Subfertility in androgen-insensitive female mice is rescued by transgenic FSH
K. A. Walters A B , M. C. Edwards A , M. Jimenez A , D. J. Handelsman A and C. M. Allan AA ANZAC Research Institute, Andrology Laboratory, University of Sydney, Concord Hospital, Hospital Road, Concord, NSW 2139, Australia.
B Corresponding author. Email: k.walters@unsw.edu.au
Reproduction, Fertility and Development 29(7) 1426-1434 https://doi.org/10.1071/RD16022
Submitted: 12 January 2016 Accepted: 30 May 2016 Published: 22 June 2016
Abstract
Androgens synergise with FSH in female reproduction but the nature of their interaction in ovarian function and fertility is not clear. In the present study, we investigated this interaction, notably whether higher endogenous FSH can overcome defective androgen actions in androgen receptor (AR)-knockout (ARKO) mice. We generated and investigated the reproductive function of mutant mice exhibiting AR resistance with or without expression of human transgenic FSH (Tg-FSH). On the background of inactivated AR signalling, which alone resulted in irregular oestrous cycles and reduced pups per litter, ovulation rates and antral follicle health, Tg-FSH expression restored follicle health, ovulation rates and litter size to wild-type levels. However, Tg-FSH was only able to partially rectify the abnormal oestrous cycles observed in ARKO females. Hence, elevated endogenous FSH rescued the intraovarian defects, and partially rescued the extraovarian defects due to androgen insensitivity. In addition, the observed increase in litter size in Tg-FSH females was not observed in the presence of AR signalling inactivation. In summary, the findings of the present study reveal that FSH can rescue impaired female fertility and ovarian function due to androgen insensitivity in female ARKO mice by maintaining follicle health and ovulation rates, and thereby optimal female fertility.
Additional keywords: androgen receptor, female fertility, ovarian function.
Introduction
Androgens play a key role in female fertility because testosterone is an obligatory precursor for aromatisation into oestradiol, the canonical female sex steroid that is critical to female reproductive function (Hillier et al. 1994). In addition, direct androgen receptor (AR)-mediated actions of androgen are essential to optimise ovarian function and fertility (Walters 2015). The AR is expressed throughout the hypothalamic–pituitary–gonadal axis and is evolutionarily conserved. Within the ovary, the AR is expressed at most stages of follicular development, most prominently in granulosa cells (Walters et al. 2008). Using global and cell-specific AR-knockout (ARKO) female mice, we and others have proven a direct involvement of ARs in the regulation of female reproductive function (Hu et al. 2004; Shiina et al. 2006; Walters et al. 2007, 2012; Sen and Hammes 2010). Global ARKO females are subfertile and exhibit irregular oestrous cycles, an impaired ovulatory LH surge, abnormal follicular development, fewer preovulatory follicles and corpora lutea (CL) and more atretic follicles (Hu et al. 2004; Shiina et al. 2006; Walters et al. 2007; Cheng et al. 2013). Findings from analysis of granulosa cell-specific ARKO (GCARKO) female mice has identified that granulosa cells are a key site for AR-mediated actions involved in optimising female fertility, by maintaining normal follicle development (Sen and Hammes 2010; Walters et al. 2012). Furthermore, an extraovarian (neuroendocrine) role for AR-mediated actions in maintaining female fertility was proven by the findings that transplantation of wild-type control mouse ovaries into ovariectomised ARKO female mice led to irregular oestrous cycles and reduced fertility, whereas cross-transplantation of control or ARKO ovaries into ovariectomised wild-type control hosts had no effect on oestrous cycles or fertility (Walters et al. 2009). In addition, pituitary-specific ARKO (PitARKO) female mice are subfertile and exhibit reduced ovulatory FSH and LH levels, together with impaired follicle health and ovulation (Wu et al. 2014).
FSH secreted by pituitary gonadotrophs in a cyclic manner, driven by patterned hypothalamic gonadotrophin-releasing hormone (GnRH) secretion and related neuroendocrine feedback mechanisms, plays a major role in the recruitment and development of healthy ovarian follicles through to the preovulatory stage (McGee and Hsueh 2000). FSH and its receptor (FSHR) are necessary for normal follicle development because FSH-deficient female mice are infertile due to a block in early (preantral) follicle development and follicle atresia (Kumar et al. 1997). FSHRs, also localised with ARs on granulosa cells, are responsible for FSH-dependent granulosa cell proliferation, aromatisation and the appearance of LH receptor expression on theca cells in expanded antral follicles (Oktem and Urman 2010). The complex reciprocal interaction between FSH and androgen action in ovarian function is well established. FSH regulates AR expression in follicles according to developmental stage (Tetsuka and Hillier 1996) and, conversely, both testosterone (an aromatisable androgen) and dihydrotestosterone (DHT; a non-aromatisable androgen) increase FSH receptor expression in non-human primates, gilts and mice (Weil et al. 1999; Cárdenas et al. 2002; Sen et al. 2014). In primates, testosterone enhances downstream mediators of FSH effects, such as aromatase activity and cAMP formation (Hillier and De Zwart 1981; Hillier and Tetsuka 1997). In addition, androgens may synergise with FSH to stimulate follicle growth and responsiveness, because, in mice, testosterone increases preantral follicle responsiveness to FSH (Wang et al. 2001) and the non-aromatisable androgen DHT stimulates FSH-mediated mouse preantral–antral follicle growth (Sen et al. 2014). In women, it is hypothesised that the progressive decrease in circulating androgens (testosterone, dehydroepiandrosterone (DHEA) and androstenedione (A4)) over a woman’s reproductive life (Davison et al. 2005) may diminish the aging ovary’s responsiveness to FSH-based fertility stimulation. On that basis, IVF centres have instigated the use of treatment with the pro-androgen DHEA (Wiser et al. 2010) and testosterone (Fábregues et al. 2009) for women who experience poor ovarian response to FSH stimulation, with reportedly beneficial effects (Balasch et al. 2006; Fábregues et al. 2009), although convincing controlled studies are lacking (Massin et al. 2006; Sipe et al. 2010; Bosdou et al. 2012; Yeung et al. 2014).
Given the colocation of ARs and FSHRs in granulosa cells and their convergent effects on follicle development, we sought to analyse this hormonal interaction by using a transgenic (Tg) mouse line expressing bioactive heterodimeric human FSH (hFSH; Allan et al. 2001). These Tg-FSH mice exhibit a significant increase in litter size and ovulation rates up to 6 months of age (McTavish et al. 2007). In order to decipher the nature of the interaction between androgen and FSH actions on female fertility, in particular whether FSH can overcome deficiencies of androgen action, we combined our global ARKO and Tg-FSH mouse models and assessed key components (cycling, fertility, follicle development and health) of female fertility.
Materials and methods
Mice
Mice were maintained under standard housing conditions (free access to food and water in a temperature- and humidity-controlled, 12-h light cycle environment) at the ANZAC Research Institute. All procedures were performed in mice anaesthetised with ketamine–xylazine (100 mg kg–1 + 10 mg kg–1). All procedures were approved by the Sydney Local Health District Animal Welfare Committee within National Health and Medical Research Council (NHMRC) guidelines for animal experimentation.
Generation of Tg-FSH and ARKO female mice
Female mice expressing pituitary-independent Tg-FSH driven by the rat insulin II gene promoter on a C3H background (Allan et al. 2001; McTavish et al. 2007) and homozygous ARKO mice on a C57Bl/6J background (Walters et al. 2007; Simanainen et al. 2012) were generated as described previously. The two genetically modified strains were cross-bred to obtain control wild-type (WT), ARKO, Tg-FSH and Tg-FSH + ARKO mice, with the F1 generation being used for analysis. Ovarian histomorphology was assessed and circulating Tg-FSH levels were measured at 6 months of age.
DNA and RNA extraction, genotyping and reverse transcription–polymerase chain reaction
Genomic DNA isolated from tail biopsy was used as a template for polymerase chain reaction (PCR) genotyping to detect Cre–loxP-mediated excision in the mouse Ar gene and the presence of Tg-FSH, as described previously (Allan et al. 2001; Walters et al. 2007). The loss of Ar exon 3 was identified using two forward PCR primers within Ar exon 3 (AREx3-F, CTTCTCTCAGGGAAACAGAAGT) or the Neo cassette (ARNeo-F, TAGATCTCTCGTGGGATCATTG) and a common reverse primer located within intron 3 (AR-R, GGGAGACACAGGATAGGAAATT). Two product sizes were obtained: 613 bp for intact Ar and 289 bp for floxed Ar. Mice containing the SRY-box containing gene 2 (Sox2)–Cre Tg were PCR genotyped as described previously (Schwenk et al. 1995). Global ARKO males and females were distinguished by PCR genotyping the mouse Y chromosome sex-determining region Y (Sry) gene as described previously (Notini et al. 2005; Walters et al. 2007). The PCR primers used to identify the human FSH β-subunit transgene (AATGCTCAGCCAAGGACAAAGA and AACTTAATGAAACCGGCCTAAT) produced a product of 500 bp (Allan et al. 2001).
Hormone assays
Blood collected by cardiac exsanguination under ketamine–xylazine anaesthesia was allowed to clot at room temperature for 20 min, then centrifuged at 5000 rpm for 5 min at room temperature to collect serum, which was stored at –20°C until assay. Serum hFSH was determined using species-specific dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) kits (Perkin-Elmer), with a detection limit of 0.05 IU L–1, as described previously (Allan et al. 2001; McTavish et al. 2007).
Serum levels of progesterone (P4), testosterone and DHT were measured in extracts of 100 µL mouse serum by liquid chromatography–tandem mass spectrometry (LC-MS/MS; Harwood and Handelsman 2009) as adapted for mouse serum (McNamara et al. 2010) and further modified by Caldwell et al. (2014). Serum was extracted with 1 mL methyl tert-butyl ether fortified with testosterone-1,2,3-d3, dihydrotestosterone-16,16,17-d3 and oestradiol-2,4,16,16-d4 as internal standards. The organic layer, separated by freezing the aqueous layer, was dried and reconstituted in 75 µL of 20% methanol and 50 µL was injected onto the Kinetex XB C18, 1.7µ column for analysis. The limits of quantitation (defined as the lowest level that can be detected with a CV <20%) were 0.1 ng mL–1 for P4, 0.025 ng mL–1 for testosterone and 0.1 ng mL–1 for DHT.
Assessment of the oestrous cycle
Oestrous cycling was determined for a 2-week period in mice at 10 weeks of age. Oestrous cycle stage was detected by analysis of vaginal epithelial cell smears collected daily (1000 hours) in 20 μL sterile phosphate-buffered saline (PBS) and then transferred to glass slides, air dried and stained with 0.05% Trypan blue for light microscopy (Walters et al. 2012). The stage of the oestrous cycle was defined according to the presence or absence of leucocytes and cornified epithelial and nucleated epithelial cells, as described previously (Caldwell et al. 2014). Briefly, pro-oestrus was characterised by the presence of mostly nucleated and some cornified epithelial cells; at the oestrous stage mostly cornified epithelial cells were present; at metoestrus both cornified epithelial cells and leucocytes were present; and at dioestrus primarily leucocytes were visible.
Assessment of fertility
To estimate natural fertility, 6- to 8-week-old female mice (WT = 7; ARKO = 5; ARKO + Tg-FSH = 7; Tg-FSH = 6) were mated continuously with an individual mature (at least 8 weeks of age) fertile male stud for a 6-month period. Cages were monitored daily and the number of pups and litters recorded.
Ovary collection and follicle classification, enumeration and health
Ovaries were collected from 6-month-old female mice at the dioestrus stage of the oestrous cycle. Dissected ovaries were weighed, fixed in 4% paraformaldehyde at 4°C overnight and stored in 70% ethanol before histological processing. Ovaries were processed through a graded series of alcohol into glycol methacrylate resin (Technovit 7100; Heraeus Kulzer). Ovaries were serially sectioned at 20 μm, stained with periodic acid-Schiff and counterstained with haematoxylin. Total numbers of growing follicles per ovary at different developmental stages were determined as described previously (Myers et al. 2004; Walters et al. 2007). Follicles were classified as small preantral (oocyte with 1.5–2 layers of cuboidal granulosa cells), large preantral (oocyte surrounded by more than two and up to five layers of granulosa cells), small antral (oocyte surrounded by more than five layers of granulosa cells and/or one or two small areas of follicular fluid) and large antral (contained a single large antral cavity), with CL identified by morphological properties consistent with luteinised follicles visible throughout several serial sections.
Follicles were enumerated on all serial sections throughout each ovary using an Olympus microscope with Stereo Investigator software (MicroBrightField; Myers et al. 2004; Walters et al. 2007, 2012). For all histological analyses, repetitive counting of follicles was avoided by only counting or measuring follicles containing an oocyte with a visible nucleolus. To avoid bias, all ovaries were analysed without knowledge of sample genotypes. Follicles were classified as unhealthy if they contained a degenerate oocyte and/or >10% of the granulosa cells were pyknotic in appearance (Walters et al. 2007). The proportion of unhealthy follicles per ovary was estimated as the percentage of all follicles at that developmental stage.
Statistical analysis
Statistical analysis was performed using NCSS software (NCSS Statistical Software). Data that were not normally distributed were transformed to achieve normality and homoscedasticity (equal variances) before analysis based on a Box–Cox analysis to identify the optimal power transform. Unless stated otherwise, all results are expressed as the mean ± s.e.m. Statistical differences were tested by two-way analysis of variance (ANOVA) with genotype main effects AR inactivation and the presence of Tg-FSH and their AR inactivation × Tg-FSH interaction with post hoc testing using Fisher’s least significant difference multiple-comparison test. Main effects of AR and FSH are reported, with interaction results omitted if not significant. All parametric tests were confirmed by non-parametric equivalent tests. Two-sided P < 0.05 was considered significant.
Results
Verification of Tg female mice
The presence of the exon 3 deletion in the Ar gene and Tg-FSH were confirmed by PCR genotyping. Ar with intact exon 3 was undetectable in genomic DNA from ARKO + Tg-FSH and ARKO mice, whereas Tg-FSH was only detectable in Tg-FSH and ARKO + Tg-FSH mice (Fig. 1a). Tg-FSH expression was verified by detection of serum hFSH levels in all females carrying the Tg-FSH genotype, but not in its absence, noting the ARKO genotype had no significant effect on circulating concentrations of serum Tg-FSH (Fig. 1b).
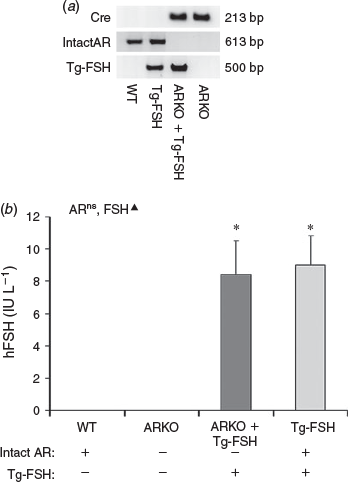
|
Oestrous cycle analysis
The loss of AR signalling significantly reduced oestrous cycling, based on the number of cycles assessed from serial vaginal cytology smears (P < 0.01), but the presence of Tg-FSH had no significant main effect on the number of oestrous cycles in 2 weeks (Fig. 2a). Compared with WT (2.8 ± 0.3 cycles) and Tg-FSH (2.3 ± 0.3) female mice, ARKO whether alone or in the presence of Tg-FSH resulted in disrupted vaginal cytology, with fewer completed normal oestrous cycles in 2 weeks (1.7 ± 0.2 and 1.0 ± 0.6 cycles respectively; Fig. 2). Although there was no significant effect of FSH on cycle number, oestrous cycle pattern appeared less aberrant in ARKO + Tg-FSH compared with ARKO mice (Fig. 2b).
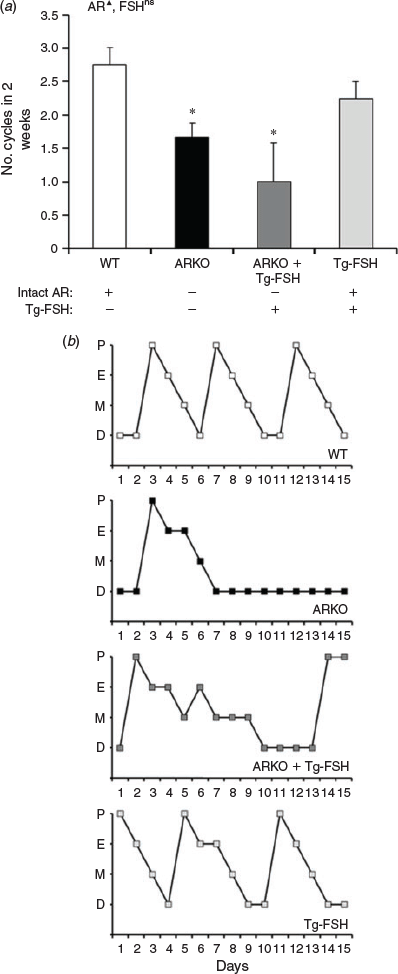
|
Serum P4, testosterone and DHT concentrations
Neither AR inactivation nor the presence of Tg-FSH had any effect on serum P4, testosterone and DHT concentrations (Fig. 3).
Fertility
Over a 6-month breeding trial, fertility was significantly affected by the loss of AR signalling (P < 0.01) and the presence of Tg-FSH (P < 0.01). Compared with WT females (8.8 ± 0.9 pups/litter), the loss of AR signalling reduced, whereas the presence of Tg-FSH restored, normal fertility in ARKO females (5.0 ± 0.6 and 9.2 ± 0.7 pups per litter respectively); Tg-FSH alone caused the expected increase in litter size (12.4 ± 0.8 pups per litter; Fig. 4a). Moreover, whereas only 40% of ARKO female breeders bore 40 pups in the breeding trial, the presence of Tg-FSH restored fertility in ARKO females (ARKO + Tg-FSH) to levels that were not significantly different from those of WT females (Fig. 4b). The number of litters during the 6-month breeding trial was reduced by the loss of AR actions (P < 0.01) as well as the presence of Tg-FSH (P < 0.05). ARKO (–24%), ARKO + Tg-FSH (–25%) and Tg-FSH (–18%) females exhibited a reduction in the number of litters compared with WT females (Fig. 4c).
Ovarian weight, follicle and CL populations
Ovarian weight was affected by the presence of Tg-FSH (P < 0.05) but not AR inactivation (P = 0.2; Fig. 5a). Ovary weights of Tg-FSH females were increased compared with WT, ARKO + Tg-FSH and ARKO females (9.8 ± 1.3 vs 6.4 ± 0.5, 7.5 ± 0.5 and 6.1 ± 0.7 mg respectively). All stages of follicle development were present in all genotypes, with overall normal morphology. Large preantral follicle counts were reduced by loss of AR signalling (P < 0.05) but not by the presence of Tg-FSH (P = 0.9; Fig. 5b). For all other stages of follicle development, there was no significant effect of the loss of AR signalling or the presence of Tg-FSH on growing follicle populations (Fig. 5b, c). Both the loss of AR signalling (P < 0.05) and the presence of Tg-FSH (P < 0.05) had significant effects on CL number. Numbers of CL were reduced in ARKO compared with WT control female mice (6.8 ± 1.8 vs 11.3 ± 2.2 respectively; P < 0.05). The presence of Tg-FSH restored CL numbers in ARKO ovaries (9.5 ± 4.5) to levels comparable with those in WT controls, whereas Tg-FSH alone resulted in the expected increase in CL numbers (25.0 ± 5.4; Fig. 5c, d).
Follicle health
Follicle health of large antral follicles was impaired by the loss of AR signalling (P < 0.05) but not by the presence of Tg-FSH (P = 0.1; Fig. 6a), with a notably much higher prevalence of pyknotic granulosa cells in ARKO alone follicles (Fig. 6b). In contrast, neither loss of AR signalling nor the presence of Tg-FSH had any significant effect on small preantral, large preantral or small antral follicle health (Fig. 6a).
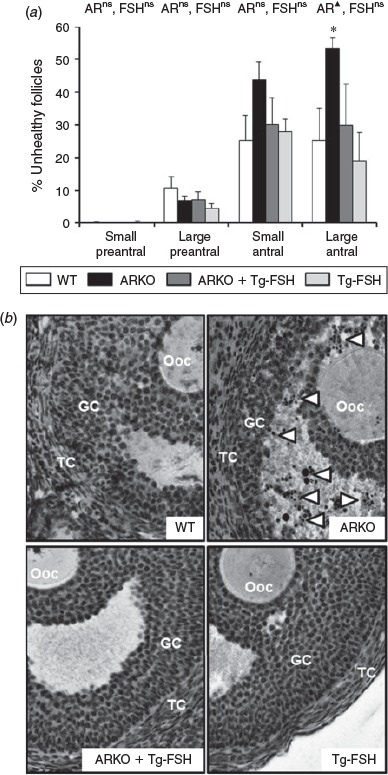
|
Discussion
Androgens and FSH have key convergent and complementary roles in the ovary as major regulators of follicle development and ovarian function (Kumar et al. 1997; McGee and Hsueh 2000; Walters 2015). In the present study, to unravel the interaction between AR- and FSH-mediated actions in female fertility, we combined two mouse models to produce mice that lack functional AR signalling and overexpress FSH activity. The findings show that Tg-FSH can rescue impaired female fertility and ovarian function due to androgen insensitivity in female ARKO mice, because ARKO + Tg-FSH female mice exhibit an ovulation rate and litter size comparable to those of WT females.
AR-mediated actions play a direct role in ovarian function (Walters 2015), as well as in extraovarian neuroendocrine function by maintaining normal hypothalamic–pituitary–ovarian function (Hu et al. 2004; Walters et al. 2009; Cheng et al. 2013). In the present study, despite FSH rescuing the reduced fertility in ARKO mice, as indicated by pups per litter, the serial vaginal smear patterns remain aberrant in ARKO + Tg-FSH females, most likely due to dysfunctional CL, as indicated by the reduced P4 concentrations. Thus, the addition of FSH was unable to fully restore defects due to AR inactivation, which lends support to a central neuroendocrine role for AR actions independent, at least in part, of FSH in the regulation of female fertility. This supposition is further vindicated by findings that both global (Cheng et al. 2013) and pituitary-specific (Wu et al. 2014) loss of AR actions alters negative and positive feedback mechanisms, and significantly reduces the magnitude of the ovulatory LH surge.
In the present study, and in agreement with previous findings (Walters et al. 2007), fertility was significantly impaired by the loss of AR signalling, as illustrated by reduced pups per litter, whereas the presence of elevated circulating serum FSH increased the number of pups per litter, as expected (McTavish et al. 2007). The combined ARKO + Tg-FSH mouse model revealed that the presence of Tg-FSH was sufficient to restore the reduced number of pups per litter observed in the ARKO females to that seen in WT controls. These findings imply that FSH is a component of the pathways disrupted by the loss of AR actions in ARKO ovaries. Although further studies are required to fully unravel the altered mechanisms, the maintenance of follicle health in ARKO + Tg-FSH mice indicates that key FSH-regulated pathways involved in restoring fertility are at the level of the ovary.
We and others have demonstrated that a key cause of the subfertility in ARKO female mice is dysfunctional ovulation (Hu et al. 2004; Shiina et al. 2006; Walters et al. 2007, 2009; Cheng et al. 2013). In the present study, ARKO females exhibited significantly fewer CL numbers per ovary, indicative of reduced ovulation and consistent with an ovulatory role for AR actions. Conversely, Tg-FSH mice exhibited increased numbers of CL per ovary, consistent with previous work (McTavish et al. 2007) and the well-known clinical observation that ovarian stimulation by exogenous FSH stimulates the development of multiple ovarian follicles (Howles et al. 1994). Interestingly, the presence of Tg-FSH in the ARKO females restored CL numbers to those observed in control ovaries. Thus, FSH appears to rescue the impaired fertility of ARKO females by increasing ovulation rate. However, there may be an age-dependent cut-off as to when FSH can overcome intraovarian defects due to AR deficiency, because the reduced ovulation rates observed in a mouse model with specific loss of AR signalling in granulosa cells can only be overcome by superovulation in 8- to 9-week-old mice and not 24- to 25-week-old mice (Sen and Hammes 2010). In addition, several studies indicate that androgens can synergise with FSH to improve ovarian sensitivity to FSH. For example, DHT enhanced FSH-mediated preantral-to-antral follicular growth in mice (Sen et al. 2014) and FSH-stimulated the proliferation of cumulus cells in pigs (Hickey et al. 2004), whereas testosterone increased FSH responsiveness of mouse preantral follicles (Wang et al. 2001).
It is likely that the majority of disrupted AR-regulated pathways overcome by the presence of Tg-FSH are intraovarian and involve improved follicle health. Both AR-mediated and FSH actions are involved in maintaining follicle health, because ARKO ovaries exhibit increased levels of follicular atresia (Shiina et al. 2006; Walters et al. 2007), whereas late stage (beyond preantral) follicles exhibit increased atresia in FSH-knockout ovaries (Kumar et al. 1997). Furthermore, recently DHT and testosterone have been shown to increase microRNA-125b expression, which suppresses pro-apoptotic protein (Bcl-2 homologous antagonist/killer (BAK1), Bcl-2 modifying factor (BMF), Bcl-2-associated X protein (BAX) and tumor suppressor protein p53 (TRP53)) expression (Sen et al. 2014). In the present study, although ARKO females exhibited a significant increase in histologically defined unhealthy follicles, connoting a higher level of apoptosis at the large antral stage, the presence of Tg-FSH restored follicle health to levels comparable with WT controls. Thus, FSH is sufficient to restore reduced granulosa cell health due to a loss of AR signalling, implying that androgenic mechanisms that attenuate follicular atresia can also be modulated by FSH.
In conclusion, the findings of the present study demonstrate that AR-mediated and FSH actions play key roles in regulating female fertility, and provide strong evidence to support the concept that the interaction between FSH and AR is synergistic, with FSH actions able to rescue dysfunctional ovarian function observed in ARKO females mice, notably by maintaining follicle health and ovulation rates, and thereby optimal female fertility. Furthermore, these data imply that the rescue of ARKO female mouse subfertility is most likely due to a rescue in disrupted androgenic mechanisms involved in regulating follicle health and atresia.
Acknowledgements
The authors thank Jenny Spaliviero, Linda Middleton and Lucy Yang for technical support. This work was supported by Australian National Health and Medical Research Council grants 632678, 512545 and 1008160.
References
Allan, C. M., Haywood, M., Swaraj, S., Spaliviero, J., Koch, A., Jimenez, M., Poutanen, M., Levallet, J., Huhtaniemi, I., Illingworth, P., and Handelsman, D. J. (2001). A novel transgenic model to characterize the specific effects of follicle-stimulating hormone on gonadal physiology in the absence of luteinizing hormone actions. Endocrinology 142, 2213–2220.| 1:CAS:528:DC%2BD3MXjvFGgur8%3D&md5=052dbb38462db00327e093ead21419d2CAS | 11356665PubMed |
Balasch, J., Fabregues, F., Penarrubia, J., Carmona, F., Casamitjana, R., Creus, M., Manau, D., Casals, G., and Vanrell, J. A. (2006). Pretreatment with transdermal testosterone may improve ovarian response to gonadotrophins in poor-responder IVF patients with normal basal concentrations of FSH. Hum. Reprod. 21, 1884–1893.
| Pretreatment with transdermal testosterone may improve ovarian response to gonadotrophins in poor-responder IVF patients with normal basal concentrations of FSH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xms1Ontrg%3D&md5=398d28ef9f822c94b9eef867d5c19508CAS | 16517559PubMed |
Bosdou, J. K., Venetis, C. A., Kolibianakis, E. M., Toulis, K. A., Goulis, D. G., Zepiridis, L., and Tarlatzis, B. C. (2012). The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Hum. Reprod. Update 18, 127–145.
| The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtFKrtb4%3D&md5=fd9e66d470be391f69ed6c3462a11f5aCAS | 22307331PubMed |
Caldwell, A. S., Middleton, L. J., Jimenez, M., Desai, R., McMahon, A. C., Allan, C. M., Handelsman, D. J., and Walters, K. A. (2014). Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 155, 3146–3159.
| Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1egsrbF&md5=f87c8fb6501ea8e158ae09c50d4b3f87CAS | 24877633PubMed |
Cárdenas, H., Herrick, J. R., and Pope, W. F. (2002). Increased ovulation rate in gilts treated with dihydrotestosterone. Reproduction 123, 527–533.
| Increased ovulation rate in gilts treated with dihydrotestosterone.Crossref | GoogleScholarGoogle Scholar | 11914115PubMed |
Cheng, X. B., Jimenez, M., Desai, R., Middleton, L. J., Joseph, S. R., Ning, G., Allan, C. M., Smith, J. T., Handelsman, D. J., and Walters, K. A. (2013). Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice. Am. J. Physiol. Endocrinol. Metab. 305, E717–E726.
| Characterizing the neuroendocrine and ovarian defects of androgen receptor-knockout female mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1Sqsb7F&md5=fc8ce0cc192201c6462fd10ff5bdda3fCAS | 23880317PubMed |
Davison, S. L., Bell, R., Donath, S., Montalto, J. G., and Davis, S. R. (2005). Androgen levels in adult females: changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 90, 3847–3853.
| Androgen levels in adult females: changes with age, menopause, and oophorectomy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtlCisLk%3D&md5=f66732605c41d2780a518f381100b902CAS | 15827095PubMed |
Fábregues, F., Peñarrubia, J., Creus, M., Manau, D., Casals, G., Carmona, F., and Balasch, J. (2009). Transdermal testosterone may improve ovarian response to gonadotrophins in low-responder IVF patients: a randomized, clinical trial. Hum. Reprod. 24, 349–359.
| Transdermal testosterone may improve ovarian response to gonadotrophins in low-responder IVF patients: a randomized, clinical trial.Crossref | GoogleScholarGoogle Scholar | 19054777PubMed |
Harwood, D. T., and Handelsman, D. J. (2009). Development and validation of a sensitive liquid chromatography–tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin. Chim. Acta 409, 78–84.
| Development and validation of a sensitive liquid chromatography–tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1aju7%2FI&md5=b09661ffb4d5a4e05e296c25027618bbCAS | 19747904PubMed |
Hickey, T. E., Marrocco, D. L., Gilchrist, R. B., Norman, R. J., and Armstrong, D. T. (2004). Interactions between androgen and growth factors in granulosa cell subtypes of porcine antral follicles. Biol. Reprod. 71, 45–52.
| Interactions between androgen and growth factors in granulosa cell subtypes of porcine antral follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFKktLg%3D&md5=62fe4fe7c3b574dfad9f3ad7430daf1fCAS | 14973257PubMed |
Hillier, S. G., and De Zwart, F. A. (1981). Evidence that granulosa cell aromatase induction/activation by follicle-stimulating hormone is an androgen receptor-regulated process in-vitro. Endocrinology 109, 1303–1305.
| Evidence that granulosa cell aromatase induction/activation by follicle-stimulating hormone is an androgen receptor-regulated process in-vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXls1anurg%3D&md5=89fd19de19243a7966be49535a8e5b96CAS | 6793349PubMed |
Hillier, S. G., and Tetsuka, M. (1997). Role of androgens in follicle maturation and atresia. Baillieres Clin. Obstet. Gynaecol. 11, 249–260.
| Role of androgens in follicle maturation and atresia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c7pslaksA%3D%3D&md5=ce41b9a18d917d2a9049eb6a0a2ce473CAS | 9536210PubMed |
Hillier, S. G., Whitelaw, P. F., and Smyth, C. D. (1994). Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol. Cell. Endocrinol. 100, 51–54.
| Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXktVWisLk%3D&md5=964804330efbf989330e820888f68a5dCAS | 8056158PubMed |
Howles, C. M., Loumaye, E., Giroud, D., and Luyet, G. (1994). Multiple follicular development and ovarian steroidogenesis following subcutaneous administration of a highly purified urinary FSH preparation in pituitary desensitized women undergoing IVF: a multicentre European Phase III study. Hum. Reprod. 9, 424–430.
| 1:STN:280:DyaK2c3nsVygtA%3D%3D&md5=e5840a33b9fcba6f494ce3d3218d720fCAS | 8006130PubMed |
Hu, Y. C., Wang, P. H., Yeh, S., Wang, R. S., Xie, C., Xu, Q., Zhou, X., Chao, H. T., Tsai, M. Y., and Chang, C. (2004). Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc. Natl Acad. Sci. USA 101, 11 209–11 214.
| Subfertility and defective folliculogenesis in female mice lacking androgen receptor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvVKgtro%3D&md5=9126af48d860538b00e57a3e1ad5674bCAS |
Kumar, T. R., Wang, Y., Lu, N., and Matzuk, M. M. (1997). Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 15, 201–204.
| Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtVOks7c%3D&md5=58f02df0e142e59b8b605437a8fa48d8CAS | 9020850PubMed |
Massin, N., Cedrin-Durnerin, I., Coussieu, C., Galey-Fontaine, J., Wolf, J. P., and Hugues, J. N. (2006). Effects of transdermal testosterone application on the ovarian response to FSH in poor responders undergoing assisted reproduction technique: a prospective, randomized, double-blind study. Hum. Reprod. 21, 1204–1211.
| Effects of transdermal testosterone application on the ovarian response to FSH in poor responders undergoing assisted reproduction technique: a prospective, randomized, double-blind study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsFWjtLk%3D&md5=d0a59b0d86546a828a579f92350a4710CAS | 16476678PubMed |
McGee, E. A., and Hsueh, A. J. (2000). Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 21, 200–214.
| 1:STN:280:DC%2BD3c3ksVahsw%3D%3D&md5=8f84e11125e8971773b1655663a30badCAS | 10782364PubMed |
McNamara, K. M., Harwood, D. T., Simanainen, U., Walters, K. A., Jimenez, M., and Handelsman, D. J. (2010). Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography–tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 121, 611–618.
| Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography–tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvFKqt7c%3D&md5=f153fa49c36f5457994e251123d36510CAS | 20144714PubMed |
McTavish, K. J., Jimenez, M., Walters, K. A., Spaliviero, J., Groome, N. P., Themmen, A. P., Visser, J. A., Handelsman, D. J., and Allan, C. M. (2007). Rising follicle-stimulating hormone levels with age accelerate female reproductive failure. Endocrinology 148, 4432–4439.
| Rising follicle-stimulating hormone levels with age accelerate female reproductive failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpslCnurk%3D&md5=61ac5733af2d3d23f774210a7d57023eCAS | 17540727PubMed |
Myers, M., Britt, K. L., Wreford, N. G., Ebling, F. J., and Kerr, J. B. (2004). Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127, 569–580.
| Methods for quantifying follicular numbers within the mouse ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkvFOqu78%3D&md5=4d558d79990170bcf8931596982bad38CAS | 15129012PubMed |
Notini, A. J., Davey, R. A., McManus, J. F., Bate, K. L., and Zajac, J. D. (2005). Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J. Mol. Endocrinol. 35, 547–555.
| Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XivVGntw%3D%3D&md5=4deb0c28383ea5c1f40131a9d0fe3053CAS | 16326839PubMed |
Oktem, O., and Urman, B. (2010). Understanding follicle growth in vivo. Hum. Reprod. 25, 2944–2954.
| Understanding follicle growth in vivo.Crossref | GoogleScholarGoogle Scholar | 20937745PubMed |
Schwenk, F., Baron, U., and Rajewsky, K. (1995). A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081.
| A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlsVCrsA%3D%3D&md5=50023ca086b4710c523b52b6c0738ba9CAS | 8559668PubMed |
Sen, A., and Hammes, S. R. (2010). Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol. Endocrinol. 24, 1393–1403.
| Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpt1CntrY%3D&md5=8ed3798239df8981b23ed05ba7117c8eCAS | 20501640PubMed |
Sen, A., Prizant, H., Light, A., Biswas, A., Hayes, E., Lee, H. J., Barad, D., Gleicher, N., and Hammes, S. R. (2014). Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl Acad. Sci. USA 111, 3008–3013.
| Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjtVKhsrY%3D&md5=09648f0f7b10580267acee06be343cc5CAS | 24516121PubMed |
Shiina, H., Matsumoto, T., Sato, T., Igarashi, K., Miyamoto, J., Takemasa, S., Sakari, M., Takada, I., Nakamura, T., Metzger, D., Chambon, P., Kanno, J., Yoshikawa, H., and Kato, S. (2006). Premature ovarian failure in androgen receptor-deficient mice. Proc. Natl Acad. Sci. USA 103, 224–229.
| Premature ovarian failure in androgen receptor-deficient mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xms12huw%3D%3D&md5=c1d10f4fddddbe4765a2fbca5ac71985CAS | 16373508PubMed |
Simanainen, U., Gao, Y. R., Walters, K. A., Watson, G., Desai, R., Jimenez, M., and Handelsman, D. J. (2012). Androgen resistance in female mice increases susceptibility to DMBA-induced mammary tumors. Horm. Cancer 3, 113–124.
| Androgen resistance in female mice increases susceptibility to DMBA-induced mammary tumors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmsF2ms78%3D&md5=b8819e3676c33cfca3e53fcef72c1c77CAS | 22370991PubMed |
Sipe, C. S., Thomas, M. R., Stegmann, B. J., and Van Voorhis, B. J. (2010). Effects of exogenous testosterone supplementation in gonadotrophin stimulated cycles. Hum. Reprod. 25, 690–696.
| Effects of exogenous testosterone supplementation in gonadotrophin stimulated cycles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitFeisbc%3D&md5=51ba027ca9a1d9378e4260c4d725b4c4CAS | 20031956PubMed |
Tetsuka, M., and Hillier, S. G. (1996). Androgen receptor gene expression in rat granulosa cells: the role of follicle-stimulating hormone and steroid hormones. Endocrinology 137, 4392–4397.
| 1:CAS:528:DyaK28XlvVSmsLk%3D&md5=1ef34cd20faac7be6507fb7d547abac2CAS | 8828500PubMed |
Walters, K. A. (2015). Role of androgens in normal and pathological ovarian function. Reproduction 149, R193–R218.
| Role of androgens in normal and pathological ovarian function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXns1Srtr0%3D&md5=b9789194f00411ff3047f193f2d4886aCAS | 25516989PubMed |
Walters, K. A., Allan, C. M., Jimenez, M., Lim, P. R., Davey, R. A., Zajac, J. D., Illingworth, P., and Handelsman, D. J. (2007). Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology 148, 3674–3684.
| Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1Oisbk%3D&md5=87047093c2591926b12e46f81a3d9f4aCAS | 17463055PubMed |
Walters, K. A., Allan, C. M., and Handelsman, D. J. (2008). Androgen actions and the ovary. Biol. Reprod. 78, 380–389.
| Androgen actions and the ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisFSltb8%3D&md5=64e206397e1e89ee44560ac97746493aCAS | 18003945PubMed |
Walters, K. A., McTavish, K. J., Seneviratne, M. G., Jimenez, M., McMahon, A. C., Allan, C. M., Salamonsen, L. A., and Handelsman, D. J. (2009). Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function. Endocrinology 150, 3274–3282.
| Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotlCrsLg%3D&md5=f25394de1a517144e97df37827d1c1c2CAS | 19359383PubMed |
Walters, K. A., Middleton, L. J., Joseph, S. R., Hazra, R., Jimenez, M., Simanainen, U., Allan, C. M., and Handelsman, D. J. (2012). Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol. Reprod. 87, 151.
| Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility.Crossref | GoogleScholarGoogle Scholar | 23115271PubMed |
Wang, H., Andoh, K., Hagiwara, H., Xiaowei, L., Kikuchi, N., Abe, Y., Yamada, K., Fatima, R., and Mizunuma, H. (2001). Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology 142, 4930–4936.
| Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnslGqu7s%3D&md5=a5694cc491612bc4a3c24f679526660cCAS | 11606461PubMed |
Weil, S., Vendola, K., Zhou, J., and Bondy, C. A. (1999). Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J. Clin. Endocrinol. Metab. 84, 2951–2956.
| Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltFCltbk%3D&md5=7ecafdf4bc2cbea28531d1c352a9e858CAS | 10443703PubMed |
Wiser, A., Gonen, O., Ghetler, Y., Shavit, T., Berkovitz, A., and Shulman, A. (2010). Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum. Reprod. 25, 2496–2500.
| Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFyltLjE&md5=a7c84d5ae8d4a96999a8129de2bca818CAS | 20729538PubMed |
Wu, S., Chen, Y., Fajobi, T., DiVall, S. A., Chang, C., Yeh, S., and Wolfe, A. (2014). Conditional knockout of the androgen receptor in gonadotropes reveals crucial roles for androgen in gonadotropin synthesis and surge in female mice. Mol. Endocrinol. 28, 1670–1681.
| Conditional knockout of the androgen receptor in gonadotropes reveals crucial roles for androgen in gonadotropin synthesis and surge in female mice.Crossref | GoogleScholarGoogle Scholar | 25157703PubMed |
Yeung, T. W. Y., Chai, J., Li, R. H. W., Lee, V. C. Y., Ho, P. C., and Ng, E. H. Y. (2014). A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertil. Steril. 102, 108–115.e1.
| A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnsFCjtrc%3D&md5=1f49a78840fb201f3b47f2b2a32541f5CAS |





