MicroSort® sperm sorting causes no increase in major malformation rate
Donald P. Marazzo A C D , David Karabinus A , Lawrence A. Johnson B and Joseph D. Schulman AA Genetics and IVF Institute, 3015 Williams Drive, Fairfax, VA 22031, USA.
B U.S. Department of Agriculture, 10300 Baltimore Ave, Bldg. 200, BARC-East Beltsville, MD 20705, USA.
C Institute for Healthy Reproductive Outcomes, 355 S Highland Avenue, Pittsburgh, PA 15206, USA.
D Corresponding author. Email: donm1027@gmail.com
Reproduction, Fertility and Development 28(10) 1580-1587 https://doi.org/10.1071/RD15011
Submitted: 9 January 2015 Accepted: 6 March 2015 Published: 22 April 2015
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
The purpose of the present study was to evaluate the safety of MicroSort (MicroSort Division, GIVF, Fairfax, VA, USA) sperm sorting by monitoring major malformations in infants and fetuses conceived using sorted spermatozoa. Data were collected in a prospective protocol with monitoring that began from conception through birth until 1 year of life. Comprehensive ascertainment identified fetuses and stillbirths with malformations after 16 weeks gestation, pregnancies terminated for malformations and babies with major malformations. Outcomes in MicroSort pregnancies were compared with outcomes in published studies that used active and comprehensive ascertainment of malformations in the general population and in pregnancies established after assisted reproduction. Using comprehensive outcomes from all pregnancies, the rate of major malformations in MicroSort pregnancies conceived after IVF with or without intracytoplasmic sperm injection was 7.8%; this did not differ significantly from the rates reported in the three assisted reproductive technology control studies not associated with MicroSort (8.6%, 9.2% and 8.3%). Similarly, the rate of major malformations in MicroSort pregnancies initiated with intrauterine insemination was 6.0%, not significantly different from that reported in non-assisted reproductive technology pregnancies not associated with MicroSort (6.9%, 4.6% and 5.7%). Prospective record review of pregnancy outcomes and paediatric evaluation to 1 year indicate no association between MicroSort sperm sorting and major malformations.
Additional keywords: assisted reproductive technologies, birth defects, family balancing, genetic disease prevention, intracytoplasmic sperm injection, IVF, sexual selection, X-linked diseases.
Introduction
MicroSort (MicroSort Division, GIVF, Fairfax, VA, USA) allows families to increase their chances of having a baby of an indicated sex either to balance the sex ratio within their family (family balancing) or to prevent genetic disease transmission in sex-linked disease. The MicroSort technique (Johnson et al. 1993) uses high-speed flow cytometric sorting of individual spermatozoa based on the 2.8% greater total DNA content in normal human spermatozoa bearing an X compared with Y chromosome (Sumner and Robinson 1976). Spermatozoa are briefly exposed to a dilute solution of a non-intercalating fluorescent DNA dye (Hoechst 33342), which attaches to the minor groove of DNA (Latt and Stetten 1976). Sperm separation is based on the strength of the fluorescent signal after pulsing single spermatozoa with ultraviolet light. Details of the MicroSort methodology and its effectiveness have been reported elsewhere (Johnson et al. 1989; Johnson and Schulman 1994; Schulman and Karabinus 2005; Karabinus et al. 2014).
This method for sexing semen has been a commercial reality for cattle and deer in the animal production industry, along with several other species. Sexed semen may also be applied for conservation purposes in zoo animals and other threatened or endangered wildlife species (Seidel 2012; Rath et al. 2013). Overall, several millions of insemination doses are produced annually for commercial purposes (Seidel 2012).
Morrell and Dresser (1989) found no morphological changes or loss of reproductive capacity with flow-sorted Hoechst 33342-stained spermatozoa in three generations of swine and nine generations of rabbits. In a review of the birth status of several animal species (cattle, swine, rabbits and sheep), Johnson and Schulman (1994) noted that none of more than 200 births from sorted spermatozoa was reported as having macroscopic morphological changes.
The present study focuses on the safety of this procedure in humans based on the rate of major malformations in fetuses and babies conceived using the sorted sperm procedure. Active ascertainment of major malformations with the comprehensive inclusion of fetal abnormalities indicates rates of approximately 8% in the general population, compared with approximately 4% in passive registries (Queißer-Luft et al. 2002). The present study involved pregnancies established after either intrauterine insemination (IUI) or IVF, usually with intracytoplasmic insemination (ICSI). The present study compared MicroSort data with large population-based reports (Hansen et al. 2002; Ludwig and Katalinic 2002; Davies et al. 2012) that actively ascertained rates of major malformations in pregnancies from the general population and in pregnancies conceived through assisted reproductive technologies (ARTs).
Materials and methods
Herein we report data from an Investigational Device Exemption (IDE) for testing the effectiveness and safety of MicroSort. ‘IDE’ is the terminology used by the US Food and Drug Administration (FDA) to indicate a clinical trial using a medical device that has not yet been approved for clinical use but meets the criteria of an IDE (Code of Federal Regulations Title 21; http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm080202.htm, accessed March 2015). Use of the device is limited to studies that meet the IDE criteria, and is not approved for use outside the defined IDE. The Chesapeake Institutional Review Board (CIRBI) and The Genetics and IVF Institute Institutional Review Board (IRB) approved the study protocol. The conduct of the clinical trial was in accordance with the ethical standards of these IRB entities.
Subjects
The two indications for participation in the present study were genetic disease prevention of sex-linked or sex-limited genetic disorders or family balancing, defined as an attempt to increase the less-represented gender of children within a family unit. Participants were eligible for the genetic disease prevention arm of the study if they met one of the following criteria: (1) the woman carried an X-linked disease that was only manifest in male children or was more severe in male children; (2) the man was affected by an X-linked disease that would only be passed through daughters; (3) the man carried a premutation on the X chromosome (e.g. fragile X) that would be passed to his daughters and not his sons, with possible mutation and morbidity in the children of his daughters; (4) either partner had a previous child with a disease or malformation affecting only one sex with an increased risk in subsequent children (e.g. hypospadias); or (5) offspring were at increased risk for a significant disease with the preponderance of effect in children of a particular sex (e.g. autism spectrum disorder). None of the extremely rare Y-linked genetic diseases or genetic diseases linked to pseudoautosomal regions of the X or Y chromosome was recruited or referred for participation in this study. In all, 4610 couples were recruited for the family balancing indication and 383 couples for genetic disease prevention.
Data are comprehensive over the entire period from January 1995 to October 2012. During this time, more than 1300 babies were born (993 females and 344 males) after MicroSort, including 136 infants (121 females and 15 males) in whom the primary indication was genetic disease prevention.
Couples who participated in the trial, and any gamete donors or gestational carriers, had negative laboratory tests for human immunodeficiency virus-1 antibody, hepatitis B surface antigen and hepatitis C antibody within 1 year of their attempt at pregnancy. All couples signed informed consent before being enrolled in the clinical trial. There were exclusions for clinically significant diseases, including substance abuse. This category included diseases such as diabetes, cardiac disease, active autoimmune disease, haemoglobinopathies and other maternal disease that could negatively affect the health of the mother during pregnancy or that would increase the risk for pregnancy complications. Participants signed medical release forms so we could obtain newborn and paediatric records for children resulting from their participation in the study.
Couples participating for the family balancing indication must have had at least one child and wanted to use MicroSort to have a child of the under-represented gender among all their children. The woman was required to be between the ages of 18–39 years at the time of egg retrieval or insemination. Couples were excluded if the woman or man (or gamete donor) had a history of a major congenital malformation or chromosomal abnormality in themselves or their children.
Couples participating for the genetic disease prevention indication had a family history and/or genetic testing supportive of a genetic diagnosis that made offspring susceptible to inherited disease that was X-linked or that otherwise had a greater risk of significant clinical disease in one sex relative to the other (i.e. sex limited).
Sperm sorting
Technical details of MicroSort sperm separation are provided elsewhere (Johnson et al. 1993; Johnson and Schulman 1994; Karabinus et al. 2014) and are described here briefly. Fresh or frozen–thawed semen was processed, stained with H33342 and sorted at high speed into populations enriched in either X chromosome-bearing spermatozoa (X-Sort) or Y chromosome-bearing spermatozoa (Y-Sort). During the study period, the method achieved average ratios of approximately 7 : 1 female : male spermatozoa (87.8%/12.2%) with X-Sorting and approximately 3 : 1 male : female spermatozoa (74.3%/25.7%) with Y-Sorting, as determined by fluorescence in-situ hybridisation (FISH). Freshly sorted samples were immediately used for reproductive procedures such as IUI, ICSI and IVF. Samples frozen after sorting could be shipped and were used after thawing solely for ICSI.
Procedures to initiate pregnancy after MicroSort
IUI was timed using conventional methods in natural cycles or after ovarian stimulation with clomiphene and occasionally with gonadotrophins (<5%). Ovarian stimulation with a combination of gonadotrophins and pituitary suppression preceded IVF or ICSI. Fresh or cryopreserved sorted sperm samples were used at the two study sites in Virginia and California. Collaborators outside the two study sites exclusively used cryopreserved sorted specimens. Participants were counselled with regard to their options for achieving pregnancy (IUI, ICSI and IVF) and were allowed to choose whatever method they preferred. Table 1 summarises treatment procedures associated with sort type (X-Sort or Y-Sort) and sort indication (family balancing or genetic disease prevention) in the study population. Individual couples that continued to meet study criteria were allowed multiple treatment procedures.
Pregnancy follow-up
A clinical pregnancy was defined as any pregnancy with an ultrasound-detected fetal sac, with or without fetal heart activity, a miscarriage more than 35 days after insemination or fertilisation, documented presence of fetal tissue or birth. Biochemical pregnancies or pregnancies with demise before 35 days after insemination or fertilisation and no evidence of fetal tissue were not counted as clinical pregnancies. If a participant did not become pregnant, she could elect to undergo another attempt or discontinue study participation.
Pregnant women were advised to obtain routine obstetric care and were contacted after each trimester whenever possible, once at 14–15 weeks, again at 27–28 weeks and again after delivery due date. The pregnant woman was asked whether she had had a fetal ultrasound, an invasive diagnostic test, such as an amniocentesis or chorionic villus sampling (CVS), and whether the pregnancy was ongoing or whether there was a termination or any loss of a fetus. If she had had any of the tests or if there was a fetal loss or pregnancy termination, records were immediately requested from the obstetrician. Informative events during the pregnancy were recorded, including ultrasound findings and any specialised or abnormal test results. The sex of the fetus and reports of any genetic tests were also recorded. At the conclusion of pregnancy all prenatal records were again requested, including the results of prenatal ultrasounds, genetic tests and autopsies. The outcome of each pregnancy was documented, whether a live birth, stillbirth, spontaneous abortion, ectopic pregnancy or elective termination. A trained clinician reviewed records and requested additional records as needed. The principal investigator (D. P. M.), as well as the paediatric geneticists (A. L., H. S., D. B., K. G., S. S., M. J.), when indicated, reviewed these records.
Participants who had a spontaneous abortion or elective pregnancy termination were encouraged to have a chromosome analysis of the fetal tissue. In cases of pregnancy losses during the second and third trimester, including stillborn infants, chromosome analysis and autopsy by a pathologist were requested.
Newborn and infant follow-up
Within several weeks after birth, the newborn’s medical records were requested. Two of the six physicians who were board certified in Clinical Genetics and Paediatrics reviewed each of the available neonatal records independently and requested additional records as necessary to identify major malformations or to clarify significant clinical outcomes. The presence or absence of congenital malformations was the primary safety end-point and of paramount importance during the review of the newborns records. Careful evaluation for anomalies, including head, fontanelles, sutures, neck, eyes, red reflex, nose, ears, mouth, palate, heart, circulation, abdomen, umbilicus, genitalia, anus, spine, extremities, hips, neurological system and skin, was undertaken. Further testing was requested if indicated, including karyotyping for any infant who had or was suspected to have a major congenital malformation.
Approximately half of all major malformations are identified at birth (Graham 1991), so we selected follow-up at 1 year of age to more fully identify major malformations that become manifest in the first year after birth. Patients with live births were contacted more than 12 months after delivery to retrieve records on the health and development of their infant (or infants). Medical records were obtained from the infant’s paediatrician. Again, two of the board-certified paediatricians, who were also board-certified in Clinical Genetics, reviewed each of the available records for the presence or absence of congenital malformations. The infant’s growth and development was evaluated from well baby examinations. Any infant illnesses were reviewed and additional information requested if indicated.
Each infant was classified into one of the following categories: (1) no congenital malformation; (2) major congenital malformation; (3) minor congenital malformation; (4) developmental variant; or (5) other. Other adverse events were recorded. The two physicians made their classifications independently. If there was disagreement between their classifications, attempts were made to reach concurrence; if there was persistent disagreement, the more severe assessment was assigned.
Classification of major malformations in births and fetuses
For the present study, ascertainment of major malformation consistently included live births and stillbirths with major congenital malformations, major malformations identified in any fetus after 16 weeks gestation and pregnancy terminations performed for fetal abnormalities at any gestational age. The term ‘major malformations and terminations’ (MM&T) has been used in tables, and occasionally in the text, to reflect the inclusive ascertainment of malformations in the pregnancies of the clinical trial participant, as well as the studies used for comparison. Classification of major versus minor malformations in live births was based on a widely accepted consensus definition of major malformations (i.e. malformations that generally cause functional impairment or require surgical correction; Bonduelle et al. 2002). Congenital malformations included a structural abnormality or set of abnormalities present at birth regardless of aetiology: chromosomal, Mendelian, multifactorial or sporadic. All chromosomal anomalies detected were considered major malformations regardless of anticipated clinical or developmental impact, including balanced translocations that resulted in pregnancy terminations regardless of identification of parental inheritance, and karyotypes with two Y-chromosomes (XYY). Malformations that did not meet criteria for major malformations were considered minor if the specific malformation occurred in <4% of infants in the specific ethnic group, and a normal variant if the population incidence was >4%.
Two specific minor abnormalities were clarified for consistency with historical studies (Bonduelle et al. 2002). Ankyloglossia was considered a minor malformation even if treatment included a minor surgical release; in addition, small muscular ventricular septal defects (VSD) were considered minor malformations. Small muscular VSDs are exceedingly common, occurring in 2.5%–4.4% of asymptomatic neonates, and demonstrate spontaneous closure rates that approximate 80%–90% (Sands et al. 1999; Chang et al. 2011).
Outcomes that were reported after 1 year were included in the major malformation statistics if they fit the criteria for major malformations, whether or not the malformation developed after the established 1-year planned follow-up. The board-certified paediatric geneticists independently reviewed all paediatric records, requested additional information as necessary and established the severity of the malformations.
Identification of appropriate control studies
Control studies used for comparison were identified through a Medline search including terms congenital malformations, congenital anomalies, assisted reproduction, in vitro fertilisation/IVF and intracytoplasmic sperm injection/ICSI. Only articles in English were included in the search. Control studies were identified using four criteria. The first three criteria were that: (1) the study evaluated the rates of major malformations in IVF and/or ICSI pregnancies, as well as malformation rates in natural conceptions or the general population; (2) the method of ascertainment was active rather than the collection of passive reports; and (3) major malformation rates had to include all appropriate outcomes: live births and stillbirths with major malformations, fetuses with major malformations from 16 weeks to term and terminations performed for fetal abnormalities. The final criterion used to identify control studies was that the study was designed to evaluate live births for malformations up to and including at least 1 year of life. Only by selecting studies that met these four criteria could the studies provide comparable data. The key discriminators of active versus passive ascertainment are the prospective follow-up, the thoroughness of the examination or the record review and the skill of the individual who recognises the malformation. In the present study, board-certified paediatric geneticists identified all malformations by detailed review of all paediatric and prenatal records from conception through, at minimum, to 1 year of life. To ensure completeness of the search for control studies, all references in the recent meta-analysis of malformations in ART (Wen et al. 2012) were also reviewed to identify any additional studies that would meet the criteria for control studies.
The total search identified three studies that met the criteria (Hansen et al. 2002; Ludwig and Katalinic 2002; Davies et al. 2012). The rate of MM&T after MicroSort sperm sorting was compared with the rate in control studies without MicroSort in three categories: (1) all babies with at least one thorough physical examination reviewed by the paediatric geneticists in the MicroSort trial versus all babies conceived in control studies regardless of means of conception; (2) ART babies with a minimum of 1 year follow-up in the MicroSort trial versus ART babies in control studies; and (3) babies with a minimum of 1 year follow-up resulting from IUI procedures following MicroSort sperm sorting versus natural conceptions. All the control studies used the same standard definition for major malformations that was used in the MicroSort cohort.
Statistical analysis
Rates of MM&T from the MicroSort clinical trial were compared with controls with comparable modes of pregnancy initiation. Thus, the outcomes of pregnancies arising from MicroSort ART procedures (IVF and IVF with ICSI) were compared with the outcomes of pregnancies from ART procedure controls, and rates of MM&T in pregnancies after MicroSort with IUI were compared with rates in the general population or in naturally occurring pregnancies. For the purposes of comparing ART outcomes in the present study with ART outcomes in the designated controls, only fresh embryo cycles were used. Cases of frozen embryo transfer (FET) and the resulting outcomes were included in the cumulative (all babies) outcomes. This approach was taken because the number of FET cases is relatively small, and pregnancies may occur many years after egg retrieval. Previous studies have suggested that malformation rates associated with FET are similar to rates from fresh embryo transfers (Li et al. 2010; Pelkonen et al. 2014).
No statistical testing was done for individual major malformations because the study was not powered to detect an increase in rare individual events.
Two-sided Pearson’s Chi-squared test without continuity correction was used to make comparisons between proportions. Differences reaching a significance level of 5% were considered significant. Relative risks for ART babies compared with non-ART babies were calculated using our own MicroSort data. Respective malformation rates were compared between MicroSort and each of the three control studies (Hansen et al. 2002; Ludwig and Katalinic 2002; Davies et al. 2012) for each of three categories respectively (i.e. ART outcomes, non-ART outcomes and for all outcomes inclusive of ART and non-ART pregnancies) using a two-sided Pearson’s Chi-squared test without continuity correction and assessing whether the two-sided 95% confidence interval on their relative risk includes 1.0.
Results
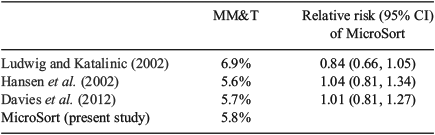
Table 2 includes all MicroSort babies who had at least one thorough examination either at birth or at 1 year and compares the incidence of major malformations in this group with the three control studies that met the study criteria (Hansen et al. 2002; Ludwig and Katalinic 2002; Davies et al. 2012). The rate of MM&T following MicroSort was not significantly different than the rate in any of the three control studies. The rate of MM&T in MicroSort (5.8%; 95% confidence interval (CI) 4.6%–7.3%) was similar to the incidence found in studies of non-MicroSort general populations by Hansen et al. (2002) and Davies et al. (2012), as well as the rate reported by Ludwig and Katalinic (2002).
The relative risks of major malformations for MicroSort pregnancies compared with pregnancies that were not exposed to the MicroSort sperm sorting technology (Table 3) range from 0.84 (Ludwig and Katalinic 2002) to 1.01 (Davies et al. 2012) and to 1.04 (Hansen et al. 2002). In all cases, the 95% CIs included 1.0 and differences were not statistically significant.
|
We were unable to obtain follow-up at 1 year on all babies born in the study. Of the 1358 babies born who reached at least 1 year of age, 90.2% (1225/1358) had birth records submitted and reviewed, and 76.0% (1032/1358) had both their postnatal (1 year of life or more) and birth records evaluated.
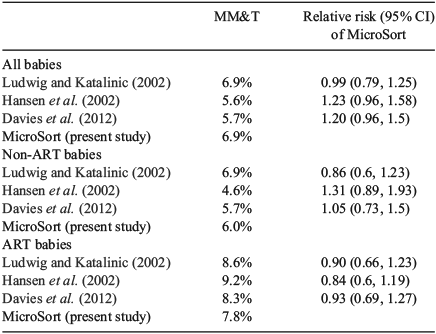
When we included only those babies with completed follow-up examinations to at least 1 year of life (Table 4), the MM&T rate of major malformations following MicroSort sperm sorting (6.9%; 95% CI 5.4%–8.6%) was not significantly different from the other studies among all pregnancies regardless of the conception mode.
Looking at the effect of mode of conception in detail (Tables 4, 5), there was good agreement among rates of major malformation in all studies when restricted to non-ART pregnancies, as well as when restricted to ART-initiated pregnancies. In the non-ART cases, the MicroSort MM&T rate was 6.0% (95% CI 4.0%–8.5%), which did not differ significantly from MM&T rates in the studies of natural conceptions by Hansen et al. (2002), Ludwig and Katalinic (2002), and Davies et al. (2012). In pregnancies resulting from IVF or ICSI, the MicroSort major malformation rate, as measured by an MM&T rate of 7.8% (95% CI 5.6%–10.4%), did not differ significantly from the ART MM&T rates in the three control studies.
|
The MicroSort study population itself demonstrated that the difference in malformation rates between MicroSort babies conceived through ART versus non-ART was not significant (7.8% vs 6.0%, respectively, with a relative risk of 1.31 (95% CI 0.82–2.08); P = 0.258). The malformation rate in FET cycles among babies examined at 1 year of age was 5.7% (2/35).
Discussion
MicroSort sperm sorting increases the probability of selecting the chromosomal sex of offspring. Indications for participation in the present trial were genetic disease prevention and family balancing. There was a cumulative preference for daughters among the family balancing participants, and the female bias in X-linked genetic disease cases had a relatively small contribution that added to this preference. Karabinus et al. (2014) have also reported reasonable pregnancy rates and miscarriage rates associated with MicroSort sperm sorting.
Millions of doses of sexed spermatozoa are produced each year for insemination of a variety of non-human mammalian species using this technique of sperm sorting (Seidel 2012). Tubman et al. (2004) found no difference in major malformations between 1169 calves produced from the sperm sorting technology with Hoechst 33342 and 793 calves from non-sorted spermatozoa, confirming previous studies (Morrell and Dresser 1989; Johnson and Schulman 1994). The present MicroSort sperm sorting trial in humans was a one-of-a-kind study of human gametes manipulated before fertilisation that demonstrated the lack of association between major malformations in offspring and MicroSort staining and sorting of male gametes before fertilisation.
The strength of the present study is the implementation of prospective follow-up, through comprehensive record review of all pregnancies resulting from MicroSort cycles and children up to a minimum of 1 year of age. This thorough ascertainment of birth defects was inclusive and used a standard classification for defining the severity of malformations (Bonduelle et al. 2002). Board-certified paediatric geneticists identified and reviewed relevant findings and established the severity of the malformations. Data from the Western Australian (WA) Birth Defects Registry indicates that only one in 12 major malformations will be identified between 1 and 6 years of life (Bower et al. 2010). The present study, equivalent to the comparative reports that were used herein, did require reports up to at least 1 year of age but major malformations that were identified after 1 year were included to further optimise ascertainment.
The recruitment of a prospective, non-MicroSort cohort for IUI or ICSI without an infertility indication was not feasible. All the control studies included both ART-initiated pregnancies and naturally occurring pregnancies. A formalised analysis of developmental outcomes was precluded due to the lack of a control cohort and the diversity of diagnostic labelling for non-major developmental outcomes.
ARTs have been linked to rare genomic imprinting disorders (Marchesi et al. 2012), specifically Beckwith-Wiedemann, Angelmann and maternal hypomethylation syndrome (Amor and Halliday 2008). Although there was no attempt to separate genomic imprinting disorders from other malformation syndromes in the present study, no recognised imprinting disorders occurred in the MicroSort cohort.
Evidence suggests that imprinting defects from spermatozoa occur at the time that methylation is established in male gametes, which is as early as the diploid spermatogonium (Manipalviratn et al. 2009; Owen and Segars 2009). Furthermore, primordial germ cells maintain the methylation status of the differentially methylated regions until they enter the germinal ridge (Stringer et al. 2013). Therefore, the staining of mature male gametes with Hoechst 33342 before fertilisation is unlikely to affect the epigenetics of future generations.
The meta-analysis by Wen et al. (2012) has supported data indicating a greater rate of major malformations in pregnancies conceived through ART. The studies used as controls in the present study (Hansen et al. 2002; Ludwig and Katalinic 2002; Davies et al. 2012) also demonstrate a significant increase (P < 0.05) in major malformation rates when pregnancies are conceived through the use of ART. The cause of this difference is contentious and it may be due, in part, to the underlying infertility of couples who use ART. Due to the lack of a significant increase in the MM&T rate among MicroSort ART cases (7.8%) compared with MicroSort non-ART cases (6.0%) and the unknown number of infertile couples in the present study, this study does not clarify the relationship between ART and major malformations. However, the higher rates of major malformations in ART cases in the literature suggest that the relatively high fraction of ART babies in the MicroSort study (51.1%; 527/1032) may have had a marginal upward effect on the rate of major malformations seen in all MicroSort babies at 1 year (6.9%).
Other than the initiation of pregnancy using ART, other potential confounding factors on malformation rates (Duong et al. 2012), such as the age of the woman whose eggs were fertilised, singleton versus multifetal gestation and the rate of nulliparity, could not be compared because these data were not available for the three control studies. The high percentage of family balancing cases makes it likely that the study group had a lower rate of nulliparity and a greater median age than the women in the control studies used for comparison. If true, these two confounders would have had qualitatively opposite net effects.
Despite years of clinical use, questions of safety and efficacy remain unresolved for many reproductive interventions at the onset of conception, including ART and associated gamete and embryo manipulations (Evers 2013). The present study of MicroSort sperm sorting was designed with sufficient power and comprehensive ascertainment so as to demonstrate its safety for an intervention involving gametes before widespread clinical adoption. There were no American studies that could be used for comparison because none met the comprehensive ascertainment criteria used for the present study. The population in the MicroSort study was ethnically diverse; country of origin for control studies was limited to countries that had both a well-developed facility for assisted reproduction and active and comprehensive population ascertainment of malformations. The three control studies used for this paper were conducted in Germany (one study) and Australia (two studies). Despite potential ethnic disparities between studies, there is no evidence that the rates would be different based on ethnic differences in our cohort.
The UK has the largest scope of reproductive data and the oldest assisted reproduction database, and the Scandinavian countries have been leaders in linking population-based health and reproductive treatment records (Brison et al. 2013). The lack of American studies available for comparison demonstrates the need to develop cost-effective and thorough evaluation methods related to ART and its modifications in the US. Although the Society for Assisted Reproductive Technology (SART) database would be the most logical starting point for the construction of the reproductive database, more comprehensive, active ascertainment is needed. The ascertainment protocol used in the present study could serve as a prototype for the active follow-up of assisted reproduction outcomes in the US. Ideally, comprehensive population health data should be also linked to a comprehensive reproductive database. This large clinical trial over 16 years and approximately 7500 MicroSort sperm separations, which resulted in the birth of 1358 babies, was conducted to assess the safety of MicroSort sperm separation for family balancing and genetic disease prevention. This comprehensive analysis of pregnancy outcomes from conception to 1 year of life demonstrates that MicroSort sperm separation does not increase the risk of major congenital malformations.
Acknowledgements
The authors are grateful to Daniel Molina for organised data collection and administrative advice and consultation, Eugene Heyman for statistical planning, analysis and guidance, Wayne Stanley for review of the article and scientific advice, Harvey Stern for paediatric genetic consultation, Marisa Cole as the clinical trial coordinator and Nancy Gallucci as physician and patient liaison. Daniel Potter was the Medical Director for the MicroSort West site. The Genetics and IVF Institute, USA, was the sole source of financial support for this research.
References
Amor, D. J., and Halliday, J. (2008). A review of known imprinting syndromes and their association with assisted reproduction technologies. Hum. Reprod. 23, 2826–2834.| A review of known imprinting syndromes and their association with assisted reproduction technologies.Crossref | GoogleScholarGoogle Scholar | 18703582PubMed |
Bonduelle, M., Liebaers, I., Deketelaere, V., Derde, M. P., Camus, M., Devroey, P., and Van Steirteghem, A. (2002). Neonatal data on a cohort of 2889 babies born after ICSI (1991–1999) and of 2995 infants born after IVF (1983–1999). Hum. Reprod. 17, 671–694.
| Neonatal data on a cohort of 2889 babies born after ICSI (1991–1999) and of 2995 infants born after IVF (1983–1999).Crossref | GoogleScholarGoogle Scholar | 11870121PubMed |
Bower, C., Rudy, E., Callaghan, A., Quick, J., and Nassar, N. (2010). Age at diagnosis of birth defects. Birth Defects Res. A Clin. Mol. Teratol. 88, 251–255.
| Age at diagnosis of birth defects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksV2ktbc%3D&md5=48b06e165698eb0c43aacb9b9b808babCAS | 20213697PubMed |
Brison, D. R., Roberts, S. A., and Kimber, S. J. (2013). How should we assess the safety of IVF technologies? Reprod. Biomed. Online 27, 710–721.
| How should we assess the safety of IVF technologies?Crossref | GoogleScholarGoogle Scholar | 24145118PubMed |
Chang, J.-K., Jien, W.-Y., Chen, H.-L., and Hsieh, K.-S. (2011). Color Doppler echocardiographic study on the incidence and natural history of early infancy muscular ventricular septal defect. Pediatr. Neonatol. 52, 256–260.
| Color Doppler echocardiographic study on the incidence and natural history of early infancy muscular ventricular septal defect.Crossref | GoogleScholarGoogle Scholar | 22036220PubMed |
Davies, M. J., Moore, V. M., Willson, K. J., Van Essen, P., Priest, K., Scott, H., Haan, E. A., and Chan, A. (2012). Reproductive technologies and the risk of birth defects. N. Engl. J. Med. 366, 1803–1813.
| Reproductive technologies and the risk of birth defects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xnt1Gjsrk%3D&md5=d51f492861570a8cc6ec93ae7129ae6eCAS | 22559061PubMed |
Duong, HT, Hoyt, AT, Carmichael, SL, Gilboa, SM, Canfield, MA, Case, A., McNeese, ML, Waller, DK, the National Birth Defects Prevention Study (2012). Is maternal parity an independent risk factor for birth defects? Birth Defects Res. A Clin. Mol. Teratol. 94, 230–236.
| Is maternal parity an independent risk factor for birth defects?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivVGgtb4%3D&md5=1ff1a0d4857881893e4f99f66c51f442CAS | 22371332PubMed |
Evers, J. L. H. (2013). The wobbly evidence base of reproductive medicine. Reprod. Biomed. Online 27, 742–746.
| The wobbly evidence base of reproductive medicine.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c%2FjtV2hsg%3D%3D&md5=baeaa4cdfc9fe8992b13be69acc4e421CAS |
Graham, J. M. (1991). Clinical approach to structural defects. Semin. Perinatol. 15, 2–15.
| 1:STN:280:DyaK3M3ntF2gtQ%3D%3D&md5=94aa78c17ca7e7de009629ad0c4c3c8fCAS | 2052946PubMed |
Hansen, M., Kurinczuk, J. J., Bower, C., and Webb, S. (2002). The risk of major birth defects after intra-cytoplasmic sperm injection and in vitro fertilization. N. Engl. J. Med. 346, 725–730.
| The risk of major birth defects after intra-cytoplasmic sperm injection and in vitro fertilization.Crossref | GoogleScholarGoogle Scholar | 11882727PubMed |
Johnson, L. A., and Schulman, J. D. (1994). The safety of sperm selection by flow cytometry. Hum. Reprod. 9, 758–759.
| 1:STN:280:DyaK2M%2FhsVyruw%3D%3D&md5=4a8e6840574bb9b33fb14245e2b90befCAS | 7929718PubMed |
Johnson, L. A., Flook, J. P., and Hawk, H. W. (1989). Sex preselection in rabbits: live births from X and Y sperm separated by DNA and cell sorting. Biol. Reprod. 41, 199–203.
| Sex preselection in rabbits: live births from X and Y sperm separated by DNA and cell sorting.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c%2FivVGisA%3D%3D&md5=fd6d26defc4abdc32e6d8c86583932c4CAS | 2804212PubMed |
Johnson, L. A., Welch, G. R., Keyvanfar, K., Dorfmann, A., Fugger, E. F., and Schulman, J. D. (1993). Gender preselection in humans? Flow cytometric separation of X and Y spermatozoa for the prevention of X-linked disease. Hum. Reprod. 8, 1733–1739.
| 1:STN:280:DyaK2c7jt1aqsQ%3D%3D&md5=c68cabf83d3269188483166da528fc16CAS | 8300839PubMed |
Karabinus, D. S., Marazzo, D. P., Stern, H. S., Potter, D. A., Opanga, C., Cole, M.,, Johnson, L. A., and Schulman, J. D. (2014). The effectiveness of flow cytometric sorting of human sperm (MicroSort) for influencing a child’s sex. Reprod. Biol. Endocrinol. 12, 106.
| The effectiveness of flow cytometric sorting of human sperm (MicroSort) for influencing a child’s sex.Crossref | GoogleScholarGoogle Scholar | 25420620PubMed |
Latt, S. A., and Stetten, G. (1976). Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J. Histochem. Cytochem. 24, 24–33.
| Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28Xos1ensw%3D%3D&md5=adab8d48e02a78df3ee2406351aa90e0CAS | 943439PubMed |
Li, H. Z., Qiao, J., Chi, H. B., Chen, X. N., Liu, P., and Ma, C. H. (2010). Comparison of the major malformation rate of children conceived from cryopreserved embryos and fresh embryos. Chin. Med. J. (Engl). 123, 1893–1897.
| 20819574PubMed |
Ludwig, M., and Katalinic, A. (2002). Malformation rates in fetuses and children conceived after ICSI. Reprod. BioMed. Online 5, 171–178.
| Malformation rates in fetuses and children conceived after ICSI.Crossref | GoogleScholarGoogle Scholar | 12419043PubMed |
Manipalviratn, S., DeCherney, A., and Segars, J. (2009). Imprinting disorders and assisted reproductive technology. Fertil. Steril. 91, 305–315.
| Imprinting disorders and assisted reproductive technology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnsF2ns7Y%3D&md5=ecbfcc80b6998a82793e6ceee0d35ba4CAS | 19201275PubMed |
Marchesi, D. E., Qiao, J., and Feng, H. L. (2012). Embryo manipulation and imprinting. Semin. Reprod. Med. 30, 323–334.
| Embryo manipulation and imprinting.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1WksLfO&md5=8f076e7c67a71f4fabe29c0e4e759088CAS | 22740058PubMed |
Morrell, J. M., and Dresser, D. W. (1989). Offspring from inseminations with mammalian sperm stained with Hoechst 33342, either with or without flow cytometry. Mutat. Res. 224, 177–183.
| Offspring from inseminations with mammalian sperm stained with Hoechst 33342, either with or without flow cytometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXisVSm&md5=ae9d3434019228b26632921ae00732a0CAS | 2797035PubMed |
Owen, C. M., and Segars, J. H. (2009). Imprinting disorders and assisted reproductive technology. Semin. Reprod. Med. 27, 417–428.
| Imprinting disorders and assisted reproductive technology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOis7zL&md5=1285d242c03bf1c41a84fbcc311dd205CAS | 19711252PubMed |
Pelkonen, S., Hartikainen, A. L., Ritvanen, A., Koivunen, R., Martikainen, H., Gissler, M., and Tiitinen, A. (2014). Major congenital anomalies in children born after frozen embryo transfer: a cohort study 1995–2006. Hum. Reprod. 29, 1552–1557.
| Major congenital anomalies in children born after frozen embryo transfer: a cohort study 1995–2006.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cjhtV2htQ%3D%3D&md5=15e1fbf5cf5ed5c4bab2178717ef2ea3CAS | 24812318PubMed |
Queißer-Luft, A., Stolz, G., Wiesel, A., Schlaefer, K., and Spranger, J. (2002). Malformations in newborn: results based on 30940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990–1998). Arch. Gynecol. Obstet. 266, 163–167.
| Malformations in newborn: results based on 30940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990–1998).Crossref | GoogleScholarGoogle Scholar | 12197558PubMed |
Rath, D., Barcikowski, S., de Graaf, S., Garrels, W., Grossfeld, R., Klein, S., Knabe, W., Knorr, C., Kues, W., Meyer, H., Michi, J., Moench-Tegeder, G., Rehbock, C., Taylor, U., and Washausen, S. (2013). Sex selection of sperm in farm animals: status report and developmental prospects. Reproduction 145, R15–R30.
| Sex selection of sperm in farm animals: status report and developmental prospects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslGisLk%3D&md5=d8d7429bf174c0d409100205ae0fcbe7CAS | 23148085PubMed |
Sands, A. J., Casey, F. A., Craig, B. G., Dornan, J. C., Rogers, J., and Mulholland, H. C. (1999). Incidence and risk factors for ventricular septal defect in ‘low risk’ neonates. Arch. Dis. Child. Fetal Neonatal Ed. 81, F61–F63.
| Incidence and risk factors for ventricular septal defect in ‘low risk’ neonates.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzoslWntA%3D%3D&md5=9dd3319f52815eb7439ace69d6db749dCAS | 10375365PubMed |
Schulman, J. D., and Karabinus, D. S. (2005). Scientific aspects of preconception gender selection. Reprod. Biomed. Online 10, 111–115.
| Scientific aspects of preconception gender selection.Crossref | GoogleScholarGoogle Scholar | 15820020PubMed |
Seidel, G. E. (2012). Sexing mammalian sperm: where do we go from here? J. Reprod. Dev. 58, 505–509.
| Sexing mammalian sperm: where do we go from here?Crossref | GoogleScholarGoogle Scholar | 23124700PubMed |
Stringer, J. M., Barrand, S., and Western, P. (2013). Fine-tuning evolution: germ-line epigenetics and inheritance. Reproduction 146, R37–R48.
| Fine-tuning evolution: germ-line epigenetics and inheritance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFejsLbO&md5=29e966a95d2db3e3b2de6517bd0f3e40CAS | 23633622PubMed |
Sumner, A. T., and Robinson, J. A. (1976). A difference in dry mass between the heads of X- and Y-bearing human spermatozoa. J. Reprod. Fertil. 48, 9–15.
| A difference in dry mass between the heads of X- and Y-bearing human spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXhtlCisw%3D%3D&md5=bbd2bf9227c19015853b252eca56c103CAS | 966214PubMed |
Tubman, L. M., Brink, Z., Suh, T. K., and Seidel, G. E. (2004). Characteristics of calves produced with sperm sexed by flow cytometry/cell sorting J. Anim. Sci. 82, 1029–1036.
| 1:CAS:528:DC%2BD2cXis1arsb8%3D&md5=78df6639e8723fb9a0030f083c7af315CAS | 15080324PubMed |
Wen, J., Jiang, J., Ding, C., Dai, J., Liu, Y., Xia, Y., Liu, J., and Hu, Z. (2012). Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil. Steril. 97, 1331–1337.
| Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 22480819PubMed |





