Reductions in the number of mid-sized antral follicles are associated with markers of premature ovarian senescence in dairy cows
Silvia C. Modina A B , Irene Tessaro A , Valentina Lodde A , Federica Franciosi A , Davide Corbani A and Alberto M. Luciano AA Reproductive and Developmental Biology Laboratory, Department of Health, Animal Sciences and Food Safety, Università degli Studi di Milano, via G. Celoria 10, 20133, Milano, Italy.
B Corresponding author. Email: silvia.modina@unimi.it
Reproduction, Fertility and Development 26(2) 235-244 https://doi.org/10.1071/RD12295
Submitted: 13 September 2012 Accepted: 26 November 2012 Published: 18 January 2013
Abstract
High-producing dairy cows are subfertile; however, the mechanisms responsible for the decreased fertility are unknown. The aim of the present study was to test the hypothesis that culled dairy cows (4–8 years old) characterised by ‘Lo’ ovaries (i.e. those with <10 mid-antral follicles) are affected by premature ovarian senescence. Cows in which both ovaries were ‘Lo’ ovaries represented 5% of the total population analysed, and exhibited reduced ovarian size (P < 0.001) and increased perifollicular stroma (P < 0.05) compared with age-matched controls (i.e. cows in which both ovaries had >10 mid-antral follicles; ‘Hi’ ovaries). The total number of follicles, including healthy and atretic primordial, primary, secondary and small antral follicles, was lower in Lo ovaries (P < 0.01). Interestingly, the primordial follicle population in Lo ovaries was lower (P < 0.05) than in the control. Finally, the follicular fluid of mid-antral follicles from Lo ovaries had reduced oestradiol and anti-Müllerian hormone levels (P < 0.05), but increased progesterone concentrations (P < 0.05). Together, these data account for the reduced fertility of cows with Lo ovaries and are in agreement with previous observations that oocytes isolated from Lo ovaries have reduced embryonic developmental competence. Cows with a specific Lo ovary condition may represent a suitable model to address the causes of low fertility in high-yielding dairy cows, as well as the condition of premature ovarian aging in single-ovulating species.
Additional keywords: anti-Müllerian hormone, infertility, ovarian aging, ovary, premature ovarian failure.
Introduction
The antral follicle count (AFC) is considered one of the most reliable, non-invasive methods of determining ovarian reserve and is a good indicator of the size of the remaining primordial pool in women with proven natural fertility (Hansen et al. 2011). Diminished ovarian reserve, associated with a drop in ovarian volume and vascularity, generally predicts a poor response to gonadotropin, limits the possibility of successful pregnancy and is correlated with the occurrence of menopausal transition (Lass and Brinsden 1999; Kwee et al. 2007; La Marca et al. 2012). A low AFC has been described in women during the perimenopausal period (Hansen et al. 2008; Broekmans et al. 2009), as well as in young infertile and subfertile women affected by premature ovarian failure (POF; De Vos et al. 2010; Monget et al. 2012), suggesting that the factors affecting the size of the follicular reserve also affect the quality of the remaining oocytes and the likelihood of conception (Rosen et al. 2011).
Chronological age also affects fertility in cattle. Mature cows (13–16 years old) have reduced follicle numbers (Malhi et al. 2005, 2006) and decreased oocyte competence (Malhi et al. 2007) compared with young cows (1–6 years old). Interestingly, in young adult heifers (1–3 years old), the AFC is positively correlated with ovarian size, the total quantity of healthy follicles and healthy oocytes (Ireland et al. 2007, 2008) and pregnancy rate (Cushman et al. 2009; Mossa et al. 2012). Moreover variations in biomarkers of human follicular differentiation and function, as well as those for ovarian aging, such as serum concentrations of FSH, oestradiol (E2), anti-Müllerian hormone (AMH) and inhibin-B (Lambalk et al. 2009; La Marca et al. 2012), have been shown to be associated with the AFC and reproductive potential in cattle (for a review, see Ireland et al. 2011).
In both dairy and beef cows, reduced fertility is due to a high incidence of abnormal ovarian activity (Macmillan et al. 2003; Yimer et al. 2010). In previous studies, we demonstrated that the oocytes of 4- to 8-year-old dairy cows that had ovaries with fewer than 10 mid-antral follicles (2–6 mm in diameter) and no follicles ≥10 mm in diameter (‘Lo’ ovaries) exhibited reduced developmental capability compared with age-matched controls in which both ovaries had >10 mid-antral follicles (‘Hi’ ovaries; Modina et al. 2007). Moreover, defective endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) signalling was found in Lo ovaries in addition to reduced follicle vasculature, which could adversely affect oocyte quality and early embryonic in vitro development (Tessaro et al. 2011). Therefore, the aim of the present study was to assess whether in a population of culled dairy cows of reproductive age those cows with Lo ovaries had morphological and endocrine markers commonly associated with premature ovarian aging that could, in turn, lead to a premature decline in fertility.
Materials and methods
Collection of Hi and Lo ovaries and evaluation of gross ovarian anatomy
Unless stated otherwise, all chemicals and reagents were purchased from Sigma Chemical (St Louis, MO, USA). Bovine ovaries were obtained from a local abattoir (INALCA, Ospedaletto Lodigiano, Italy) from 4- to 8-year-old dairy cows, subjected to routine veterinary inspection and consistent with specific health requirements as stated in EEC Directive 89/556 and the following modifications. The ovaries were transported, on ice, to the laboratory within 2 h from collection.
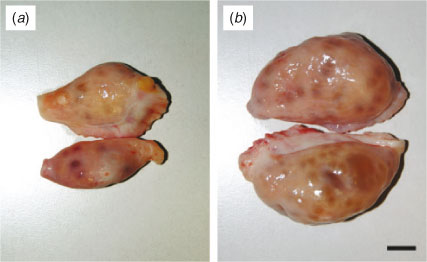
The ovaries from each cow were isolated and classified into one of two categories, as described previously (Modina et al. 2007; Tessaro et al. 2011). Briefly, ovaries were classified as Lo (= low efficiency) ovaries when they were found to contain <10 follicles between 2 and 6 mm in diameter and no follicles ≥10 mm or as Hi (= high efficiency) ovaries when they contained >10 follicles between 2 and 6 mm in diameter (Fig. 1).
|
Each ovary was subjected to gross examination, with mid-antral follicles visible on the ovarian surface counted and measured using a ruler. In addition, ovaries were weighed and ovarian length and height were determined using a ruler.
In the present study, we only used ovaries isolated from cows containing either both Lo or both Hi ovaries. Ovaries from cows with one Lo and one Hi ovary were not used. The Hi ovaries were used as the age-matched control group. In both categories, the presence or absence of a corpus luteum was not taken into account, as stated previously (Gandolfi et al. 1997; Modina et al. 2007; Tessaro et al. 2011). It has been demonstrated that the AFC is not affected by the stage of the oestrous cycle (Cushman et al. 2009). In addition, the corpus luteum does not exert a local effect on the developmental competence of oocytes derived from subordinate follicles (Vassena et al. 2003).
Microscopic evaluation of ovaries
Ovarian sections were evaluated microscopically to: (1) confirm that the number of mid-antral follicles (‘mid-AFC’ hereafter) reflects the actual number of antral follicles in the entire organ; (2) count the number of follicles at earlier stages of folliculogenesis (i.e. primordial, primary, secondary and small antral follicles) in the entire organ; (3) evaluate follicular health status; and (4) evaluate the organisation of collagen fibres in the cortical and perifollicular ovarian stroma.
Ovaries were cut into eight equal longitudinal strips using a scalpel, as described previously (Ireland et al. 2008) with slight modifications. All strips were processed for histological evaluation as follows: strips were fixed in B5 fixative (Bio-Optica, Milan, Italy), dehydrated in a graded series of ethanol, cleared with xylene, embedded in paraffin and sectioned at 8 μm. The sections were then placed on glass microscope slides that had been treated with Vectabond (Vector Laboratories, Burlingame, CA, USA) to enhance the adherence of tissues, stained with haematoxylin and eosin and then analysed as described below.
All slides were examined under a microscope and the mid-AFC was determined by counting the number of mid-antral follicles present throughout the entire organ. The number of follicles at earlier stages of folliculogenesis was determined in every 40th section of a randomly chosen strip, as described previously (Tilly 2003; Ireland et al. 2007). Only follicles that contained a cross-section of the oocyte nucleus were counted. To obtain an estimate of the entire follicular population in the ovary, the number of follicles counted was multiplied for 320, a correction factor that takes into consideration the counting follicles in every 40th section in one of the eight strips (40 × 8 = 320). It has been demonstrated previously that this is a reliable estimate of the total number of follicles at early stages of development (Ireland et al. 2008).
Oocytes surrounded by a single layer of flattened granulosa cells were classified as primordial follicles. Primary follicles consisted of an oocyte surrounded by one layer of cuboidal granulosa cells. Secondary follicles contained an oocyte surrounded usually by two to six layers of cuboidal granulosa cells and no antrum. Small antral follicles consisted of an oocyte surrounded by several layers of cuboidal granulosa cells with a fully formed theca interna and an antral cavity (<2 mm; Ireland et al. 2008; Rodgers and Irving-Rodgers 2010). A follicle was considered morphologically healthy if it exhibited an intact basal membrane, organised granulosa cell layers with only occasional pyknotic nuclei or atretic bodies in the granulosa cells or follicular antrum, and an intact oocyte and nucleus (Lussier et al. 1987; Yang and Rajamahendran 2000; Ireland et al. 2008; Tessaro et al. 2011).
Additional fragments of Lo and Hi ovaries were fixed and processed as described above and sections were stained with Heidenhain’s Azan Trichrome (Bio-Optica), which specifically stains collagen fibres, to assess the density of collagen fibres in the ovarian cortical stroma and perifollicular stroma of healthy mid-antral follicles. The ovarian and perifollicular stroma of Hi and Lo ovaries were analysed and the percentage of follicles enclosed in thick and thin layers of collagen fibres was determined.
Hormone concentrations in follicular fluid
Follicular fluid was aspirated from mid-antral follicles of Lo and Hi ovaries using a 2-mL syringe with a 26.5-gauge needle. For each ovary, five follicles were aspirated and the follicular fluid recovered was pooled. Each pool was centrifuged for 30 s at 5000g to separate the follicular cells. The supernatant was stored at –20°C until use.
Quantitative analyses of E2 (sensitivity 10 pg mL–1) and progesterone (P4; sensitivity 0.1 ng mL–1) were performed on 400-µL samples of follicular fluid using an Architect i1000SR Immunoassay Analyzer (Abbott Diagnostics, Abbott Park, IL, USA) according to the manufacturer’s instructions (Stricker et al. 2006; Plati et al. 2010).
Concentrations of AMH were determined using the Active MIS/AMH ELISA kit (Diagnostic Systems Laboratories, Webster, TX, USA; sensitivity 0.006 ng mL–1), according to the manufacturer’s instructions. This kit is an enzymatically amplified two-site immunoassay containing materials for the quantitative measurement of AMH in human serum; it recently has been validated in bovine follicular fluid (Monniaux et al. 2008; Ireland et al. 2009; Rico et al. 2009). Concentrations of AMH were determined in 10-µL samples of follicular fluid diluted 1 : 200 using a protein-based buffer included in the kit. Analyses of the colorimetric reaction were performed using the Multiskan Reader MS plate reader (MTX Laboratory Systems, Vienna, VA, USA; sensitivity 0.006 ng mL–1) at a wavelength of 450 nm.
Statistical analysis
Data are presented as the mean ± s.e.m. Mean values of two groups were compared using t-tests, preceded by the Levene test to assess the equality of variances in the different samples. For correlation studies, the significance of differences was determined using Pearson’s correlation coefficient (r). All analyses were performed using SPSS version 17.0 (SPSS, Chicago, IL, USA). P < 0.05 was considered significant.
Results
Evaluation of gross ovarian anatomy: mid-AFC and ovarian size and weight
In the present study, a total of 475 cows aged 4–8 years were examined. Ovaries were obtained from cows culled at the local abattoir in four different slaughtering sessions. Of the 475 cows examined, 4.66 ± 1.79% were found to have both Lo ovaries (<10 mid-antral follicles 2–6 mm in diameter and no follicles ≥10 mm in diameter).
Gross ovarian anatomy (i.e. confirmation of mid-AFC and evaluation of ovarian size and weight) was assessed in 28 Lo and 48 Hi ovaries. Figure 1 shows representative images of the gross ovarian anatomy of Lo (Fig. 1a) and Hi (Fig. 1b) ovaries. As indicated in Table 1, there was a significant difference in the mean number of mid-antral follicles between Lo and Hi ovaries (4.51 ± 0.32 vs 31.93 ± 1.67, respectively; P < 0.001). Similarly, there was a significant reduction in ovarian wet weight and size (length and height) in Lo compared with Hi ovaries (P < 0.001).

|
Microscopic evaluation of the ovaries: follicular population count and health status
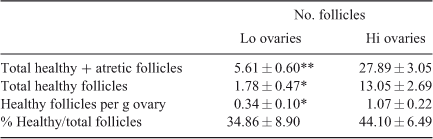
Twenty ovaries from each of the Lo and Hi groups were evaluated histologically. The results of the microscopic evaluation of mid-antral follicles are given in Table 2 and are in agreement with the results obtained by counting mid-antral follicles on the ovarian surface, confirming the accuracy of the experimental approach used in the present study. The number of mid-antral follicles differed significantly between Lo and Hi ovaries (5.61 ± 0.60 vs 27.89 ± 3.05 respectively; P < 0.001). Interestingly, the number of healthy mid-antral follicles and the number of healthy mid-antral follicles per g ovary were lower in Lo compared with Hi ovaries (P < 0.01). However, this difference was no longer significant if the number of healthy follicles as a proportion of the total number of follicles (healthy + atretic) was considered, rather than the absolute number or the number of follicles per g ovary.
|
Table 3 gives results of the microscopic evaluation of follicles at earlier stages of folliculogenesis (as the sum of primordial, primary, secondary and small antral follicles). The absolute number of follicles at these stages of folliculogenesis and the number of healthy follicles per g ovary were lower in Lo compared with Hi ovaries, but the number of healthy follicles as a percentage of total follicles did not differ between the two groups.

|
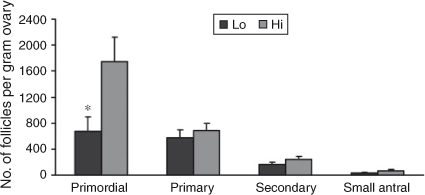
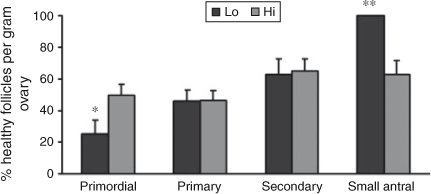
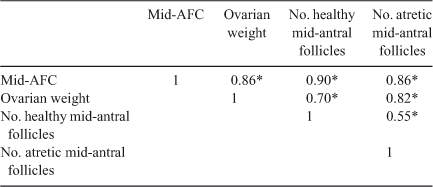
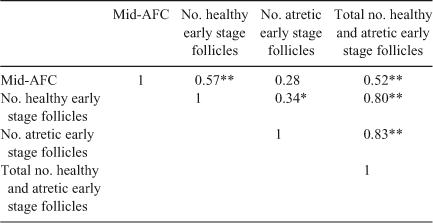
Interestingly, comparing the number of follicles at each stage of folliculogenesis between the two groups revealed that the total number of primordial follicles per g ovary was lower in Lo compared with Hi ovaries (P < 0.05; Fig. 2), whereas there were no differences in follicle numbers in the other follicle classes. In addition, the percentage of primordial healthy follicles per g ovary was lower in Lo compared with Hi ovaries, whereas there were no differences in the health status between the two groups of primary and secondary follicles. At the small antral stage, there was a lower percentage of healthy follicles in the Hi compared with Lo ovaries (P < 0.01; Fig. 3). Variations in mid-AFC exhibited significant positive correlations with ovarian weight (r = 0.86; P < 0.01), the number of healthy mid-antral follicles (r = 0.90; P < 0.01) and the number of atretic mid-antral follicles (r = 0.86; P < 0.01; Table 4). Finally, variations in mid-AFC were positively correlated with the number of healthy (r = 0.57; P < 0.01) and healthy plus atretic (r = 0.52; P < 0.01) follicles at earlier stages of development (as the sum of primordial, primary, secondary and small antral follicles; Table 5).
|
|
|
|
Evaluation of ovarian cortical and perifollicular stroma
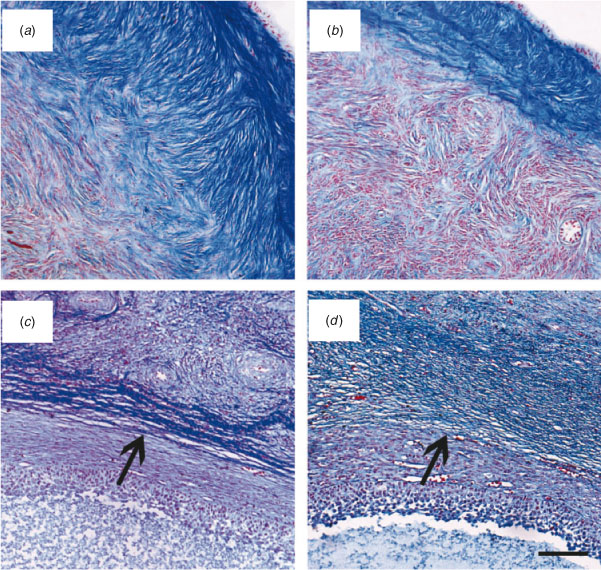
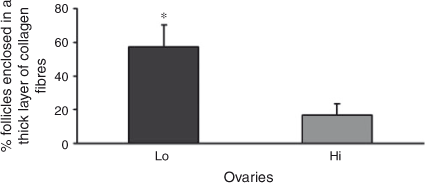
Histochemical evaluation of ovarian cortical and perifollicular stroma was conducted on 22 ovaries in each of the Lo and Hi groups. In Lo ovaries, the cortical stroma was generally characterised by dense and compacted collagen fibres arranged in thick bundles (Fig. 4a). In contrast, in Hi ovaries, the ovarian stroma had mostly a fine organisation (Fig. 4b). Further analysis revealed that the perifollicular stroma of mid-antral follicles was organised in a network of collagen fibres of various densities. Thus, mid-antral follicles were classified as enclosed in a thick (Fig. 4c) or thin (Fig. 4d ) layer of collagen fibres. The percentage of follicles enclosed in a thick layer of collagen fibres was significantly higher in Lo compared with Hi ovaries (P < 0.05; Fig. 5).
|
|
Hormone concentrations in follicular fluid
Hormone concentrations were determined in the follicular fluid aspirated from mid-antral follicles of 44 Lo and 60 Hi ovaries. Lower concentrations of E2 and AMH were detected in Lo compared with Hi ovaries, in addition to a decreased E2/P4 ratio (P < 0.05). In contrast, P4 concentrations were higher in Lo compared with Hi ovaries (P < 0.05; Fig. 6).
Discussion
The present study demonstrates a direct relationship between reductions in mid-AFC and markers of ovarian premature senescence in dairy cows of reproductive age. In the present study, 4- to 8-year-old dairy cows with Lo ovaries exhibited decreased ovarian size and an increase in the ovarian stroma, a decline in the ovarian reserve and a reduction in AMH concentrations in the follicular fluid compared with the age-matched control group (Hi ovaries). Remarkably, these features, in addition to decreased E2 and increased P4 concentrations in the follicular fluid, correlate with embryonic developmental competence because oocytes collected from animals with Lo ovaries have a significantly lower in vitro developmental competence than oocytes from animals with Hi ovaries (Gandolfi et al. 1997; Modina et al. 2007; Tessaro et al. 2011).
Over the past two decades, significant fertility reduction in dairy cows has been described. Currently, up to 50% of dairy cows exhibit abnormal post partum oestrous cycles and ovarian dysfunction resulting in an increase in the calving to first insemination interval (Sakaguchi 2011; Walsh et al. 2011) and a decrease in conception rates (Garnsworthy et al. 2009). This is an important issue because reproductive inefficiency has serious economic impacts by decreasing milk yield and the number of calves born, and by increasing the cost of veterinary services and the culling rate (Gröhn and Rajala-Schultz 2000; Sakaguchi 2011).
Several lines of evidence indicate that ovarian dysfunction and/or reduced oocyte competence may play an important part in reducing fertility (Lucy 2001; Lucy 2007). For example, Opsomer et al. (1998) analysed ovarian dysfunction in 335 high-yielding dairy cows of reproductive age (after calving) and found that a high percentage of cows (almost 23%) suffered delayed cyclicity or anovulation and a prolonged luteal phase. Interestingly, most of these animals had inactive ovaries that were, as determined by rectal palpation, small and hard, with limited or absent follicular development. In the present study, approximately 5% of the dairy cows examined were found to have comparable macroscopic characteristics: they had ovaries with a reduced number of mid-antral follicles and were fibrous. In the present study, specific histochemical analyses were used to better characterise the Lo phenotype ovary. The analyses revealed the presence of a compact stroma encapsulating the few healthy mid-antral follicles localised in Lo ovaries; this increased perifollicular ovarian stroma may contribute to the scarce ingrowth of capillaries into the theca, as reported recently for these follicles (Tessaro et al. 2011), and contributes to the isolation of the mid-antral follicles from ovarian environment.
Interestingly, healthy mid-antral follicles from Lo ovaries are also characterised by reduced eNOS expression, which may be related to increased oxidative stress due to vascular defects, and reduced NO availability in the follicular fluid (Tessaro et al. 2011). Thus, we can hypothesise that changes in the uptake of nutritional and regulatory molecules may contribute to the low quality of oocytes from Lo ovaries of dairy cows of reproductive age, as suggested for menopausal woman (Tatone et al. 2008). Conversely, the depletion of ovarian follicles would likely result in an increase in cortical ovarian stroma, accounting for the changes in ovarian morphology of Lo ovaries.
In the present study, Lo ovaries exhibited a phenotype resembling menopausal gonads that have been described in young women affected by POF (Haidar et al. 1994). POF is a heterogeneous disorder characterised by hypergonadotropic hypogonadism before the age of 40, which is preceded by a phase of accelerated ovarian senescence (Kokcu 2010) that results in follicle reduction (Shelling 2010). Two major reasons for the development of POF have been proposed: (1) failure to acquire an adequate number of initial primordial follicles, which normally takes place during fetal life; and (2) excessive clearance of primordial follicles, together with the suppressed activation and further development of primordial follicles (Jagarlamudi et al. 2010). The aetiology of most of cases of POF is idiopathic; however, this condition has a heterogenous background (Nelson 2009; De Vos et al. 2010) and, in the past decade, an increasing number of genes has been implicated in premature ovarian failure (Goswami and Conway 2005; Skillern and Rajkovic 2008; van Dooren et al. 2009; Persani et al. 2010; Cordts et al. 2011).
Light and transmission electron microscopy studies have led to the identification of two POF phenotypes: follicular and afollicular POF ovaries (Haidar et al. 1994; Massin et al. 2004, 2008). Massin et al. (2004) described POF follicular ovaries as normal-sized ovaries characterised by the presence of both non-growing and growing follicles at various stages of development, whereas POF afollicular ovaries are smaller in size and do not contain any follicles. In contrast, Haidar et al. (1994) described both follicular and afollicular ovaries as diminished in volume, fibrous in consistency and rich in irregular spirals of cells and fibres in the cortical region. Regardless of these classifications, our observations suggest that bovine ovaries with low mid-AFC are comparable to POF ovaries, indicating early ovarian senescence. This idea is supported by the fact that, in our model (population of cows with Lo ovaries), the quantity of mid-antral follicles on the ovarian surface exhibited a significant positive correlation with the total number of primordial follicles, as described previously in dairy cows (Cushman et al. 1999) and in women during the perimenopausal period (Hansen et al. 2008; Broekmans et al. 2009) or affected by POF (De Vos et al. 2010; Monget et al. 2012). This observation is important because exhaustion of the pool of primordial follicles within the ovarian cortex is the cause of primary ovarian insufficiency in most young women (De Vos et al. 2010).
The percentage of atresia was higher in animals with Lo than Hi ovaries; it is thus reasonable to assume that, in our model, there is an accelerated clearance of the pool of primordial follicles and, in turn, the reduced follicle count could explain the decreased fertility in cows with Lo ovaries. However, the mechanisms responsible for ovarian insufficiency and primordial follicle depletion remain to be determined and several factors may be involved.
In a previous study, Ireland et al. (2008) observed that young adult cattle with a high AFC had a greater number of morphologically healthy follicles in all follicular stages analysed than their age-matched counterparts with a low AFC. In contrast, our data show that the percentage of healthy follicles in all follicular stages analysed was similar between the two groups, whereas the population of healthy primordial follicles was significantly compromised in Lo ovaries. However, in their study, Ireland et al. (2008) used cross-bred beef heifers that were 10–14 months old, previously synchronised. Thus, breed, lactating status, age and hormonal treatments may account for the discrepancies between these two studies, including the unexpected higher percentage of healthy early antral follicles in Lo compared with Hi ovaries.
Analysing the follicles as individual classes, as described previously (Rodgers and Irving-Rodgers 2010), we observed a certain level of atresia at all stages of folliculogenesis, including the mid-antral follicle stage, in both Lo and Hi ovaries. Moreover, at the mid-antral follicle stage, the percentage of healthy (and atretic) follicles was similar between the Lo and Hi ovaries. Thus, we would have expected the follicular fluid of mid-antral follicles from both categories to have similar concentrations of E2, P4 and AMH, because these hormones are known to be related to follicular health status (Irving-Rodgers et al. 2003; Rico et al. 2011). However, we found higher P4 and lower E2 concentrations (and a lower E2 : P4 ratio) in Lo compared with Hi ovaries. These observations are consistent with the main features of ‘basal atresia’ described by Irving-Rodgers et al. (2001), which has been defined as a form of atresia in which cell death commences within the basal regions of the membrana granulosa and differs from ‘antral atresia’, in which cell death is initiated within the antral compartment. In particular, basal atretic follicles have been described as those <5 mm in diameter that have substantially elevated P4 and decreased androstenedione, testosterone and E2 concentrations compared with healthy follicles and follicles affected by ‘antral atresia’ (Irving-Rodgers et al. 2003). It is important to mention that the early morphological features of basal atresia can be recognised unequivocally only at the ultrastructural level and that is difficult to distinguish these follicles using standard histological evaluation; in addition, oocytes collected from these follicles are of a significantly poorer quality than either healthy follicles or follicles affected by ‘antral atresia’ (Irving-Rodgers et al. 2010).
Analysis of the follicular fluid revealed lower AMH concentrations in mid-antral follicles from Lo ovaries, further supporting the hypothesis that these follicles were affected by basal atresia. We assume that the reduced AMH concentrations may be the consequence of decreased protein production by the mural cells lying immediately on the basal membrane of the follicles. In fact, it has been demonstrated that, in antral follicles, the outer layer of granulosa cells close to the theca is one of the regions of high AMH expression where expression declines sharply in follicles undergoing atresia (Rico et al. 2009, 2011).
There were no significant differences in AMH content in the follicular fluid of 3- to 5-mm diameter antral follicles from dairy cows with either a high or low number of this follicles size class in the ovaries (Rico et al. 2011). However, in that study, the cows were classified as ‘high’ or ‘low’ on the basis of their responses to superovulation and, using this classification, resulted in differences in the number of 3- to 5-mm follicle class. This approach and the absence of further information regarding the characteristics of the ovaries and the healthy and/or atretic status of 3- to 5-mm diameter antral follicles do not enable the results of that study to be compared with those of the present study.
Decreased AMH expression has been observed in granulosa cells of early antral follicles from women affected by POF (Meduri et al. 2007), in addition to low plasma AMH concentrations (Dumesic and Abbott 2008; La Marca et al. 2009), as a result of a reduction in the number of growing follicles (Knauff et al. 2009). Thus, we can speculate that, in our model, AMH concentrations in the follicular fluid are predictive of oocyte quality and, in turn, may provide further evidence of precocious ovarian aging. This condition may be involved in the premature decline in fertility in dairy cows of reproductive age.
The present study has shown, for the first time, that within the population of culled dairy cows, those cows with ovaries in which there is a reduced number of mid-antral follicles may be affected by POF. Moreover, the results of the present study contribute to the identification of the mechanisms and factors involved in reduced fertility. This is particularly relevant because, in the dairy industry, reproductive disorders are a major cause of economic losses. Finally, considering that the number of antral follicles in cattle is very highly repeatable and stable within individuals (Burns et al. 2005), dairy cows with a Lo phenotype ovary may represent a suitable model and be a readily accessible source of ovaries and oocytes for future studies into the mechanisms controlling premature ovarian aging and oocyte developmental competence in single-ovulating species.
Acknowledgements
The authors thank Professors John J. Peluso and Carla Tatone for their critical review of the manuscript. This work was supported by Marie Curie Actions FP7-Reintegration-Grants within the 7th European Community Framework Program (Contract: 303640, ‘Pro-Ovum’). IT was funded by ‘Dote Ricercatori’ and ‘Dote Ricerca Applicata’ (FSE, Regione Lombardia, Italy).
References
Broekmans, F. J., Soules, M. R., and Fauser, B. C. (2009). Ovarian aging: mechanisms and clinical consequences. Endocr. Rev. 30, 465–493.| Ovarian aging: mechanisms and clinical consequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFaisbnP&md5=529fa24a063969d8a1af826f7a0024d3CAS | 19589949PubMed |
Burns, D. S., Jimenez-Krassel, F., Ireland, J. L., Knight, P. G., and Ireland, J. J. (2005). Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol. Reprod. 73, 54–62.
| Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXls1Srtrk%3D&md5=7acd1cb6f72a58e79b37e64ea03e6a31CAS | 15744026PubMed |
Cordts, E. B., Christofolini, D. M., Dos Santos, A. A., Bianco, B., and Barbosa, C. P. (2011). Genetic aspects of premature ovarian failure: a literature review. Arch. Gynecol. Obstet. 283, 635–643.
| Genetic aspects of premature ovarian failure: a literature review.Crossref | GoogleScholarGoogle Scholar | 21188402PubMed |
Cushman, R. A., DeSouza, J. C., Hedgpeth, V. S., and Britt, J. H. (1999). Superovulatory response of one ovary is related to the micro- and macroscopic population of follicles in the contralateral ovary of the cow. Biol. Reprod. 60, 349–354.
| Superovulatory response of one ovary is related to the micro- and macroscopic population of follicles in the contralateral ovary of the cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXotlyhuw%3D%3D&md5=80a83199d18ac7870d6269048eeca580CAS | 9916001PubMed |
Cushman, R. A., Allan, M. F., Kuehn, L. A., Snelling, W. M., Cupp, A. S., and Freetly, H. C. (2009). Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: investigation of influence of stage of the estrous cycle, age, and birth weight. J. Anim. Sci. 87, 1971–1980.
| Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: investigation of influence of stage of the estrous cycle, age, and birth weight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXms1eju7w%3D&md5=343ca94d61659176453a0a163134b50dCAS | 19286826PubMed |
De Vos, M., Devroey, P., and Fauser, B. C. (2010). Primary ovarian insufficiency. Lancet 376, 911–921.
| Primary ovarian insufficiency.Crossref | GoogleScholarGoogle Scholar | 20708256PubMed |
Dumesic, D. A., and Abbott, D. H. (2008). Implications of polycystic ovary syndrome on oocyte development. Semin. Reprod. Med. 26, 53–61.
| Implications of polycystic ovary syndrome on oocyte development.Crossref | GoogleScholarGoogle Scholar | 18181083PubMed |
Gandolfi, F., Luciano, A. M., Modina, S., Ponzini, A., Pocar, P., Armstrong, D. T., and Lauria, A. (1997). The in vitro developmental competence of bovine oocytes can be related to the morphology of the ovary. Theriogenology 48, 1153–1160.
| The in vitro developmental competence of bovine oocytes can be related to the morphology of the ovary.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28zgtV2mtw%3D%3D&md5=dfcc7725197b700578f878de2dfb0cc9CAS | 16728204PubMed |
Garnsworthy, P. C., Fouladi-Nashta, A. A., Mann, G. E., Sinclair, K. D., and Webb, R. (2009). Effect of dietary-induced changes in plasma insulin concentrations during the early post partum period on pregnancy rate in dairy cows. Reproduction 137, 759–768.
| Effect of dietary-induced changes in plasma insulin concentrations during the early post partum period on pregnancy rate in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosl2nur4%3D&md5=0dfdf335d83289ee748c708a12634abdCAS | 19129370PubMed |
Goswami, D., and Conway, G. S. (2005). Premature ovarian failure. Hum. Reprod. Update 11, 391–410.
| Premature ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmvVaksb0%3D&md5=d78ffc9a1ed015330e38b4916ec50826CAS | 15919682PubMed |
Gröhn, Y. T., and Rajala-Schultz, P. J. (2000). Epidemiology of reproductive performance in dairy cows. Anim. Reprod. Sci. 60–61, 605–614.
| Epidemiology of reproductive performance in dairy cows.Crossref | GoogleScholarGoogle Scholar | 10844228PubMed |
Haidar, M. A., Baracat, E. C., Simoes, M. J., Focchi, G. R., Evencio Neto, J., and de Lima, G. R. (1994). Premature ovarian failure: morphological and ultrastructural aspects. Sao Paulo Med. J. 112, 534–538.
| 1:STN:280:DyaK2MzjsVKmtg%3D%3D&md5=c41ef0ec629e72d3741618463e0d53b5CAS | 7610321PubMed |
Hansen, K. R., Knowlton, N. S., Thyer, A. C., Charleston, J. S., Soules, M. R., and Klein, N. A. (2008). A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 23, 699–708.
| A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause.Crossref | GoogleScholarGoogle Scholar | 18192670PubMed |
Hansen, K. R., Hodnett, G. M., Knowlton, N., and Craig, L. B. (2011). Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil. Steril. 95, 170–175.
| Correlation of ovarian reserve tests with histologically determined primordial follicle number.Crossref | GoogleScholarGoogle Scholar | 20522327PubMed |
Ireland, J. J., Ward, F., Jimenez-Krassel, F., Ireland, J. L., Smith, G. W., Lonergan, P., and Evans, A. C. (2007). Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum. Reprod. 22, 1687–1695.
| Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1ejtLo%3D&md5=fd72acc2474712d3e0d7578ea8ea02bcCAS | 17468258PubMed |
Ireland, J. L., Scheetz, D., Jimenez-Krassel, F., Themmen, A. P., Ward, F., Lonergan, P., Smith, G. W., Perez, G. I., Evans, A. C., and Ireland, J. J. (2008). Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol. Reprod. 79, 1219–1225.
| Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVCltL3M&md5=60d206909bf0010a7640c68c04a83ab1CAS | 18768912PubMed |
Ireland, J. J., Zielak-Steciwko, A. E., Jimenez-Krassel, F., Folger, J., Bettegowda, A., Scheetz, D., Walsh, S., Mossa, F., Knight, P. G., Smith, G. W., Lonergan, P., and Evans, A. C. (2009). Variation in the ovarian reserve is linked to alterations in intrafollicular estradiol production and ovarian biomarkers of follicular differentiation and oocyte quality in cattle. Biol. Reprod. 80, 954–964.
| Variation in the ovarian reserve is linked to alterations in intrafollicular estradiol production and ovarian biomarkers of follicular differentiation and oocyte quality in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsVajsr4%3D&md5=90eec3699aa7003c27c07b999f994ba3CAS | 19164170PubMed |
Ireland, J. J., Smith, G. W., Scheetz, D., Jimenez-Krassel, F., Folger, J. K., Ireland, J. L., Mossa, F., Lonergan, P., and Evans, A. C. (2011). Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Mullerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod. Fertil. Dev. 23, 1–14.
| Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Mullerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3ktFajtw%3D%3D&md5=e2aea670a34b3ade46497e0e036c4a08CAS | 21366975PubMed |
Irving-Rodgers, H. F., van Wezel, I. L., Mussard, M. L., Kinder, J. E., and Rodgers, R. J. (2001). Atresia revisited: two basic patterns of atresia of bovine antral follicles. Reproduction 122, 761–775.
| Atresia revisited: two basic patterns of atresia of bovine antral follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXos1Ohu70%3D&md5=a8da830c7640e71af48520d590e2c5d3CAS | 11690537PubMed |
Irving-Rodgers, H. F., Krupa, M., and Rodgers, R. J. (2003). Cholesterol side-chain cleavage cytochrome P450 and 3beta-hydroxysteroid dehydrogenase expression and the concentrations of steroid hormones in the follicular fluids of different phenotypes of healthy and atretic bovine ovarian follicles. Biol. Reprod. 69, 2022–2028.
| Cholesterol side-chain cleavage cytochrome P450 and 3beta-hydroxysteroid dehydrogenase expression and the concentrations of steroid hormones in the follicular fluids of different phenotypes of healthy and atretic bovine ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsVCnsb0%3D&md5=e97569d4291928def259b7127c9aa276CAS | 12930727PubMed |
Irving-Rodgers, H. F., Hummitzsch, K., Murdiyarso, L. S., Bonner, W. M., Sado, Y., Ninomiya, Y., Couchman, J. R., Sorokin, L. M., and Rodgers, R. J. (2010). Dynamics of extracellular matrix in ovarian follicles and corpora lutea of mice. Cell Tissue Res. 339, 613–624.
| Dynamics of extracellular matrix in ovarian follicles and corpora lutea of mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXis1yjsrc%3D&md5=2564333755cc43c54cefe7eb753d5fb6CAS | 20033213PubMed |
Jagarlamudi, K., Reddy, P., Adhikari, D., and Liu, K. (2010). Genetically modified mouse models for premature ovarian failure (POF). Mol. Cell. Endocrinol. 315, 1–10.
| Genetically modified mouse models for premature ovarian failure (POF).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFyms7nO&md5=10c7719e63c0190af8e8ff74ae91a987CAS | 19643165PubMed |
Knauff, E. A., Eijkemans, M. J., Lambalk, C. B., ten Kate-Booij, M. J., Hoek, A., Beerendonk, C. C., Laven, J. S., Goverde, A. J., Broekmans, F. J., Themmen, A. P., de Jong, F. H., and Fauser, B. C. (2009). Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J. Clin. Endocrinol. Metab. 94, 786–792.
| Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt1Wmsbw%3D&md5=89d10546c5c377ca4d855d9b91ba6607CAS | 19066296PubMed |
Kokcu, A. (2010). Premature ovarian failure from current perspective. Gynecol. Endocrinol. 26, 555–562.
| Premature ovarian failure from current perspective.Crossref | GoogleScholarGoogle Scholar | 20500113PubMed |
Kwee, J., Elting, M. E., Schats, R., McDonnell, J., and Lambalk, C. B. (2007). Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reprod. Biol. Endocrinol. 5, 9.
| Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization.Crossref | GoogleScholarGoogle Scholar | 17362511PubMed |
La Marca, A., Marzotti, S., Brozzetti, A., Stabile, G., Artenisio, A. C., Bini, V., Giordano, R., De Bellis, A., Volpe, A., and Falorni, A. (2009). Primary ovarian insufficiency due to steroidogenic cell autoimmunity is associated with a preserved pool of functioning follicles. J. Clin. Endocrinol. Metab. 94, 3816–3823.
| Primary ovarian insufficiency due to steroidogenic cell autoimmunity is associated with a preserved pool of functioning follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht12ru7jL&md5=eaae6eadb71b44cc124c5776852b7936CAS | 19622621PubMed |
La Marca, A., Argento, C., Sighinolfi, G., Grisendi, V., Carbone, M., D’Ippolito, G., Artenisio, A. C., Stabile, G., and Volpe, A. (2012). Possibilities and limits of ovarian reserve testing in ART. Curr. Pharm. Biotechnol. 13, 398–408.
| Possibilities and limits of ovarian reserve testing in ART.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XktFWis74%3D&md5=7c9de8f58a899959be32d1be91b62265CAS | 21657996PubMed |
Lambalk, C. B., van Disseldorp, J., de Koning, C. H., and Broekmans, F. J. (2009). Testing ovarian reserve to predict age at menopause. Maturitas 63, 280–291.
| Testing ovarian reserve to predict age at menopause.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsF2itrk%3D&md5=8f8a2b96b51b67ec12f863fa3c52765fCAS | 19631481PubMed |
Lass, A., and Brinsden, P. (1999). The role of ovarian volume in reproductive medicine. Hum. Reprod. Update 5, 256–266.
| The role of ovarian volume in reproductive medicine.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzmsVyhsw%3D%3D&md5=948e4bc40b8c42cbf4daa520a3c14686CAS | 10438110PubMed |
Lucy, M. C. (2001). Reproductive loss in high-producing dairy cattle: where will it end? J. Dairy Sci. 84, 1277–1293.
| Reproductive loss in high-producing dairy cattle: where will it end?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXktlKhu7Y%3D&md5=6ab35dfb1b22fc84882f1ea85e7e215aCAS | 11417685PubMed |
Lucy, M. C. (2007). Fertility in high-producing dairy cows: reasons for decline and corrective strategies for sustainable improvement. Soc. Reprod. Fertil. Suppl. 64, 237–254.
| 1:STN:280:DC%2BD2s3otlKitg%3D%3D&md5=d55b8373f07552bc9d6f5803d4717182CAS | 17491151PubMed |
Lussier, J. G., Matton, P., and Dufour, J. J. (1987). Growth rates of follicles in the ovary of the cow. J. Reprod. Fertil. 81, 301–307.
| Growth rates of follicles in the ovary of the cow.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c7hvVGisg%3D%3D&md5=3bca8e7125334c3995d239dbb2f4fa0eCAS | 3430454PubMed |
Macmillan, K. L., Segwagwe, B. V., and Pino, C. S. (2003). Associations between the manipulation of patterns of follicular development and fertility in cattle. Anim. Reprod. Sci. 78, 327–344.
| Associations between the manipulation of patterns of follicular development and fertility in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXksF2gtrc%3D&md5=92aa4dafb775102adc71905ab06b9590CAS | 12818652PubMed |
Malhi, P. S., Adams, G. P., and Singh, J. (2005). Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics. Biol. Reprod. 73, 45–53.
| Bovine model for the study of reproductive aging in women: follicular, luteal, and endocrine characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXls1Srtrg%3D&md5=df381b9986fb47ef9f1941eacf6aac6cCAS | 15744017PubMed |
Malhi, P. S., Adams, G. P., Pierson, R. A., and Singh, J. (2006). Bovine model of reproductive aging: response to ovarian synchronization and superstimulation. Theriogenology 66, 1257–1266.
| Bovine model of reproductive aging: response to ovarian synchronization and superstimulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xotleqsbk%3D&md5=5fd8ada089961aa70b44660ce8d95f0dCAS | 16704875PubMed |
Malhi, P. S., Adams, G. P., Mapletoft, R. J., and Singh, J. (2007). Oocyte developmental competence in a bovine model of reproductive aging. Reproduction 134, 233–239.
| Oocyte developmental competence in a bovine model of reproductive aging.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVKlsrfI&md5=7319e375e31a341a7637f00fdb9ddcaaCAS | 17660233PubMed |
Massin, N., Gougeon, A., Meduri, G., Thibaud, E., Laborde, K., Matuchansky, C., Constancis, E., Vacher-Lavenu, M. C., Paniel, B., Zorn, J. R., Misrahi, M., Kuttenn, F., and Touraine, P. (2004). Significance of ovarian histology in the management of patients presenting a premature ovarian failure. Hum. Reprod. 19, 2555–2560.
| Significance of ovarian histology in the management of patients presenting a premature ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2crmt12mtw%3D%3D&md5=b5cbbc06d0075d614f169063da4de249CAS | 15319385PubMed |
Massin, N., Meduri, G., Bachelot, A., Misrahi, M., Kuttenn, F., and Touraine, P. (2008). Evaluation of different markers of the ovarian reserve in patients presenting with premature ovarian failure. Mol. Cell. Endocrinol. 282, 95–100.
| Evaluation of different markers of the ovarian reserve in patients presenting with premature ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitVegtLg%3D&md5=16e5f463c5445afb8aa89efb13a3c0daCAS | 18191888PubMed |
Meduri, G., Massin, N., Guibourdenche, J., Bachelot, A., Fiori, O., Kuttenn, F., Misrahi, M., and Touraine, P. (2007). Serum anti-Mullerian hormone expression in women with premature ovarian failure. Hum. Reprod. 22, 117–123.
| Serum anti-Mullerian hormone expression in women with premature ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlChtb3N&md5=8f181e22b3be4bd5c741fb1e97469684CAS | 16954410PubMed |
Modina, S., Borromeo, V., Luciano, A. M., Lodde, V., Franciosi, F., and Secchi, C. (2007). Relationship between growth hormone concentrations in bovine oocytes and follicular fluid and oocyte developmental competence. Eur. J. Histochem. 51, 173–180.
| 1:STN:280:DC%2BD2srpvFOqtw%3D%3D&md5=7c165dddadc23f53b79ef8b8f81a7536CAS | 17921112PubMed |
Monget, P., Bobe, J., Gougeon, A., Fabre, S., Monniaux, D., and Dalbies-Tran, R. (2012). The ovarian reserve in mammals: a functional and evolutionary perspective. Mol. Cell. Endocrinol. 356, 2–12.
| The ovarian reserve in mammals: a functional and evolutionary perspective.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xls1emtrs%3D&md5=b666b28710748d0972314234cfe3f25aCAS | 21840373PubMed |
Monniaux, D., Clemente, N., Touze, J. L., Belville, C., Rico, C., Bontoux, M., Picard, J. Y., and Fabre, S. (2008). Intrafollicular steroids and anti-Mullerian hormone during normal and cystic ovarian follicular development in the cow. Biol. Reprod. 79, 387–396.
| Intrafollicular steroids and anti-Mullerian hormone during normal and cystic ovarian follicular development in the cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFWmsb0%3D&md5=6ccf1089da6cad24574ffe2efe78cefbCAS | 18448844PubMed |
Mossa, F., Walsh, S. W., Butler, S. T., Berry, D. P., Carter, F., Lonergan, P., Smith, G. W., Ireland, J. J., and Evans, A. C. (2012). Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J. Dairy Sci. 95, 2355–2361.
| Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvFygtLo%3D&md5=edceb69daa6d95207df12537d14c8b57CAS | 22541464PubMed |
Nelson, L. M. (2009). Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 360, 606–614.
| Clinical practice. Primary ovarian insufficiency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFyhsr4%3D&md5=1edf94810977f929c9f7c43c7c171646CAS | 19196677PubMed |
Opsomer, G., Coryn, M., Deluyker, H., and de Kruif, A. (1998). An analysis of ovarian dysfunction in high yielding dairy cows after calving based on progesterone profiles. Reprod. Domest. Anim. 33, 193–204.
| An analysis of ovarian dysfunction in high yielding dairy cows after calving based on progesterone profiles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXksVaiurw%3D&md5=c4691cd55c645478f63a26f77457c4fbCAS |
Persani, L., Rossetti, R., and Cacciatore, C. (2010). Genes involved in human premature ovarian failure. J. Mol. Endocrinol. 45, 257–279.
| Genes involved in human premature ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1arsbnN&md5=6219fb762a8e0d5f0a5cc55efcf32da9CAS | 20668067PubMed |
Plati, E., Kouskouni, E., Malamitsi-Puchner, A., Boutsikou, M., Kaparos, G., and Baka, S. (2010). Visfatin and leptin levels in women with polycystic ovaries undergoing ovarian stimulation. Fertil. Steril. 94, 1451–1456.
| Visfatin and leptin levels in women with polycystic ovaries undergoing ovarian stimulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVyrtLbN&md5=42bf493c86a7c88e8b7af874ccfb00cfCAS | 19523615PubMed |
Rico, C., Fabre, S., Medigue, C., di Clemente, N., Clement, F., Bontoux, M., Touze, J. L., Dupont, M., Briant, E., Remy, B., Beckers, J. F., and Monniaux, D. (2009). Anti-Mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 80, 50–59.
| Anti-Mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosFKq&md5=761c413ec7ae9ede1426d7afd46f6f95CAS | 18784351PubMed |
Rico, C., Medigue, C., Fabre, S., Jarrier, P., Bontoux, M., Clement, F., and Monniaux, D. (2011). Regulation of anti-Mullerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol. Reprod. 84, 560–571.
| Regulation of anti-Mullerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXis1Omt7c%3D&md5=bf6fd8da2e40961e45f1b34f75ede491CAS | 21076084PubMed |
Rodgers, R. J., and Irving-Rodgers, H. F. (2010). Morphological classification of bovine ovarian follicles. Reproduction 139, 309–318.
| Morphological classification of bovine ovarian follicles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitFKit7s%3D&md5=c78d00f2c3179d5fe4b988b9513416aaCAS | 19786400PubMed |
Rosen, M. P., Johnstone, E., Addauan-Andersen, C., and Cedars, M. I. (2011). A lower antral follicle count is associated with infertility. Fertil. Steril. 95, 1950–1954.
| A lower antral follicle count is associated with infertility.Crossref | GoogleScholarGoogle Scholar | 21376313PubMed |
Sakaguchi, M. (2011). Practical aspects of the fertility of dairy cattle. J. Reprod. Dev. 57, 17–33.
| Practical aspects of the fertility of dairy cattle.Crossref | GoogleScholarGoogle Scholar | 21422734PubMed |
Shelling, A. N. (2010). Premature ovarian failure. Reproduction 140, 633–641.
| Premature ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFCnsbfN&md5=c83a941bd219baf3da09ad091a472b1aCAS | 20716613PubMed |
Skillern, A., and Rajkovic, A. (2008). Recent developments in identifying genetic determinants of premature ovarian failure. Sex Dev. 2, 228–243.
| Recent developments in identifying genetic determinants of premature ovarian failure.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cjitV2htQ%3D%3D&md5=625ef70e4fd4208c57877abb07a9324eCAS | 18987497PubMed |
Stricker, R., Eberhart, R., Chevailler, M. C., Quinn, F. A., Bischof, P., and Stricker, R. (2006). Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin. Chem. Lab. Med. 44, 883–887.
| Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1OhtLo%3D&md5=9e50c60d4607c1c19404fa0ad5ad17cfCAS | 16776638PubMed |
Tatone, C., Amicarelli, F., Carbone, M. C., Monteleone, P., Caserta, D., Marci, R., Artini, P. G., Piomboni, P., and Focarelli, R. (2008). Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 14, 131–142.
| Cellular and molecular aspects of ovarian follicle ageing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisVKmurs%3D&md5=f82e68cdf23bd9c9c49604dcd1a78704CAS | 18239135PubMed |
Tessaro, I., Luciano, A. M., Franciosi, F., Lodde, V., Corbani, D., and Modina, S. C. (2011). The endothelial nitric oxide synthase/nitric oxide system is involved in the defective quality of bovine oocytes from low mid-antral follicle count ovaries. J. Anim. Sci. 89, 2389–2396.
| The endothelial nitric oxide synthase/nitric oxide system is involved in the defective quality of bovine oocytes from low mid-antral follicle count ovaries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXps12jsrw%3D&md5=d5b2eb893bc9946194834b7537be067fCAS | 21421835PubMed |
Tilly, J. L. (2003). Ovarian follicle counts: not as simple as 1, 2, 3. Reprod. Biol. Endocrinol. 1, 11.
| Ovarian follicle counts: not as simple as 1, 2, 3.Crossref | GoogleScholarGoogle Scholar | 12646064PubMed |
van Dooren, M. F., Bertoli-Avellab, A. M., and Oldenburg, R. A. (2009). Premature ovarian failure and gene polymorphisms. Curr. Opin. Obstet. Gynecol. 21, 313–317.
| Premature ovarian failure and gene polymorphisms.Crossref | GoogleScholarGoogle Scholar | 19610175PubMed |
Vassena, R., Mapletoft, R. J., Allodi, S., Singh, J., and Adams, G. P. (2003). Morphology and developmental competence of bovine oocytes relative to follicular status. Theriogenology 60, 923–932.
| Morphology and developmental competence of bovine oocytes relative to follicular status.Crossref | GoogleScholarGoogle Scholar | 12935869PubMed |
Walsh, S. W., Williams, E. J., and Evans, A. C. (2011). A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 123, 127–138.
| A review of the causes of poor fertility in high milk producing dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M3gt1eguw%3D%3D&md5=51937d53a3ee6cf098d104b729e5aaf2CAS | 21255947PubMed |
Yang, M. Y., and Rajamahendran, R. (2000). Morphological and biochemical identification of apoptosis in small, medium, and large bovine follicles and the effects of follicle-stimulating hormone and insulin-like growth factor-I on spontaneous apoptosis in cultured bovine granulosa cells. Biol. Reprod. 62, 1209–1217.
| Morphological and biochemical identification of apoptosis in small, medium, and large bovine follicles and the effects of follicle-stimulating hormone and insulin-like growth factor-I on spontaneous apoptosis in cultured bovine granulosa cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXisl2ht7o%3D&md5=fbf035732ff7cb968d7b3d2633b16d46CAS | 10775168PubMed |
Yimer, N., Rosnina, Y., Wahid, H., Saharee, A. A., Yap, K. C., and Ganesamurthi, P. (2010). Ovarian activity in beef and dairy cows with prolonged postpartum period and heifers that fail to conceive. Trop. Anim. Health Prod. 42, 607–615.
| Ovarian activity in beef and dairy cows with prolonged postpartum period and heifers that fail to conceive.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3c7msVWnsg%3D%3D&md5=9a2dfe087301dd82222ca49f7fc3fc1aCAS | 19809886PubMed |



