, A. (2005) ‘Morphological, Glycohistochemical, and Immunohistochemical Studies on the Embryonic and Adult Bovine Testis’. (LMU München: Munich.)Andrade, L. P., Rhind, S. M., Rae, M. T., Kyle, C. E., Jowett, J., and Lea, R. G. (2013). Maternal undernutrition does not alter Sertoli cell numbers or the expression of key developmental markers in the mid-gestation ovine fetal testis. J. Negat. Results Biomed. 12, 2.
| Maternal undernutrition does not alter Sertoli cell numbers or the expression of key developmental markers in the mid-gestation ovine fetal testis.Crossref | GoogleScholarGoogle Scholar |
Aponte, P. M., de Rooij, D. G., and Bastidas, P. (2005). Testicular development in Brahman bulls. Theriogenology 64, 1440–1455.
| Testicular development in Brahman bulls.Crossref | GoogleScholarGoogle Scholar |
Ashworth, C. J., Toma, L. M., and Hunter, M. G. (2009). Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3351–3361.
| Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability.Crossref | GoogleScholarGoogle Scholar |
Atkinson, S., and Adams, N. R. (1988). Adrenal glands alter the concentration of oestradiol-17 beta and its receptor in the uterus of ovariectomized ewes. J. Endocrinol. 118, 375–380.
| Adrenal glands alter the concentration of oestradiol-17 beta and its receptor in the uterus of ovariectomized ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXlt1aksLg%3D&md5=60d69a394e57ebc7df0501258d94b3d8CAS |
Bagu, E. T., Cook, S., Gratton, C. L., and Rawlings, N. C. (2006). Postnatal changes in testicular gonadotropin receptors, serum gonadotropin, and testosterone concentrations and functional development of the testes in bulls. Reproduction 132, 403–411.
| Postnatal changes in testicular gonadotropin receptors, serum gonadotropin, and testosterone concentrations and functional development of the testes in bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFCgt7%2FO&md5=04eecf36536d30f435cc1ee70aac61a2CAS |
Barb, C. R., and Kraeling, R. R. (2004). Role of leptin in the regulation of gonadotropin secretion in farm animals. Anim. Reprod. Sci. 82–83, 155–167.
| Role of leptin in the regulation of gonadotropin secretion in farm animals.Crossref | GoogleScholarGoogle Scholar |
Barth, A. D., and Bowman, P. A. (1994). The sequential appearance of sperm abnormalities after scrotal insulation or dexamethasone treatment in bulls. Can. Vet. J. 35, 93–102.
| 1:STN:280:DyaK2czkvFaksQ%3D%3D&md5=eacbdc29c6436d328e0977b9329e166bCAS |
Barth, A. D., and Oko, R. J. (1989) ‘Abnormal Morphology of Bovine Spermatozoa’. 1st edn. (Iowa State University Press: Ames, IA, USA.)
Barth, A. D., and Ominski, K. H. (2000). The relationship between scrotal circumference at weaning and at one year of age in beef bulls. Can. Vet. J. 41, 541–546.
| 1:STN:280:DC%2BD3cvhvVGqsA%3D%3D&md5=d18dc5c3826279ea10e82dfd9860027bCAS |
Barth, A. D., Brito, L. F., and Kastelic, J. P. (2008). The effect of nutrition on sexual development of bulls. Theriogenology 70, 485–494.
| The effect of nutrition on sexual development of bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVOksL8%3D&md5=6264dbbf9ad79413c2da1b92e340b591CAS |
Beggs, D. S., Bertram, J., Chenoweth, P. J., Entwistle, K., Fordyce, G., Johnston, H., Johnston, P., McGowan, M. R., Niethe, G., Norman, S., and Perry, V. E. A. (2013) ‘Veterinary Bull Breeding Soundness Evaluation’. (Australian Cattle Veterinarians: Brisbane.)
Bielli, A., Pérez, R., Pedrana, G., Milton, J. T. B., Lopez, Á., Blackberry, M. A., Duncombe, G., Rodriguez-Martinez, H., and Martin, G. B. (2002). Low maternal nutrition during pregnancy reduces the number of Sertoli cells in the newborn lamb. Reprod. Fertil. Dev. 14, 333–337.
| Low maternal nutrition during pregnancy reduces the number of Sertoli cells in the newborn lamb.Crossref | GoogleScholarGoogle Scholar |
Blache, D., Tellam, R. L., Chagas, L. M., Blackberry, M. A., Vercoe, P. E., and Martin, G. B. (2000). Level of nutrition affects leptin concentrations in plasma and cerebrospinal fluid in sheep. J. Endocrinol. 165, 625–637.
| Level of nutrition affects leptin concentrations in plasma and cerebrospinal fluid in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktl2qtrw%3D&md5=c8bb440f4c797d5a5f8b657b5f8714b3CAS |
Blache, D., Zhang, S., and Martin, G. B. (2003) Fertility in males: modulators of the acute effects of nutrition on the reproductive axis of male sheep. In ‘Reproduction in Domestic Ruminants V’. (Eds B. K. Campbell, R. Webb, H. Dobson and C. Doberska.) pp. 387–402. (Society for Reproduction and Fertility: Cambridge.)
Bortolussi, G., McIvor, J. G., Hodgkinson, J. J., Coffey, S. G., and Holmes, C. R. (2005). The northern Australian beef industry, a snapshot. 2. Breeding herd performance and management. Aust. J. Exp. Agric. 45, 1075–1091.
| The northern Australian beef industry, a snapshot. 2. Breeding herd performance and management.Crossref | GoogleScholarGoogle Scholar |
Breier, B. H., Gallaher, B. W., and Gluckman, P. D. (1991). Radioimmunoassay for insulin-like growth factor-1: solutions to some potential problems and pitfalls. J. Endocrinol. 128, 347–357.
| Radioimmunoassay for insulin-like growth factor-1: solutions to some potential problems and pitfalls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhtFGiur8%3D&md5=f23a3f7c2247861de37a614d568bceeaCAS |
Brito, L. F., Silva, A. E., Unanian, M. M., Dode, M. A., Barbosa, R. T., and Kastelic, J. P. (2004). Sexual development in early- and late-maturing Bos indicus and Bos indicus × Bos taurus crossbred bulls in Brazil. Theriogenology 62, 1198–1217.
| Sexual development in early- and late-maturing Bos indicus and Bos indicus × Bos taurus crossbred bulls in Brazil.Crossref | GoogleScholarGoogle Scholar |
Brito, L., Barth, A., Rawlings, N., Wilde, R., Crews, D., Mir, P., and Kastelic, J. (2007a). Circulating metabolic hormones during the peripubertal period and their association with testicular development in bulls. Reprod. Domest. Anim. 42, 502–508.
| Circulating metabolic hormones during the peripubertal period and their association with testicular development in bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFKntLjN&md5=868826449a8b7fc58d162d75daab893bCAS |
Brito, L. F., Barth, A. D., Rawlings, N. C., Wilde, R. E., Crews, D. H., Boisclair, Y. R., Ehrhardt, R. A., and Kastelic, J. P. (2007b). Effect of feed restriction during calfhood on serum concentrations of metabolic hormones, gonadotropins, testosterone, and on sexual development in bulls. Reproduction 134, 171–181.
| Effect of feed restriction during calfhood on serum concentrations of metabolic hormones, gonadotropins, testosterone, and on sexual development in bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvFGisL8%3D&md5=7434793710ed9c1bdb59cedc91751668CAS |
Brito, L. F., Barth, A. D., Rawlings, N. C., Wilde, R. E., Crews, D. H., Mir, P. S., and Kastelic, J. P. (2007c). Effect of improved nutrition during calfhood on serum metabolic hormones, gonadotropins, and testosterone concentrations, and on testicular development in bulls. Domest. Anim. Endocrinol. 33, 460–469.
| Effect of improved nutrition during calfhood on serum metabolic hormones, gonadotropins, and testosterone concentrations, and on testicular development in bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFenu73E&md5=d6726d87cf5457169e8a5b81b58fa216CAS |
Brito, L. F., Barth, A. D., Rawlings, N. C., Wilde, R. E., Crews, D. H., Mir, P. S., and Kastelic, J. P. (2007d). Effect of nutrition during calfhood and peripubertal period on serum metabolic hormones, gonadotropins and testosterone concentrations, and on sexual development in bulls. Domest. Anim. Endocrinol. 33, 1–18.
| Effect of nutrition during calfhood and peripubertal period on serum metabolic hormones, gonadotropins and testosterone concentrations, and on sexual development in bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtVGhu74%3D&md5=838aa9d47f553e921ff66bcd33494babCAS |
Burns, B. M., Fordyce, G., and Holroyd, R. G. (2010). A review of factors that impact on the capacity of beef cattle females to conceive, maintain a pregnancy and wean a calf – implications for reproductive efficiency in northern Australia. Anim. Reprod. Sci. 122, 1–22.
| A review of factors that impact on the capacity of beef cattle females to conceive, maintain a pregnancy and wean a calf – implications for reproductive efficiency in northern Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cfotFWnsA%3D%3D&md5=30dfc179d97e374b347d0e38e597ed4aCAS |
Burns, B. M., Corbet, N. J., Corbet, D. H., Crisp, J. M., Venus, B. K., Johnston, D. J., Li, Y., McGowan, M. R., and Holroyd, R. G. (2013). Male traits and herd reproductive capability in tropical beef cattle. 1. Experimental design and animal measures. Anim. Prod. Sci. 53, 87–100.
| Male traits and herd reproductive capability in tropical beef cattle. 1. Experimental design and animal measures.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXns12jsA%3D%3D&md5=fe4ca858268dd42d043380f468c49e05CAS |
Callaghan, M. J., McAuliffe, P., Rodgers, R. J., Hernandez-Medrano, J., and Perry, V. E. (2016). Subacute ruminal acidosis reduces sperm quality in beef bulls. J. Anim. Sci. 94, 3215–3228.
| Subacute ruminal acidosis reduces sperm quality in beef bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhvVylt7fO&md5=19aa20511839f587e3c5ef7d0858dba3CAS |
Chagas, L. M., Gore, P. J. S., Meier, S., Macdonald, K. A., and Verkerk, G. A. (2007). Effect of monopropylene glycol on luteinizing hormone, metabolites, and postpartum anovulatory intervals in primiparous dairy cows. J. Dairy Sci. 90, 1168–1175.
| Effect of monopropylene glycol on luteinizing hormone, metabolites, and postpartum anovulatory intervals in primiparous dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitlWntLk%3D&md5=775d71a4fde63283a1e4fb62233d0e0cCAS |
Chase, C. C. J., Chenoweth, P. J., Larsen, R. E., Hammond, A. C., Olson, T. A., West, R. L., and Johnson, D. D. (2001). Growth, puberty, and carcass characteristics of Brahman-, Senepol-, and Tuli-sired F-1 Angus bulls. J. Anim. Sci. 79, 2006–2015.
| Growth, puberty, and carcass characteristics of Brahman-, Senepol-, and Tuli-sired F-1 Angus bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtFalsb8%3D&md5=bc6c03b70e7a85402cec9579d981f489CAS |
Chavatte-Palmer, P., Dupont, C., Debus, N., and Camous, S. (2014). Nutritional programming and the reproductive function of the offspring. Anim. Prod. Sci. 54, 1166–1176.
| Nutritional programming and the reproductive function of the offspring.Crossref | GoogleScholarGoogle Scholar |
Copping, K. J., Hoare, A., Callaghan, M., McMillen, I. C., Rodgers, R. J., and Perry, V. E. A. (2014). Fetal programming in 2-year-old calving heifers: peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner. Anim. Prod. Sci. 54, 1333–1337.
| Fetal programming in 2-year-old calving heifers: peri-conception and first trimester protein restriction alters fetal growth in a gender-specific manner.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtlaktLnP&md5=67f5ddbb0bf064f0e48884743e8ac3c9CAS |
Corbet, N. J., Burns, B. M., Johnston, D. J., Wolcott, M. L., Corbet, D. H., Venus, B. K., Li, Y., McGowan, M. R., and Holroyd, R. G. (2013). Male traits and herd reproductive capability in tropical beef cattle. 2. Genetic parameters of bull traits. Anim. Prod. Sci. 53, 101–113.
| Male traits and herd reproductive capability in tropical beef cattle. 2. Genetic parameters of bull traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXns12jtg%3D%3D&md5=69fb8deca5691ccb5eb879d8e7c4d85dCAS |
Da Silva, P., Aitken, R. P., Rhind, S. M., Racey, P. A., and Wallace, J. M. (2001). Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs. Reproduction 122, 375–383.
| Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntVGksLY%3D&md5=202df959223f9b13cab399913dcde5dcCAS |
Da Silva, P., Aitken, R. P., Rhind, S. M., Racey, P. A., and Wallace, J. M. (2003). Effect of maternal overnutrition during pregnancy on pituitary gonadotrophin gene expression and gonadal morphology in female and male foetal sheep at Day 103 of gestation. Placenta 24, 248–257.
| Effect of maternal overnutrition during pregnancy on pituitary gonadotrophin gene expression and gonadal morphology in female and male foetal sheep at Day 103 of gestation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlOltA%3D%3D&md5=e73873dc961a5ea9fde813cbc27fdc48CAS |
Dupont, C., Cordier, A. G., Junien, C., Mandon-Pepin, B., Levy, R., and Chavatte-Palmer, P. (2012). Maternal environment and the reproductive function of the offspring. Theriogenology 78, 1405–1414.
| Maternal environment and the reproductive function of the offspring.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38bhs1emug%3D%3D&md5=03e798dbcd50d526125043beab544a58CAS |
Edwards, L. J., and McMillen, I. C. (2002). Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo–pituitary adrenal axis in sheep during late gestation. Biol. Reprod. 66, 1562–1569.
| Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo–pituitary adrenal axis in sheep during late gestation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjtFWnsLY%3D&md5=9b313af949889ebb8305e46892d4a077CAS |
Entwistle, K. W., and Fordyce, G. (2003) ‘Evaluating and Reporting Bull Fertility’. (Australian Association of Cattle Veterinarians: Eight Mile Plains, Qld, Australia)
Evans, A. C. O., Davies, F. J., Nasser, L. F., Bowman, P., and Rawlings, N. C. (1995). Differences in early patterns of gonadotrophin secretion between early and late maturing bulls, and changes in semen characteristics at puberty. Theriogenology 43, 569–578.
| Differences in early patterns of gonadotrophin secretion between early and late maturing bulls, and changes in semen characteristics at puberty.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXksVOgtr4%3D&md5=21091a25524d94fde6a5b491f493f7d8CAS |
Evans, A. C., Pierson, R. A., Garcia, A., McDougall, L. M., Hrudka, F., and Rawlings, N. C. (1996). Changes in circulating hormone concentrations, testes histology and testes ultrasonography during sexual maturation in beef bulls. Theriogenology 46, 345–357.
| Changes in circulating hormone concentrations, testes histology and testes ultrasonography during sexual maturation in beef bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlsVShsb4%3D&md5=4ea10e470a39272450be8c623f3472eeCAS |
Fair, T. (2010). Mammalian oocyte development: checkpoints for competence. Reprod. Fertil. Dev. 22, 13–20.
| Mammalian oocyte development: checkpoints for competence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitlagurg%3D&md5=9b3fe3fd237a07bd06aefffc4b1264ecCAS |
Fields, M. J., Hentges, J. F., and Cornelisse, K. W. (1982). Aspects of the sexual development of Brahman versus Angus bulls in Florida. Theriogenology 18, 17–31.
| Aspects of the sexual development of Brahman versus Angus bulls in Florida.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283pvVCqtA%3D%3D&md5=513070772562310c8242fb8fc73290b8CAS |
Fordyce, G., Entwistle, K., Norman, S., Perry, V., Gardiner, B., and Fordyce, P. (2006). Standardising bull breeding soundness evaluations and reporting in Australia. Theriogenology 66, 1140–1148.
| Standardising bull breeding soundness evaluations and reporting in Australia.Crossref | GoogleScholarGoogle Scholar |
Freer, M. (2007) ‘Nutrient Requirements of Domesticated Ruminants’. (CSIRO Publishing: Collingwood, Vic., Australia.)
Gardner, D. S., Van Bon, B. W., Dandrea, J., Goddard, P. J., May, S. F., Wilson, V., Stephenson, T., and Symonds, M. E. (2006). Effect of periconceptional undernutrition and gender on hypothalamic–pituitary–adrenal axis function in young adult sheep. J. Endocrinol. 190, 203–212.
| Effect of periconceptional undernutrition and gender on hypothalamic–pituitary–adrenal axis function in young adult sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Wjtrc%3D&md5=9cd58d3c31009894cc15b93fde2306b7CAS |
Godinho, H., Cardoso, M., and Nogueria, J. (1973). Patterns of parenchymal ramification of the testicular artery in some ruminants. Anat. Anz. 133, 118–124.
| 1:STN:280:DyaE3s3htF2jsg%3D%3D&md5=7bb725642510bd79b23a5799e1377d76CAS |
Griswold, M. D., and McLean, D. J. (2006) The Sertoli cell. In ‘Knobil and Neill’s Physiology of Reproduction.’ (Ed. J. D. Neill.) pp. 949–975. (Elsevier: New York.)
Grover, A., Smith, C. E., Gregory, M., Cyr, D. G., Sairam, M. R., and Hermo, L. (2005). Effects of FSH receptor deletion on epididymal tubules and sperm morphology, numbers, and motility. Mol. Reprod. Dev. 72, 135–144.
| Effects of FSH receptor deletion on epididymal tubules and sperm morphology, numbers, and motility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXnt1ygu7w%3D&md5=8aca452e6bc5c5208dbab48cf2915fd2CAS |
Hernandez-Medrano, J. H., Copping, K. J., Hoare, A., Wapanaar, W., Grivell, R., Kuchel, T., Miguel-Pacheco, G., McMillen, I. C., Rodgers, R. J., and Perry, V. E. (2015). Gestational dietary protein is associated with sex specific decrease in blood flow, fetal heart growth and post-natal blood pressure of progeny. PLoS One 10, e0125694.
| Gestational dietary protein is associated with sex specific decrease in blood flow, fetal heart growth and post-natal blood pressure of progeny.Crossref | GoogleScholarGoogle Scholar |
Holroyd, R. G., Doogan, V. J., DeFaveri, J., Fordyce, G., McGowane, M. R., Bertram, J. D., Vankang, D. M., Fitzpatrick, L. A., Jayawardhanai, G. A., and Miller, R. G. (2002). Bull selection and use in northern Australia 4. Calf output and predictors of fertility of bulls in multiple-sire herds. Anim. Reprod. Sci. 71, 67–79.
| Bull selection and use in northern Australia 4. Calf output and predictors of fertility of bulls in multiple-sire herds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD383kvFSrtw%3D%3D&md5=2f09a9b085dbafc60fb901f00108edd5CAS |
Hopper, R. M. (2014) ‘Bovine Reproduction’. 1st edn. (John Wiley & Sons, Inc.: Ames, IA, USA.)
Jégou, B. (1992). The Sertoli cell in vivo and in vitro. Cell Biol. Toxicol. 8, 49–54.
| The Sertoli cell in vivo and in vitro.Crossref | GoogleScholarGoogle Scholar |
Kaneko, H., Noguchi, J., Kikuchi, K., Akagi, S., Shimada, A., Taya, K., Watanabe, G., and Hasegawa, Y. (2001). Production and endocrine role of inhibin during the early development of bull calves. Biol. Reprod. 65, 209–215.
| Production and endocrine role of inhibin during the early development of bull calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXkslWhtbk%3D&md5=2780502270000498a3856c4987c672a3CAS |
Klonisch, T., Fowler, P. A., and Hombach-Klonisch, S. (2004). Molecular and genetic regulation of testis descent and external genitalia development. Dev. Biol. 270, 1–18.
| Molecular and genetic regulation of testis descent and external genitalia development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsl2gt7w%3D&md5=d7683271608743f6d4b02fb868e67500CAS |
Knight, P. G., Muttukrishna, S., and Groome, N. P. (1996). Development and application of a two-site enzyme immunoassay for the determination of ‘total’ activin-A concentrations in serum and follicular fluid. J. Endocrinol. 148, 267–279.
| Development and application of a two-site enzyme immunoassay for the determination of ‘total’ activin-A concentrations in serum and follicular fluid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtVCrsrg%3D&md5=c2fcf3f024e28b1b5903af4db4491212CAS |
Kotsampasi, B., Balaskas, C., Papadomichelakis, G., and Chadio, S. E. (2009). Reduced Sertoli cell number and altered pituitary responsiveness in male lambs undernourished in utero. Anim. Reprod. Sci. 114, 135–147.
| Reduced Sertoli cell number and altered pituitary responsiveness in male lambs undernourished in utero.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms1yqu7c%3D&md5=de3622544aa6b3e3b1bbcf7e009f1fe7CAS |
Lunstra, D. D., and Echternkamp, S. E. (1982). Puberty in beef bulls: acrosome morphology and semen quality in bulls of different breeds. J. Anim. Sci. 55, 638–648.
| Puberty in beef bulls: acrosome morphology and semen quality in bulls of different breeds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s%2FjtVGmuw%3D%3D&md5=a48d3563465d1d464b47f262eaffff22CAS |
Lunstra, D. D., Ford, J. J., and Echternkamp, S. E. (1978). Puberty in beef bulls: hormone concentrations, growth, testicular development, sperm production and sexual aggressiveness in bulls of different breeds. J. Anim. Sci. 46, 1054–1062.
| Puberty in beef bulls: hormone concentrations, growth, testicular development, sperm production and sexual aggressiveness in bulls of different breeds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1cXksVSjsLo%3D&md5=ca757ca7cf56c1c555b1db753f209656CAS |
Mather, J. P., Woodruff, T. K., and Krummen, L. A. (1992). Paracrine regulation of reproductive function by inhibin and activin. Proc. Soc. Exp. Biol. Med. 201, 1–15.
| Paracrine regulation of reproductive function by inhibin and activin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XmtVGgsrk%3D&md5=af91bbc4ffbbd2aeca9860dadcc3637fCAS |
Matsuzaki, S., Uenoyama, Y., Okuda, K., Watanabe, G., Kitamura, N., Taya, K., and Yamada, J. (2000). Age-related changes in the serum levels of inhibin, FSH, LH and testosterone in Holstein bulls. J. Reprod. Dev. 46, 245–248.
| Age-related changes in the serum levels of inhibin, FSH, LH and testosterone in Holstein bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnvV2qs7Y%3D&md5=6a219055bd631145633c1ed58d8b65a6CAS |
Mayhew, T. M. (1991). The new stereological methods for interpreting functional morphology from slices of cells and organs. Exp. Physiol. 76, 639–665.
| The new stereological methods for interpreting functional morphology from slices of cells and organs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38%2FnsFanuw%3D%3D&md5=ce3f3db4ebb723b787039eb326183483CAS |
Mayhew, T. M. (2011). Mapping the distributions and quantifying the labelling intensities of cell compartments by immunoelectron microscopy: progress towards a coherent set of methods. J. Anat. 219, 647–660.
| Mapping the distributions and quantifying the labelling intensities of cell compartments by immunoelectron microscopy: progress towards a coherent set of methods.Crossref | GoogleScholarGoogle Scholar |
McAuliffe, P., Johnston, H., Johnston, P., and Perry, V. (2010). ‘Electroejaculators, Morphology and Microscopes’. (The Australian Cattle Veterinarians: Eight Mile Plains, Qld, Australia.)
McLachlan, R. I., Robertson, D. M., Burger, H. G., and de Kretser, D. M. (1986). The radioimmunoassay of bovine and human follicular fluid and serum inhibin. Mol. Cell. Endocrinol. 46, 175–185.
| The radioimmunoassay of bovine and human follicular fluid and serum inhibin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XkvFWnu70%3D&md5=eb5b4c922c03f405a111d963d42d3496CAS |
McMillen, I. C., and Robinson, J. S. (2005). Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633.
| Developmental origins of the metabolic syndrome: prediction, plasticity, and programming.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjt12lsLw%3D&md5=5055c371085737e166335faf9a33ad88CAS |
McMillen, I. C., MacLaughlin, S. M., Muhlhausler, B. S., Gentili, S., Duffield, J. L., and Morrison, J. L. (2008). Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin. Pharmacol. Toxicol. 102, 82–89.
| Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhvF2gsrg%3D&md5=b7850b6d0c8bf0859b90d5316f1a8bb1CAS |
Micke, G. C., Sullivan, T. M., Gatford, K. L., Owens, J. A., and Perry, V. E. (2010). Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Anim. Reprod. Sci. 121, 208–217.
| Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtV2hsrrE&md5=287a6ca6c483242f79fd844dd68220ebCAS |
Micke, G. C., Sullivan, T. M., McMillen, I. C., Gentili, S., and Perry, V. E. (2011). Heifer nutrient intake during early- and mid-gestation programs adult offspring adiposity and mRNA expression of growth-related genes in adipose depots. Reproduction 141, 697–706.
| Heifer nutrient intake during early- and mid-gestation programs adult offspring adiposity and mRNA expression of growth-related genes in adipose depots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFCis78%3D&md5=bb9ea69900d59ce658310aea77e13159CAS |
Micke, G. C., Sullivan, T. M., Kennaway, D. J., Hernandez-Medrano, J., and Perry, V. E. (2015). Maternal endocrine adaptation throughout pregnancy to nutrient manipulation: consequences for sexually dimorphic programming of thyroid hormones and development of their progeny. Theriogenology 83, 604–615.
| Maternal endocrine adaptation throughout pregnancy to nutrient manipulation: consequences for sexually dimorphic programming of thyroid hormones and development of their progeny.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhvFKmsb7F&md5=d3e50885502b4ac3571c7f15064574f7CAS |
Mossa, F., Carter, F., Walsh, S. W., Kenny, D. A., Smith, G. W., Ireland, J. L. H., Hildebrandt, T. B., Lonergan, P., Ireland, J. J., and Evans, A. C. O. (2013). Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88, 92.
| Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring.Crossref | GoogleScholarGoogle Scholar |
Mossa, F., Walsh, S. W., Ireland, J. J., and Evans, A. C. O. (2015). Early nutritional programming and progeny performance: is reproductive success already set at birth? Anim. Front. 5, 18–24.
| Early nutritional programming and progeny performance: is reproductive success already set at birth?Crossref | GoogleScholarGoogle Scholar |
Moura, A. A., and Erickson, B. H. (1997). Age-related changes in peripheral hormone concentrations and their relationships with testis size and number of Sertoli and germ cells in yearling beef bulls. J. Reprod. Fertil. 111, 183–190.
| Age-related changes in peripheral hormone concentrations and their relationships with testis size and number of Sertoli and germ cells in yearling beef bulls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlsFelsQ%3D%3D&md5=b8b5753c1f0010bfee2096c0a7eabf22CAS |
Norman, M. (1963). Dry season protein and energy supplements for beef cattle on native pastures at Katherine, N.T. Aust. J. Exp. Agric. 3, 280–283.
| Dry season protein and energy supplements for beef cattle on native pastures at Katherine, N.T.Crossref | GoogleScholarGoogle Scholar |
O’Shaughnessy, P. J. (2014). Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 29, 55–65.
| Hormonal control of germ cell development and spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXksFWms7g%3D&md5=f9b16f9cc07a8569d214d3e35d2d7e12CAS |
O’Shaughnessy, P. J., and Fowler, P. A. (2011). Endocrinology of the mammalian fetal testis. Reproduction 141, 37–46.
| Endocrinology of the mammalian fetal testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisVehs78%3D&md5=3114961b9b2fda2755c3a78d26a263e2CAS |
Perry, V. E. A., Munro, R. K., Chenoweth, P. J., Bodero, D. A. V., and Post, T. B. (1990). Relationships among bovine male and female reproductive traits. Aust. Vet. J. 67, 4–5.
| Relationships among bovine male and female reproductive traits.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c3ktFWjtA%3D%3D&md5=03e9d8e9daba7a4d8aca1e2a0dad866aCAS |
Perry, V. E. A., Chenoweth, P. J., Post, T. B., and Munro, R. K. (1991). Patterns of development of gonads, sex-drive and hormonal responses in tropical beef bulls. Theriogenology 35, 473–486.
| Patterns of development of gonads, sex-drive and hormonal responses in tropical beef bulls.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283pvFKrsg%3D%3D&md5=a7388be421dfe4d0ec34744f9842307cCAS |
Polguj, M., Wysiadecki, G., Podgorski, M., Szymanski, J., Olbrych, K., Olewnik, L., and Topol, M. (2015). Morphological variations of intra-testicular arterial vasculature in bovine testis – a corrosion casting study. BMC Vet. Res. 11, 263.
| Morphological variations of intra-testicular arterial vasculature in bovine testis – a corrosion casting study.Crossref | GoogleScholarGoogle Scholar |
Rae, M. T., Kyle, C. E., Miller, D. W., Hammond, A. J., Brooks, A. N., and Rhind, S. M. (2002). The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim. Reprod. Sci. 72, 63–71.
| The effects of undernutrition, in utero, on reproductive function in adult male and female sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XltFahsLw%3D&md5=e1326288eed29faa7518bc0ba8274fb8CAS |
Rawlings, N. C., Fletcher, P. W., Henricks, D. M., and Hill, J. R. (1978). Plasma luteinizing hormone (LH) and testosterone levels during sexual maturation in beef bull calves Biol. Reprod. 19, 1108–1112.
| Plasma luteinizing hormone (LH) and testosterone levels during sexual maturation in beef bull calvesCrossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhtFyrurk%3D&md5=b010b4e2a8e4c90b198816eb0cedf8adCAS |
Rawlings, N., Evans, A. C., Chandolia, R. K., and Bagu, E. T. (2008). Sexual maturation in the bull. Reprod. Domest. Anim. 43, 295–301.
| Sexual maturation in the bull.Crossref | GoogleScholarGoogle Scholar |
Rey, R., and Josso, N. (1996). Regulation of testicular anti-Müllerian hormone secretion. Eur. J. Endocrinol. 135, 144–152.
| Regulation of testicular anti-Müllerian hormone secretion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlsVOgs78%3D&md5=e3120b84a730c337f9a85fb875777bb1CAS |
Rey, R., Lukas-Croisier, C., Lasala, C., and Bedecarrás, P. (2003). AMH/MIS: what we know already about the gene, the protein and its regulation. Mol. Cell. Endocrinol. 211, 21–31.
| AMH/MIS: what we know already about the gene, the protein and its regulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXptlOrsL0%3D&md5=410d951d8fbaaae8e2c8c5278ab5fc01CAS |
Robertson, D. M., Foulds, L. M., Prisk, M., and Hedger, M. P. (1992). Inhibin/activin β-subunit monomer: isolation and characterization. Endocrinology 130, 1680–1687.
| 1:CAS:528:DyaK38Xhs1Kmt74%3D&md5=e0ad7d7e4e4047077a9e91478110675bCAS |
Rodríguez-González, G. L., Reyes-Castro, L. A., Vega, C. C., Boeck, L., Ibáñez, C., Nathanielsz, P. W., Larrea, F., and Zambrano, E. (2014). Accelerated aging of reproductive capacity in male rat offspring of protein-restricted mothers is associated with increased testicular and sperm oxidative stress. Age (Dordr.) 36, 9721.
| Accelerated aging of reproductive capacity in male rat offspring of protein-restricted mothers is associated with increased testicular and sperm oxidative stress.Crossref | GoogleScholarGoogle Scholar |
Ross, D. G., Bowles, J., Hope, M., Lehnert, S., and Koopman, P. (2009). Profiles of gonadal gene expression in the developing bovine embryo. Sex Dev. 3, 273–283.
| Profiles of gonadal gene expression in the developing bovine embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFaqtrvE&md5=e56c0a4177f1613a0dcfb62c72dceb5aCAS |
Rota, A., Ballarin, C., Vigier, B., Cozzi, B., and Rey, R. (2002). Age dependent changes in plasma anti-Müllerian hormone concentrations in the bovine male, female, and freemartin from birth to puberty: relationship between testosterone production and influence on sex differentiation. Gen. Comp. Endocrinol. 129, 39–44.
| Age dependent changes in plasma anti-Müllerian hormone concentrations in the bovine male, female, and freemartin from birth to puberty: relationship between testosterone production and influence on sex differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotFGhu7k%3D&md5=2657c64742f35cc76a95b87cf3ca6339CAS |
Sharpe, R. M., McKinnell, C., Kivlin, C., and Fisher, J. S. (2003). Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125, 769–784.
| Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXltFelt7c%3D&md5=25d41a1e73758453519bbf502574169cCAS |
Sinclair, K. D., Rutherford, K. M., Wallace, J. M., Brameld, J. M., Stoger, R., Alberio, R., Sweetman, D., Gardner, D. S., Perry, V. E., Adam, C. L., Ashworth, C. J., Robinson, J. E., and Dwyer, C. M. (2016). Epigenetics and developmental programming of welfare and production traits in farm animals. Reprod. Fertil. Dev. 28, 1443–1478.
| Epigenetics and developmental programming of welfare and production traits in farm animals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xhtl2msbbK&md5=159023c5e0742ac0b883c533fd076ecaCAS |
Spencer, T. E., and Hansen, T. R. (2015). Implantation and establishment of pregnancy in ruminants. In ‘Regulation of Implantation and Establishment of Pregnancy in Mammals: Tribute to 45-Year Anniversary of Roger V. Short’s “Maternal Recognition of Pregnancy”. Advances in Anatomy, Embryology and Cell Biology 216’. (Eds R. D. Geisert and F. W. Bazer.) pp. 105–135. (Springer International Publishing: Cham.)
Sullivan, T. M., Micke, G. C., Greer, R. M., Irving-Rodgers, H. F., Rodgers, R. J., and Perry, V. E. (2009a). Dietary manipulation of Bos indicus × heifers during gestation affects the reproductive development of their heifer calves. Reprod. Fertil. Dev. 21, 773–784.
| Dietary manipulation of Bos indicus × heifers during gestation affects the reproductive development of their heifer calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVOhu7Y%3D&md5=8aa8f27b90f08b628438cebda4c281c3CAS |
Sullivan, T. M., Micke, G. C., Magalhaes, R. S., Phillips, N. J., and Perry, V. E. (2009b). Dietary protein during gestation affects placental development in heifers. Theriogenology 72, 427–438.
| Dietary protein during gestation affects placental development in heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXptl2nsr4%3D&md5=2d4ebbf970e29b7bb7bdb6dfedd4e7a5CAS |
Sullivan, T. M., Micke, G. C., Greer, R. M., and Perry, V. E. (2010). Dietary manipulation of Bos indicus × heifers during gestation affects the prepubertal reproductive development of their bull calves. Anim. Reprod. Sci. 118, 131–139.
| Dietary manipulation of Bos indicus × heifers during gestation affects the prepubertal reproductive development of their bull calves.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVGltbo%3D&md5=cbca06ba06ba451ccfef1c0878eef75eCAS |
Toledo, F. C., Perobelli, J. E., Pedrosa, F. P., Anselmo-Franci, J. A., and Kempinas, W. D. (2011). In utero protein restriction causes growth delay and alters sperm parameters in adult male rats. Reprod. Biol. Endocrinol. 9, 94.
| In utero protein restriction causes growth delay and alters sperm parameters in adult male rats.Crossref | GoogleScholarGoogle Scholar |
Vergouwen, R. P. F. A., Jacobs, S. G. P. M., Huiskamp, R., Davids, J. A. G., and de Rooij, D. G. (1991). Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J. Reprod. Fertil. 93, 233–243.
| Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38%2FhsFSmsg%3D%3D&md5=65fb9defd824e3e32adf2e130bc90eb3CAS |
Wathes, D. C., and Wooding, F. B. (1980). An electron microscope study of implantation in the cow. Am. J. Anat. 159, 285–306.
| An electron microscope study of implantation in the cow.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M7ls12qtQ%3D%3D&md5=a3b840d281483722bcaa31212873f42aCAS |
Wolf, F. R., Almquist, J. O., and Hale, E. B. (1965). Prepubertal behavior and pubertal characteristics of beef bulls on high nutrient allowance. J. Anim. Sci. 24, 761–765.
| Prepubertal behavior and pubertal characteristics of beef bulls on high nutrient allowance.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF2M7hvVequw%3D%3D&md5=f1d1aba1de0516dc222e5be700ad2d20CAS |
Wooding, F. B. P., and Flint, A. P. F. (1994). Placentation. In ‘Marshall’s Physiology of Reproduction’. 4th edn. (Ed. G. E. Lamming.) pp. 233–460. (Chapman & Hall: London.)
Wrobel, K. H. (2000). Prespermatogenesis and spermatogoniogenesis in the bovine testis. Anat. Embryol. (Berl.) 202, 209–222.
| Prespermatogenesis and spermatogoniogenesis in the bovine testis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvksVKhsg%3D%3D&md5=89bb62e82940ab6e65864eb6942af187CAS |
Wrobel, K.-H., Sinowatz, F., and Mademann, R. (1981). Intratubular topography in the bovine testis. Cell Tissue Res. 217, 289–310.
| Intratubular topography in the bovine testis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M3htVWmuw%3D%3D&md5=258c982bb2dd59d8ea1a44a00c213679CAS |
Yilmaz, A., Davis, M. E., and Simmen, R. C. (2006). Analysis of female reproductive traits in Angus beef cattle divergently selected for blood serum insulin-like growth factor I concentration. Theriogenology 65, 1180–1190.
| Analysis of female reproductive traits in Angus beef cattle divergently selected for blood serum insulin-like growth factor I concentration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XitVKjsrw%3D&md5=8eb9363c56f1e949d958a550faf47854CAS |
Zambrano, E., Rodriguez-Gonzalez, G. L., Guzman, C., Garcia-Becerra, R., Boeck, L., Diaz, L., Menjivar, M., Larrea, F., and Nathanielsz, P. W. (2005). A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J. Physiol. 563, 275–284.
| A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXitVSnuro%3D&md5=211834c81ec652953060c6ea7a310692CAS |
Zieba, D. A., Amstalden, M., and Williams, G. L. (2005). Regulatory roles of leptin in reproduction and metabolism: a comparative review. Domest. Anim. Endocrinol. 29, 166–185.
| Regulatory roles of leptin in reproduction and metabolism: a comparative review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltVehu70%3D&md5=5a6fc0a29aebb59852835d4c972ce253CAS |