Evaluation of the uterine environment early in pregnancy establishment to characterise cows with a potentially superior ability to support conceptus survival
A. M. Ledgard A C , S. Meier B and A. J. Peterson AA AgResearch, Ruakura Research Centre, East Street, Hamilton 3240, New Zealand.
B DairyNZ Limited, Cnr Ruakura and Morrinsville Roads, Hamilton 3240, New Zealand.
C Corresponding author. Email: anita.ledgard@agresearch.co.nz
Reproduction, Fertility and Development 23(6) 737-747 https://doi.org/10.1071/RD10183
Submitted: 22 July 2010 Accepted: 29 January 2011 Published: 21 June 2011
Journal Compilation © CSIRO Publishing 2011 Open Access CC BY-NC-ND
Abstract
During previous investigations, the capacity of the cow to secrete prostaglandin in response to oxytocin has been linked with pregnancy outcome. The objective of the present study was to evaluate the predictive value of prostaglandin release to identify groups of cows as potentially superior (SR, low prostaglandin release) or inferior (IR, high prostaglandin release) for pregnancy outcome and to utilise these cows to investigate factors that contribute to optimum uterine conditions for early pregnancy. Animals were synchronised and received an in vitro-derived blastocyst on Day 7 post-oestrus. Tissues (trophoblast and endometrial) and uterine luminal fluid (ULF) were recovered 10 days later. Pregnancy rates were 94 and 78% for SR and IR cows, respectively. Of the pregnant SR cows, 69% had larged conceptuses (>24 cm) in contrast to 43% IR of cows. IR cows with small conceptuses (<12 cm) had significantly lower mean Day 3 and 5 post-oestrous progesterone concentrations than cows with large conceptuses. The expression of factors involved in the prostaglandin pathway, pregnancy and conceptus development were analysed via quantitative RT-PCR and Western blot analysis. Investigation of 16 endometrial gene transcripts indicated no differences between IR and SR cows except for osteopontin expression which, in uteri with large conceptuses, was 2-fold greater in SR than IR cows (P = 0.02). There was greater expression of CTGF, OXTR, PGES, PGHS2 and UTMP mRNA in uteri of SR and IR cows that had large compared with small conceptuses (P < 0.05). More IFNT protein was recovered in SR compared with IR ULF (P < 0.03). SR cows with large conceptuses had less TIMP2 and legumain protein in their gravid, compared with their non-gravid horns (P ≤ 0.02) whereas IR cows did not. The predictive value of prostaglandin release in response to oxytocin challenge does not appear to be an effective indicator of subsequent pregnancy rates in cows. Differences between the two groups appear to be associated with subtle differences in progesterone and uterine protein concentrations that may be related to differences in conceptus size.
Additional keywords: CTGF, endometrium, legumain, osteopontin, prostaglandin pathway, TIMP2, trophoblast.
Introduction
Pregnancy establishment in cattle is vulnerable during the period of maternal recognition of pregnancy prior to the establishment of the cotyledonary placenta (Sreenan and Diskin 1986; Thatcher et al. 2001), with 24–28% of pregnancy failures occurring between 8 and 16 days after insemination (Diskin et al. 2006). Previously, two groups of recipient cows were identified using serial transfer of in vitro-produced (IVP) embryos, as having either a high or low pregnancy rate at Day 35 of gestation (McMillan and Donnison 1999). Minimal differences in their ovarian follicular parameters or progesterone profiles were detected (McMillan et al. 1999), which suggested that differences in the uterine environment between these two groups might be influencing early embryo survival. This survival is dependent on the endometrium receiving the pregnancy signal interferon tau (IFNT), expressed by the elongating conceptus. Interferon tau prolongs the life of the corpus luteum to maintain the essential concentrations of circulating progesterone required for the establishment of pregnancy (Roberts et al. 1999). IFNT is thought to act directly (Mann et al. 1999; Robinson et al. 2008) by suppression of the oestrogen-induced expression of the oxytocin receptor (OXTR), or via the inhibition of transcription of the oestrogen receptor α (Spencer et al. 2007) resulting in fewer newly formed oxytocin receptors during the late luteal phase of the oestrous cycle. The oxytocin-stimulated secretion of luteolytic pulses of prostaglandin F2α (PGF2α) is thus inhibited (Wathes and Lamming 1995). When the extreme individuals of the recipient herd were examined for their capacity to secrete prostaglandin in response to an intravenous challenge of oxytocin, it was discovered that the group with the lowest pregnancy rate had a greater mean peak plasma concentration of the PGF2α metabolite 13,14-dihydro-15-ketoprostaglandin F2α (PGFM) than that with the highest pregnancy rate (Peterson and Lee 2003). This indicated an intrinsic difference between the two groups in endometrial signalling.
The uterine endometrium provides the nutrients necessary for blastocyst development through protein secretions termed the histotroph (Bazer 1975). Variation in proteins contained in the histotroph, which can lead to detrimental effects on conceptus growth (Gray et al. 2002), may be specific to individual animals. Progesterone is one of the main regulators of the genes expressed in the endometrium that are involved in the preparation of the uterus during early pregnancy (Bazer et al. 1979; Robinson et al. 2008; Spencer et al. 2008). This progesterone influence in early pregnancy affects conceptus growth and elongation with an association between lower progesterone concentrations during the early luteal phase and conceptuses with retarded development reported (Mann et al. 2006; McNeill et al. 2006). Conversely, elevation of progesterone concentrations from Day 3.5 post-oestrus resulted in an increase in the size of Day 16 conceptuses (Carter et al. 2008). Many comprehensive studies utilising microarray technologies to characterise the changes in the bovine transcriptome that are associated with the oestrous cycle and early pregnancy have been reported (Bauersachs et al. 2005, 2008; Klein et al. 2006; McNeill et al. 2006), providing an insight into the regulators of the events during early conceptus growth.
The objective of the present study was to evaluate the predictive value of prostaglandin response in identifying cows with a superior reproductive performance and to examine the uterine environment of these cows for differences between the two groups during the peri-attachment period of bovine pregnancy. Therefore, a new group of potentially superior and inferior animals were selected using peak level of prostaglandin response to an oxytocin challenge as the criterion for selection. After IVP embryo transfer and conceptus recovery, several candidate genes involved in PGF2α production and the endometrial response to pregnancy and pregnancy-associated proteins present in the uterine luminal fluid (ULF) were quantified.
Materials and methods
Animals
Experiments were undertaken in accordance with the regulations of the New Zealand Animal Welfare Act of 1999 and had approval from the Ruakura Animal Ethics Committee. One hundred and fifty Holstein–Friesian 13–15-month-old heifers were characterised on their prostaglandin response (peak plasma PGFM concentration) to an oxytocin challenge (Parkinson et al. 1990). These animals were kept under normal dairy grazing herd management. After the oxytocin challenge they were submitted for artificial insemination (AI), with those failing to get pregnant culled. Seven weeks post-calving a subset of 40 animals, matched for calving week and body condition score (4), were selected for the trial based on their prostaglandin response to the oxytocin challenge. Twenty animals selected as potentially reproductively superior (SR) had the lowest peak plasma PGFM concentrations and 20 as potentially inferior (IR) had the highest peak plasma PGFM concentrations.
Oxytocin challenge
The oestrous cycles of each heifer were synchronised using two 500-µg injections of sodium cloprostenol (EstroPlan; Parnell Laboratories NZ Ltd, Auckland, New Zealand), administered intramuscularly 10–12 days apart. Oestrus was monitored behaviourally and recorded twice daily for 4 days after each prostaglandin injection. The day of oestrus, following the second prostaglandin injection was designated Day 0 of the oxytocin challenge cycle. On Day 16 of this oestrous cycle, 100 IU of synthetic oxytocin (Butocin; Bomac Laboratories Ltd, Auckland, New Zealand) was administered intravenously via a jugular vein immediately after a blood sample was taken (Time 0) from the coccygeal vein. Further blood samples from the tail were taken at 10, 20, 30, 60 and 90 min into evacuated blood tubes (Vaccutainer; Becton & Dickinson, Auckland, New Zealand) containing sodium heparin, placed immediately in iced water and centrifuged within 3 h (15 min at 1500g at 4°C) after collection. Plasma samples were aspirated and stored at –20°C until analysis. Plasma concentrations of 13,14-dihydro-15-ketoprostaglandin F2α (PGFM) were measured using a radioimmunoassay (Mitchell et al. 1976). The inter- and intra-assay coefficients of variation were 20.4 and 7.2% (high), 24.1 and 10.3% (medium) and 28.1 and 16.4% (low), respectively. The lower detectable limit of the assay was 6.5 pg mL–1. Total PGFM release (measured as the area under the curve; AUC) and peak PGFM concentrations were calculated.
Uterine luminal fluid (ULF) and tissue collection
The subset of 40 animals selected for the trial as having potentially superior (20 with the lowest peak PGFM concentrations, SR) or inferior (20 with the highest peak PGFM concentrations, IR) uterine environments were confirmed, by palpation, as having normal ovarian and uterine morphology. They were then synchronised for oestrus, using a program of a 10-µg intramuscular injection of a GnRH analogue, buserelin (Receptal; Intervet Limited, Auckland, New Zealand) followed (10 days after monitored oestrus behaviour) with two 500-µg injections of EstroPlan administered intramuscularly 10–12 days apart. The day of oestrus following the second prostaglandin injection was designated Day 0 of the synchronised oestrous cycle. On Day 7 of this cycle, a grade one IVP blastocyst was transferred non-surgically into the uterine lumen ipsilateral (ipsi) to the corpus luteum of each cow. Generation of IVP embryos by in vitro fertilisation was as previously described (Thompson et al. 2000). Three SR cows were removed from the trial due to having either: peritonitis, a difficult transfer or a cystic follicle and 2 IR cows were excluded due to ill health. The remaining 35 animals were slaughtered at Day 17 of gestation and, within 1h, each horn of the uterus was flushed with 20 mL of saline and the uterine luminal fluid (ULF) from each horn was collected, kept separately, and recorded as being ipsi or contralateral (contra) to the ovary containing the corpus luteum and either gravid (conceptus present) or non-gravid. The ULFs were centrifuged at 1500g for 10 min at 4°C to remove cellular debris before being lyophilised and then reconstituted with 4 mL of distilled water containing a cocktail of protease inhibitors (Complete; Roche, Mannheim, Germany) before overnight dialysis at 4°C. The protein concentration was measured (Bradford 1976) and samples stored at –20°C until analysed. Conceptus tissue samples were collected immediately after flushing the uterine tracts and trophoblast length was measured to the nearest mm. The embryonic disc was excised before the tissues were individually snap-frozen in liquid nitrogen and stored at –80°C until RNA extraction. Intercaruncular endometrial tissue from the caudal section of the uterine horns was snap-frozen within 15 min of uterine flushing and stored at –80°C until RNA extraction.
Progesterone measurement
Blood was sampled into evacuated blood tubes every 48 h post-oestrus (from Day 1 until the day of slaughter), placed immediately in iced water, and aspirated plasma stored as described previously. Plasma progesterone concentration was measured using the commercially available Coat-A-Count Progesterone radioimmunoassay kit (Diagnostic Products Corporation, Los Angeles, CA, USA) according to the manufacturer’s instructions. The inter- and intra-assay coefficients of variation were 8.7 and 7.7% (high), 5.2 and 6.9% (medium) and 13.1 and 14.2% (low), respectively. The minimum detectable concentration of the assay was 0.07 ng mL–1.
Total RNA and reverse transcription
Total RNA was isolated from intercaruncular endometrial tissue and the whole trophoblast tissue from conceptuses ≥24 cm in length using the standard TRIzol protocol (Invitrogen, Carlsbad, CA, USA). On-column DNase digestion of 50–100 µg of each RNA sample was performed with RNeasy mini spin columns (Qiagen, Valencia, CA, USA). Briefly, 450 µL of buffered sample was mixed with 250 µL ethanol then applied to a column and centrifuged for 15 s at 8000g at room temperature. The column was washed with 500 µL of the kit’s DNase buffer before 80 µL of 0.34 U µL–1 RNase-free DNase1 (Qiagen) was added and samples incubated for 15 min at room temperature. The sample columns were washed twice in 70% buffered ethanol and RNA eluted in 50 µL diethylpyrocarbonate (DEPC)-treated water. The concentration of mRNA was determined by spectrophotometry and integrity was assessed by gel electrophoresis before storage at –80°C.
For reverse transcription (RT), 5 µg of each sample in 15 µL water, 2.5 µL of 10 mM dNTPs and 5 µL of 5 µM anchored oligo dT: 5′-GAGCTCGAGTCTAGATTTTTTTTTTTTTTT (Sigma-Aldrich, Castle Hill, NSW, Australia) were incubated at 65°C for 5 min. Ten microlitres of 5× first-strand buffer (Invitrogen), 2.5 µL of 40 U µL–1 RNase OUT inhibitor (Invitrogen), 2.5 µL of 0.1 M dithiothreitol and 2.5 µL of 200 U µL–1 Superscript III (Invitrogen) were added and the samples incubated at 50°C for 60 min, then 70°C for 15 min. A RT negative control of non-reverse transcribed samples was included. Prior to real-time RT–PCR, samples were diluted 1 : 10 in DEPC-treated water for use as templates.
Real-time RT–PCR
SYBR green-based real-time RT–PCR was used for quantification of candidate endometrial genes involved in the prostaglandin pathway (Arosh et al. 2004), associated with preimplantation ruminant pregnancy (Bauersachs et al. 2006), associated with embryonic growth (Wathes et al. 1998; Frolova et al. 2009) and expressed by the early trophoblast (Ushizawa et al. 2004) using a Corbett Rotor-Gene 600 instrument with 10-µL reactions containing 5 µL TaKaRa Sybr Premix Ex Taq mix (Takara Bio Inc., Otsu, Japan), 0.1 μM of each primer and 2 µL template. The thermal program included a 1-min incubation at 95°C to activate the TaKaRa Ex Taq HS polymerase followed by 40 cycles of 95°C for 10 s, annealing temperature of 60°C for 15 s and extension at 60°C for 20 s with ramp speed 20°C s–1. A no-template control, a RT negative control and a standard dilution series were included in each real-time run. PCR primers were designed using Vector NTI (Invitrogen) or obtained from the literature (Table 1). Where possible, primers were designed to flank putative introns. The products were analysed by gel electrophoresis on first usage of primer pair to ensure that the correct gene fragment was amplified and the PCR product was sequenced commercially (DNA Sequence Facility, Waikato University, Hamilton, NZ) to verify correct identity. PCR results were calculated with predicted values using an equation that relates reaction efficiency, Ct value (cycle number corresponding to 20% of the apex of second derivative of fluorescence intensity curve) and copy number (Wilkening and Bader 2004). Values are expressed as the relative copy number normalised against the geometric mean of three housekeeping genes (GAPDH, ubiquitin and HPRT) for each sample. All values are presented as mean + s.e.m.

|
Western blot analysis
IFNT, legumain, tissue inhibitor of matrix metalloproteinase 2 (TIMP2) and ubiquitin cross-reactive protein (UCRP/ISG15) were quantified in ULF using western blotting procedures as previously described (Ledgard et al. 2009a). Briefly, samples containing 20 μg of total ULF protein in SDS-gel loading buffer (0.5 M TRIS-HCl, 4% SDS, 50% glycerol and 2% β-mercaptoethanol) were separated on 15% SDS-polyacrylamide gels and then electroblotted onto reinforced nitrocellulose membrane (BioTrace NT, Pall, Port Washington, NY, USA). Proteins were detected using: anti-IFNT (1 : 2000; gift from Dr R. Roberts, University of Missouri, Columbia, MO, USA) and anti-TIMP2 (1 : 2000; Sigma-Aldrich, St Louis, MO, USA) with goat anti-rabbit horse-radish peroxidise (HRP) as the secondary (1 : 20 000; Sigma-Aldrich); anti-legumain (1 : 2000; R&D Systems, Minneapolis, MN, USA) and anti-ISG15 (1 : 200; gift from Dr T. Hansen, University of Wyoming, Laramie, WY, USA) with rabbit anti-goat HRP as the secondary antibody (1 : 20 000; Sigma-Aldrich). Immunoreactivity was detected by chemiluminescence (Luminol; Sigma-Aldrich, Auckland, New Zealand) or enhanced chemiluminescence (SuperSignal West Pico Luminol System; Pierce Chemical Co., Rockford, IL, USA) according to the manufacturer’s recommendations. Bands were quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA) and expressed as optical density (O.D.) units.
Statistical analysis
The total area under the curve (AUC) was calculated for PGFM concentrations in each heifer with data for each group (SR, IR) analysed by analysis of variance (ANOVA). Progesterone data were analysed by ANOVA using GenStat regression, with effect of group (SR, IR), conceptus size and their interaction tested. Gene and protein expression was analysed log-transformed using a mixed model in GenStat 13, specifying Animal as a random effect and Grouping (SR v. IR), Horn (gravid v. non-gravid), Conceptus Size (small v. large) and NP (NP v. gravid) and their interactions as fixed effects. Bar charts present means and standard errors of means (s.e.m.). Correlation between progesterone concentration on Days 3 and 5 with gene expression in gravid horns (log-transformed) was calculated.
Results
PGFM concentrations of selected animals
Mean (AUC ± s.e.m.) PGFM release for all 150 heifers was 6216 ± 558 pg mL–1 per 90 min, ranging from 585 to 24 320 pg mL–1 (data not presented). Animals selected for this trial as extreme IR had peak plasma PGFM concentrations greater than 280 pg mL–1, and as extreme SR had peak plasma PGFM concentrations lower than 140 pg mL–1. The mean PGFM profile of SR cows differed compared with IR cows (Fig. 1).
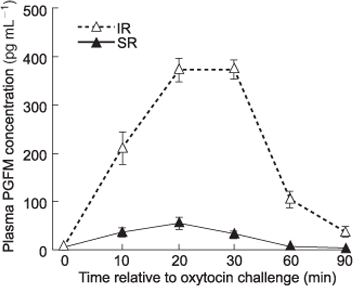
|
Pregnancy rates
Of the 35 cows receiving an IVP blastocyst, thirty were pregnant and five non-pregnant (NP) at Day 17 of gestation. Sixteen of the 17 SR cows were pregnant (94%) and 81% (13/16) of the conceptuses recovered from SR cows were >12 cm in length (medium to large). Fourteen of the 18 IR cows were pregnant (78%) with 50% (7/14) of IR conceptuses classified as medium to large. For analysis, the cows were grouped according to the size of conceptus recovered to match for stage of conceptus development: that is either small (<12 cm) or large (>24 cm), with three cows excluded from the analysis because they had a conceptus of a medium size, two (17 and 18 cm) SR and one (16 cm) IR cow (Fig. 2). One IR cow with a small conceptus was also excluded due to incomplete sample collection.
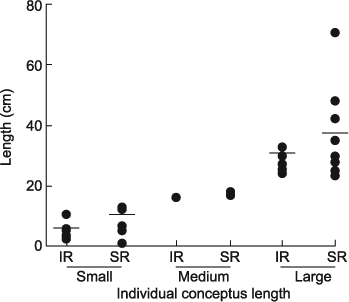
|
Comparison of mean progesterone profiles
The mean progesterone profile of all SR cows compared with all IR cows was not different (Fig. 3a). However, mean progesterone profiles of both SR and IR cows with large conceptuses (n = 9 and 6, respectively) differed compared with SR and IR cows with small (n = 5 and 6, respectively) conceptuses (Fig. 3b). SR and IR cows with large conceptuses had a 3-fold greater mean Day 3 progesterone concentration compared with those that had small conceptuses (0.47 ± 0.08 and 0.56 ± 0.13 ng mL–1 compared with 0.16 ± 0.06 and 0.16 ±0.10 ng mL–1; respectively, P < 0.002) and a 1.7-fold greater mean Day 5 progesterone concentration compared with IR cows that had small conceptuses (1.87 ± 0.23 and 1.83 ±0.06 ng mL–1, respectively compared with 1.09 ± 0.21 ng mL–1, P < 0.006). In addition, the IR cows with small conceptuses still had a 1.6-fold lower mean progesterone concentration on Day 7 compared with those from which a large conceptus was recovered (2.39 ± 0.37 ng mL–1 compared with 3.44 ± 0.45 ng mL–1 and 3.77 ± 0.33 ng mL–1, SR and IR, respectively), but this difference did not reach significance (P = 0.11).
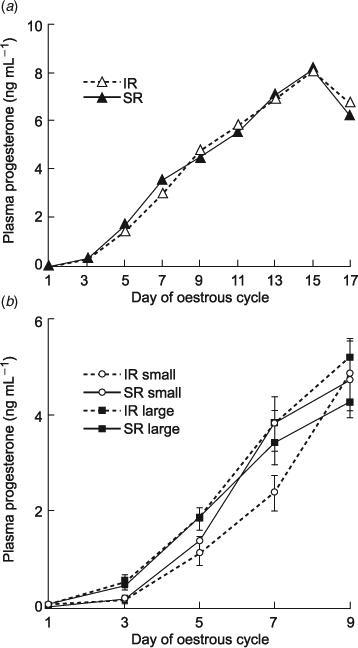
|
Gene expression levels in endometrial tissues
There was no significant difference in endometrial mean mRNA expression between pregnant SR and IR cows for OXTR or genes of the prostaglandin (PG) pathway: PGE synthase (PGES), PGF synthase (PGFS, also known as AKR1B5), PGH synthase (PGHS2, also known as cyclooxygenase 2), 15-hydroxyprostaglandin dehydrogenase (PGDH) and phospholipase A2 group VI (PLA2G6) (Fig. 4). Expression of OXTR was 3.7-fold (P < 0.001), PGES 2-fold (P < 0.04) and PGHS2 2-fold (P < 0.05) greater in uteri of pregnancies with large compared with small conceptuses. Expression of PLA2G6 mRNA in gravid horns was 1.5-fold lower compared with the non-gravid horns (P = 0.004), especially in SR and IR pregnancies with small conceptuses (5- and 2.5-fold, respectively). Expression of PGFS mRNA was 1.5-fold lower (P < 0.001) and of PGDH mRNA 2-fold lower (P = 0.003) in gravid compared with non-gravid horns. There were no other significant differences.
There was no difference in mean mRNA expression in the endometrium of pregnant SR and IR cows of pregnancy-associated genes: galectin 9 (LGALS9), glycosylation-dependent cell adhesion molecule 1 (GLYCAM1) and tissue inhibitor of matrix metalloproteinase 2 (TIMP2) or growth-associated genes: glucose transporter 1 (GLUT1), insulin-like growth factor 2 (IGF2), insulin-like growth factor binding protein 2 (IGFBP2) and IGFBP3 when comparing SR with IR or large with small conceptuses (data not presented). There was no difference in mean connective tissue growth factor (CTGF) mRNA or uterine serpins/uterine milk protein (UTMP) expression between SR and IR cows but the expression level in SR and IR cows with large conceptuses was 2-fold greater (P < 0.05) than those with small conceptuses, especially when comparing gravid horns (Fig. 4). Osteopontin (SPP1) mean mRNA expression levels were 1.8-fold greater (P < 0.05) in horns of SR compared with IR cows in pregnancies with large conceptuses (Fig. 4). This difference was not evident in horns of cows with a small conceptus. There were no other significant differences in pregnancy-associated genes.
Mean expression in NP endometrium of OXTR mRNA was 2.5-fold (P = 0.04) and PGFS mRNA 1.9-fold greater (P = 0.004), whereas UTMP mRNA was 3-fold (P < 0.001) and SPP1 mRNA 2-fold lower (P < 0.05) compared with gravid horns of SR and IR cows (Fig. 4). The only other significant difference in NP endometrial mRNA expression compared with gravid horns was in expression of GLUT1 (2-fold lower, P = 0.04) and LGALS9 (2-fold lower, P < 0.05).
The only correlations between progesterone concentration and gene expression (log-transformed) of gravid horns were between Day 3 progesterone concentration and UTMP (corr. = 0.53, P = 0.007), CTGF (corr. = 0.47, P = 0.020) and PGES (corr. = 0.43, P = 0.048) and between Day 5 progesterone concentration and PGES (corr. = 0.51, P = 0.014).
Protein expression levels in ULF
Total mean IFNT protein recovered in uteri of all pregnant SR cows was 1.4-fold greater compared with IR cows (P < 0.03). However, in all pregnancies with large conceptuses, the amount in gravid compared with non-gravid was not different (Fig. 5). In pregnancies with small conceptuses: IFNT protein in gravid horns of both SR and IR cows was 2.5-fold lower compared with gravid horns of pregnancies with large conceptuses (P < 0.03); and in non-gravid horns was 10-fold (SR) and 226-fold (IR) lower compared with horns of SR cows with large conceptuses (P < 0.001). There was no difference in mean ISG15 recovered in ULF of SR compared with IR cows or large compared with small conceptuses but ISG15 protein was 3- to 5-fold lower in non-gravid compared with gravid horns (P < 0.001; Fig. 5). There was no difference in mean TIMP2 recovered from SR compared with IR horns when pregnancies had small conceptuses (Fig. 5). In pregnancies with large conceptuses, there was 3.5-fold greater TIMP2 in the non-gravid compared with the gravid horns of SR cows (P < 0.02), but the horns of IR cows were not different. Legumain was present in ULF, predominantly as the active 49/56-kDa doublet with small amounts of the 35-kDa mature form (Fig. 5b). Total mean 49/56-kDa legumain recovered in horns of SR and IR pregnancies with small conceptuses and IR cows with large conceptuses was not different (Fig. 5). The gravid horns from SR cows with large pregnancies had 85-fold lower mean 49/56-kDa legumain compared with all other horns (P < 0.001). Total 35-kDa legumain in any of the ULFs was not different except for SR horns from large pregnancies where virtually none was detected (P = 0.009).
Gene expression levels in trophoblast tissue
Gene expression was examined in trophoblast tissue of large conceptuses derived from SR (n = 8) and IR (n = 6) pregnancies using real-time RT–PCR. The mean of total RNA isolated per whole IR conceptus trophoblast tissue was less than SR (135 and 268 μg, respectively; P < 0.05). The means of total IFNT, placental lactogen (CSH1), dickkopf 1 (DKK1), prolactin-related protein 1 (PRP1) and trophoblast kunitz domain protein 1 (TKDP1) mRNA transcripts isolated per conceptus were 1.5- to 3-fold lower in IR derived trophoblast tissue compared with SR (Fig. 6), but this did not reach significance (P = 0.1). Pregnancy-associated glycoprotein 9 (PAG9) expression was detected in only one trophoblast sample (the largest conceptus, from a SR pregnancy).
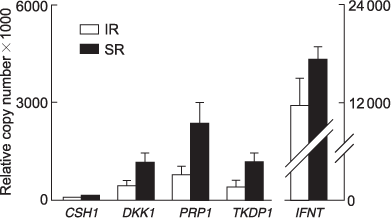
|
Discussion
Two groups of cattle were selected using the amplitude of their prostaglandin response to an oxytocin challenge as a potential indicator of the ability to achieve a successful pregnancy. Their uterine environments were then compared at Day 17 of gestation to determine variations in maternal and conceptus factors that may account for such a capacity. Although the IR cows (with greater peak PGFM release in response to oxytocin) in this trial were associated with a poorer pregnancy rate and had a greater number of pregnancies with small conceptuses compared with SR cows, the single oxytocin challenge does not appear to be an effective indicator of subsequent pregnancy success.
When mean progesterone profiles of the IR and SR cows were examined, lower concentrations on Days 3 and 5 of the cycle were evident in cows from which small conceptuses were recovered compared with those that had large conceptuses. This relationship has been documented in other reports of higher progesterone concentrations on Day 5 of the pregnant cycle being related to more advanced embryo development (Green et al. 2005; Carter et al. 2008). Although early post-ovulatory differences in progesterone concentration did not definitively distinguish IR and SR groups, it is interesting that the IR cows from which small conceptuses were recovered had a lower mean progesterone concentration on Day 7 as well as Days 3 and 5 compared with pregnancies from which large conceptuses were recovered. While small variations in early luteal phase progesterone concentrations of individual cows may not wholly account for their reproductive performance, it has been reported that expression of uterine proteins such as retinol-binding protein are susceptible to subtle changes in post-ovulatory progesterone concentrations (McNeill et al. 2006). These modulations of the primary environment to which the conceptus is exposed may be of such a degree that pregnancy outcome could be influenced. Recent microarray studies have examined the role of elevated progesterone concentrations at this critical stage of the cycle and have reported differences in subsequent endometrial gene expression (Forde et al. 2009).
Contributions to the uterine environment from the conceptus were apparent with the abundance of IFNT protein greater in the SR and IR uteri with larger conceptuses, consistent with the size of the trophoblast tissue being correlated with the amount of IFNT protein secreted (Robinson et al. 2006). A gradation of IFNT was present with less in non-gravid horns of IR pregnancies with large conceptuses compared with gravid horns and less again in gravid horns of pregnancies with small conceptuses followed by very little in their non-gravid horns. This has several implications, as IFNT stimulates a plethora of pregnancy-related endometrial genes (see review, Spencer et al. 2008) including ISG15 (Austin et al. 1996), whose protein levels were greater in the gravid compared with non-gravid horns in the present study. Increased stimulation of the uterus would, in theory, provide a more favourable environment for the growth of the conceptus and, as a consequence, an increase in the numbers of binucleate cells available to secrete pregnancy-enhancing proteins such as CSH1 (Wooding 1992) and PAG9 (Green et al. 2000). In the present study, trophoblast tissue of the large conceptuses appeared to be at a very early developmental stage, with binucleate cells just beginning to differentiate, as there were more transcripts of DKK1 and PRP1 than CSH1 present in the trophoblast tissue (Yamada et al. 2002; Ledgard et al. 2009a). The large conceptuses from SR cows tended to have greater mean total numbers of transcripts for all the trophoblast-related genes examined, indicating that it was possible that SR conceptuses had progressed slightly more in development than those from IR cows. Overall, an interaction between progesterone-enhanced stimulation of the endometrium to encourage growth of the conceptus and the subsequent timely production of trophoblast proteins to induce gene expression in the endometrium could produce a more favourable uterine environment. Slower development of the blastocyst, in a less-prepared uterus would have a compounding effect, which could account for the greater number of smaller conceptuses found in IR pregnancies.
There was no difference in the level of OXTR mRNA expression between IR and SR pregnant cows, which is consistent with the results of Parkinson et al. (1990), who reported no obvious relationship between endometrial OXTR concentration and oxytocin-induced PGFM response. The level of OXTR mRNA in the endometrium of non-pregnant cows was greater compared with all pregnant cows (except for one SR cow with a large conceptus that had comparatively high amounts in its gravid horn), indicating that regulation of the receptor had successfully occurred for pregnancy recognition. However, expression of OXTR mRNA was lower in the uteri of pregnancies with small compared with large conceptuses. Similarly, PGES and PGHS2 expression was lower in the endometrium of pregnancies with smaller conceptuses. PGHS2 is a rate-limiting enzyme in the prostaglandin pathway that catalyses the transformation of arachidonic acid into the common prostaglandin precursor PGH2 which can then be converted to PGE2 by PGES or to PGF2α by PGFS (Madore et al. 2003). PGHS2 expression in sheep and cattle is transient early in the oestrous cycle and requires progesterone exposure for at least 10 days (Charpigny et al. 1997; Arosh et al. 2002). However, no correlation was evident between PGHS2 mRNA levels in the endometrium of gravid horns and post-ovulatory progesterone concentrations. Some researchers report that recombinant IFNT increases PGHS2 expression in ruminant endometrial cells in vivo and in vitro (Asselin et al. 1997; Arosh et al. 2004; Emond et al. 2004; Gray et al. 2006), whereas others have reported a decrease (Chen et al. 2006) or no effect (Kim et al. 2003). Overall, the expression of PGHS2 in uteri with large compared with small conceptuses was greater, implying an effect of IFNT on its regulation; however, as the maximum expression was not in SR gravid horns with large conceptuses (those with the greatest exposure to IFNT), the relationship is inconsistent. In contrast, the expression of PGES in gravid horns closely mirrors the IFNT levels present, agreeing with reports that it is upregulated in response to IFNT in the bovine endometrium (Arosh et al. 2004). Expression of PGES was also positively correlated with Days 3 and 5 progesterone concentrations and its increased expression may have contributed to conceptus growth as PGES has been proposed to enhance embryonic growth by action on the immune function at the fetal–maternal interface (Emond et al. 1998). Conversely, IFNT appeared to negatively influence the expression of PGFS, PLA2G6 and PGDH mRNA as there were fewer transcripts in the gravid compared with the non-gravid (with less exposure to IFNT) horns. PGFS expression is reportedly downregulated in response to IFNT in bovine endometrium (Arosh et al. 2004) as is PLA2G6 activity, a phospholipase that catalyses the rate-limiting step in prostaglandin biosynthesis by hydrolysing arachidonic acid from membrane lipids (Tithof et al. 2007). PGDH converts PGE2 and PGF2α to their inactive metabolites (PGEM and PGFM, respectively) and although Arosh et al. (2004) described PGDH protein expression as unaffected by IFNT, they subsequently reported that mRNA and protein expression were not directly related during the oestrous cycle (Parent et al. 2006). Overall the pattern of expression of the genes in the prostaglandin pathway described in the present study point to an enhancement in the production of PGES which, with its embryotropic function, would contribute to greater conceptus growth.
Expression of the candidate growth-associated genes tested (GLUT1, IGF2, IGFBP2 and IGFBP3) did not differ for group or conceptus size recovered. However, differences in expression of three pregnancy-associated genes were measured. Two had greater mRNA expression in pregnancies with large compared with small conceptuses. The first, UTMP, is the most abundant protein present in the pregnant ruminant uterus and is reported to have an immunosuppressive action (Segerson and Bazer 1989) allowing immunological tolerance of the conceptus by the maternal uterus. Concentrations of UTMP protein in cattle ULF are increased after long exposure to progesterone (Leslie and Hansen 1991) and in the present study there was a positive correlation between Day 3 post-ovulatory progesterone concentration and UTMP mRNA expression. However, in the uteri with larger conceptuses and greater UTMP expression there was also a greater IFNT exposure, although a recent study reported UTMP expression was not stimulated by IFNT in endometrial cell cultures, only by progesterone and oestrogen (Ulbrich et al. 2009). Another conceptus-related signal may be responsible for the difference in expression of UTMP in the uteri of large pregnancies. One such candidate is CSH1, as it has been reported to stimulate the number of glands present in ovine endometrium and, hence, the levels of UTMP and SPP1 mRNA present (Spencer et al. 1999).
Expression of the second pregnancy-associated gene, CTGF, present in Day 18 pregnant bovine endometrium (Klein et al. 2006), was also positively correlated with Day 3 progesterone concentrations, agreeing with evidence of CTGF mRNA regulation by progesterone (Carter et al. 2008). A member of the CNN family, CTGF has many functions, including interaction with growth factors to stimulate cell proliferation (De Winter et al. 2008), which suggests that it has played a role in the growth of the larger conceptuses in the present study.
Finally, SPP1 was the one gene expressed differentially between SR and IR cows, but only in pregnancies where a large conceptus was present. It is a glandular secretory protein that functions during implantation in the extracellular matrix as a mediator of cell-to-cell adhesion and cell migration during trophoblast attachment (Johnson et al. 2003). There is much evidence to suggest that SPP1 mRNA expression is regulated by progesterone and does not appear to be influenced by IFNT in the ovine endometrium (see review by Johnson et al. 2003). Progesterone control was not indicated in the present study and a recent report suggested that SPP1 was regulated independently of pregnancy, IFNT and progesterone in an ovine cell culture system (Ahn et al. 2009). Levels of SPP1 mRNA in NP uteri were lower compared with in the endometrium from SR and IR pregnancies with large conceptuses, but this could be attributed to other conceptus factors, such as CSH1, as mentioned previously.
It has been suggested that legumain and TIMP2 are involved in local regulation of trophoblast invasiveness and endometrial remodelling essential for pregnancy establishment (Salamonsen and Nie 2002; Ledgard et al. 2009b). The SR cows with large conceptuses had reduced concentrations of TIMP2 and legumain protein in their gravid compared with non-gravid horns. In the case of TIMP2, it was not due to transcriptional differences despite indications from sheep that IFNT regulates TIMP2 expression (Gray et al. 2006; Chen et al. 2007). In addition, legumain protein concentrations have been reported to be reduced in the presence of larger conceptus with no differences in the mRNA expression (Ledgard et al. 2009b). Therefore, any assessment of uterine environment needs to be examined at the protein, as well as gene expression, level to observe relevant post-translational interaction between the endometrium and the conceptus.
Conclusions
A comparison at Day 17 of gestation of the uterine environment of two groups of cows selected for a high or low prostaglandin response to oxytocin challenge revealed greater levels of SPP1 mRNA and IFNT protein in SR compared with IR cows. The expression in the endometrium of several prostaglandin pathway and pregnancy-associated genes differed when cows had small compared with large conceptuses. The main divergence between groups was the preponderance of larger conceptuses in SR cows, which were associated with subtle differences in progesterone and uterine protein concentrations.
Acknowledgements
We thank L. McGowan for the production of embryos, M. Berg and P. Gore for animal treatments and husbandry, Dr H. Henderson for statistical assistance and Dr M. Green for critical reading of the manuscript. This work was supported by the New Zealand Foundation for Research, Science and Technology (C10X031) and Dairy NZ Inc. (AN709).
References
Ahn, H. W., Farmer, J. L., Bazer, F. W., and Spencer, T. E. (2009). Progesterone and interferon tau-regulated genes in the ovine uterine endometrium: identification of periostin as a potential mediator of conceptus elongation. Reproduction 138, 813–825.| Progesterone and interferon tau-regulated genes in the ovine uterine endometrium: identification of periostin as a potential mediator of conceptus elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsValt7rE&md5=d73e01403376482076ca07347c2a6e65CAS | 19635739PubMed |
Arosh, J. A., Parent, J., Chapdelaine, P., Sirois, J., and Fortier, M. A. (2002). Expression of cyclooxygenases 1 and 2 and prostaglandin E synthase in bovine endometrial tissue during the oestrous cycle. Biol. Reprod. 67, 161–169.
| Expression of cyclooxygenases 1 and 2 and prostaglandin E synthase in bovine endometrial tissue during the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XkvV2itL0%3D&md5=304989dfcb1dc14f57bc88c4b9af5ed2CAS | 12080013PubMed |
Arosh, J. A., Banu, S. K., Kimmins, S., Chapdelaine, P., Maclaren, L. A., and Fortier, M. A. (2004). Effect of interferon-tau on prostaglandin biosynthesis, transport, and signalling at the time of maternal recognition of pregnancy in cattle: evidence of polycrine actions of prostaglandin E2. Endocrinology 145, 5280–5293.
| Effect of interferon-tau on prostaglandin biosynthesis, transport, and signalling at the time of maternal recognition of pregnancy in cattle: evidence of polycrine actions of prostaglandin E2.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptVygsrg%3D&md5=c1719ebbc04d0ab84ca8fffb1414bc06CAS | 15308607PubMed |
Asselin, E., Lacroix, D., and Fortier, M. A. (1997). IFN-tau increases PGE2 production and COX-2 gene expression in the bovine endometrium in vitro. Mol. Cell. Endocrinol. 132, 117–126.
| IFN-tau increases PGE2 production and COX-2 gene expression in the bovine endometrium in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlslCjt78%3D&md5=3dc37c8c57a5e47a8b121544d159dc23CAS | 9324053PubMed |
Austin, K. J., Ward, S. K., Teixeira, M. G., Dean, V. C., Moore, D. W., and Hansen, T. R. (1996). Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy. Biol. Reprod. 54, 600–606.
| Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XhtFCjt7Y%3D&md5=0850d78ee3e7e2c395228fd4bdeb57bbCAS | 8835381PubMed |
Bauersachs, S., Ulbrich, S. E., Gross, K., Schmidt, S. E., Meyer, H. H., Einspanier, R., Wenigerkind, H., Vermehren, M., Blum, H., Sinowatz, F., and Wolf, E. (2005). Gene expression profiling of bovine endometrium during the oestrous cycle: detection of molecular pathways involved in functional changes. J. Mol. Endocrinol. 34, 889–908.
| Gene expression profiling of bovine endometrium during the oestrous cycle: detection of molecular pathways involved in functional changes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlslCisL8%3D&md5=d081dd2c742e60fd0ed43902b47546a2CAS | 15956356PubMed |
Bauersachs, S., Ulbrich, S. E., Gross, K., Schmidt, S. E., Meyer, H. H., Wenigerkind, H., Vermehren, M., Sinowatz, F., Blum, H., and Wolf, E. (2006). Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity. Reproduction 132, 319–331.
| Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xpt1Wjs7s%3D&md5=33d6a11e661942e32cef6aa7017feac8CAS | 16885540PubMed |
Bauersachs, S., Mitko, K., Ulbrich, S. E., Blum, H., and Wolf, E. (2008). Transcriptome studies of bovine endometrium reveal molecular profiles characteristic for specific stages of oestrous cycle and early pregnancy. Exp. Clin. Endocrinol. Diabetes 116, 371–384.
| Transcriptome studies of bovine endometrium reveal molecular profiles characteristic for specific stages of oestrous cycle and early pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1SgtbzK&md5=ea3a81b386bdb255eed8215c1278de99CAS | 18561091PubMed |
Bazer, F. W. (1975). Uterine protein secretions: relationship to development of the conceptus. J. Anim. Sci. 41, 1376–1382.
| 1:CAS:528:DyaE28Xis1Gk&md5=40510813b8fdea383f752e7fe5df23eeCAS | 1104552PubMed |
Bazer, F. W., Roberts, R. M., and Thatcher, W. W. (1979). Actions of hormones on the uterus and effect on conceptus development. J. Anim. Sci. 49, 35–45.
| 400776PubMed |
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
| A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XksVehtrY%3D&md5=14300a26927bd8f0c2e23c515fdbb33cCAS | 942051PubMed |
Carter, F., Forde, N., Duffy, P., Wade, M., Fair, T., Crowe, M. A., Evans, A. C. O., Kenny, D. A., Roche, J. F., and Lonergan, P. (2008). Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod. Fertil. Dev. 20, 368–375.
| Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtVKksLg%3D&md5=fb0e332b80e7323eedc60036d56ef2c8CAS | 18402756PubMed |
Charpigny, G., Reinaud, P., Tamby, J. P., Créminon, C., Martal, J., Maclouf, J., and Guillomot, M. (1997). Expression of cyclooxygenase-1 and -2 in ovine endometrium during the oestrous cycle and early pregnancy. Endocrinology 138, 2163–2171.
| Expression of cyclooxygenase-1 and -2 in ovine endometrium during the oestrous cycle and early pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXislCls74%3D&md5=8a12b069b4d96f6f074f059f2fa85a64CAS | 9112416PubMed |
Chen, Y., Green, J. A., Antoniou, E., Ealy, A. D., Mathialagan, N., Walker, A. M., Avalle, M. P., Rosenfeld, C. S., Hearne, L. B., and Roberts, R. M. (2006). Effect of interferon-tau administration on endometrium of non-pregnant ewes: a comparison with pregnant ewes. Endocrinology 147, 2127–2137.
| Effect of interferon-tau administration on endometrium of non-pregnant ewes: a comparison with pregnant ewes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvFOrtb8%3D&md5=28cd9f9fb54dd5f711012b5b617aedddCAS | 16469802PubMed |
Chen, Y., Antoniou, E., Liu, Z., Hearne, L. B., and Roberts, R. M. (2007). A microarray analysis for genes regulated by interferon-tau in ovine luminal epithelial cells. Reproduction 134, 123–135.
| A microarray analysis for genes regulated by interferon-tau in ovine luminal epithelial cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvFGis7s%3D&md5=9df5aed3781645b8031adc852d638a1aCAS | 17641094PubMed |
De Winter, P., Leoni, P., and Abraham, D. (2008). Connective tissue growth factor: structure–function relationships of a mosaic, multifunctional protein. Growth Factors 26, 80–91.
| Connective tissue growth factor: structure–function relationships of a mosaic, multifunctional protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvFCgt78%3D&md5=66829c9693a4b93cf4cda1eeda704ac0CAS | 18428027PubMed |
Diskin, M. G., Murphy, J. J., and Sreenan, J. M. (2006). Embryo survival in dairy cows managed under pastoral conditions. Anim. Reprod. Sci. 96, 297–311.
| Embryo survival in dairy cows managed under pastoral conditions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nitFGgsg%3D%3D&md5=f621fa13f142ae2bc5883fc87ec717b6CAS | 16963203PubMed |
Emond, V., Fortier, M. A., Murphy, B. D., and Lambert, R. D. (1998). Prostaglandin E2 regulates both interleukin-2 and granulocyte–macrophage colony-stimulating factor gene expression in bovine lymphocytes. Biol. Reprod. 58, 143–151.
| Prostaglandin E2 regulates both interleukin-2 and granulocyte–macrophage colony-stimulating factor gene expression in bovine lymphocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhvVSjtA%3D%3D&md5=83a5d4febac4aa08244298c3ec58302dCAS | 9472935PubMed |
Emond, V., MacLaren, L. A., Kimmins, S., Arosh, J. A., Fortier, M. A., and Lambert, R. D. (2004). Expression of cyclooxygenase-2 and granulocyte–macrophage colony-stimulating factor in the endometrial epithelium of the cow is up-regulated during early pregnancy and in response to intrauterine infusions of interferon-tau. Biol. Reprod. 70, 54–64.
| Expression of cyclooxygenase-2 and granulocyte–macrophage colony-stimulating factor in the endometrial epithelium of the cow is up-regulated during early pregnancy and in response to intrauterine infusions of interferon-tau.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhvVyh&md5=cd4bf8ff1bd70459a26c946cac5842f3CAS | 13679318PubMed |
Forde, N., Carter, F., Fair, T., Crowe, M. A., Evans, A. C., Spencer, T. E., Bazer, F. W., McBride, R., Boland, M. P., O’Gaora, P., Lonergan, P., and Roche, J. F. (2009). Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol. Reprod. 81, 784–794.
| Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyhsLbM&md5=d5b87f63f4bfb6dba0d5025dcdce42adCAS | 19553605PubMed |
Frolova, A., Flessner, L., Chi, M., Kim, S. T., Foyouzi-Yousefi, N., and Moley, K. H. (2009). Facilitative glucose transporter type 1 is differentially regulated by progesterone and oestrogen in murine and human endometrial stromal cells. Endocrinology 150, 1512–1520.
| Facilitative glucose transporter type 1 is differentially regulated by progesterone and oestrogen in murine and human endometrial stromal cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisV2guro%3D&md5=0e6cfb3cb27de46b1e96ede9f6a437adCAS | 18948400PubMed |
Gray, C. A., Burghardt, R. C., Johnson, G. A., Bazer, F. W., and Spencer, T. E. (2002). Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124, 289–300.
| Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XmvVCitL8%3D&md5=8459ac1020520773b11d7c88fbd1e795CAS | 12141942PubMed |
Gray, C. A., Abbey, C. A., Beremand, P. D., Choi, Y., Farmer, J. L., Adelson, D. L., Thomas, T. L., Bazer, F. W., and Spencer, T. E. (2006). Identification of genes regulated by early pregnancy, progesterone and interferon tau in the ovine uterus. Biol. Reprod. 74, 383–394.
| Identification of genes regulated by early pregnancy, progesterone and interferon tau in the ovine uterus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1Kktg%3D%3D&md5=3d1ebd82d6acfd1ed3a5efa2a9f0f5e3CAS | 16251498PubMed |
Green, J. A., Xie, S., Quan, X., Bao, B., Gan, X., Mathialagan, N., Beckers, J. F., and Roberts, R. M. (2000). Pregnancy-associated bovine and ovine glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy. Biol. Reprod. 62, 1624–1631.
| Pregnancy-associated bovine and ovine glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsF2hsL8%3D&md5=c99aef871a0040ce6c1726dc8f148fb3CAS | 10819764PubMed |
Green, M. P., Hunter, M. G., and Mann, G. E. (2005). Relationship between maternal hormone secretion and embryo development on Day 5 of pregnancy in dairy cows. Anim. Reprod. Sci. 88, 179–189.
| Relationship between maternal hormone secretion and embryo development on Day 5 of pregnancy in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpvV2mtrc%3D&md5=ccc10a6b1e79068b6ee457fa64e21041CAS | 16143210PubMed |
Johnson, G. A., Burghardt, R. C., Bazer, F. W., and Spencer, T. E. (2003). Osteopontin: roles in implantation and placentation. Biol. Reprod. 69, 1458–1471.
| Osteopontin: roles in implantation and placentation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXosV2kur8%3D&md5=7349651cb8933d8f489827a005f5c261CAS | 12890718PubMed |
Kim, S., Choi, Y., Spencer, T. E., and Bazer, F. W. (2003). Effects of the oestrous cycle, pregnancy and interferon tau on expression of cyclooxygenase two (COX-2) in ovine endometrium. Reprod. Biol. Endocrinol. 1, 58.
| Effects of the oestrous cycle, pregnancy and interferon tau on expression of cyclooxygenase two (COX-2) in ovine endometrium.Crossref | GoogleScholarGoogle Scholar | 12956885PubMed |
Klein, C., Bauersachs, S., Ulbrich, S. E., Einspanier, R., Meyer, H. H., Schmidt, S. E., Reichenbach, H. D., Vermehren, M., Sinowatz, F., Blum, H., and Wolf, E. (2006). Monozygotic twin model reveals novel embryo-induced transcriptome changes of bovine endometrium in the pre-attachment period. Biol. Reprod. 74, 253–264.
| Monozygotic twin model reveals novel embryo-induced transcriptome changes of bovine endometrium in the pre-attachment period.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xot1Kmuw%3D%3D&md5=f1206e3fbbe53a1cb000e5a03b4e81f9CAS | 16207835PubMed |
Ledgard, A. M., Lee, R. S., Couldrey, C., and Peterson, A. J. (2009a). Dickkopf-1 expression during early bovine placentation and its down-regulation in somatic cell nuclear transfer (SCNT) pregnancies. J. Reprod. Dev. 55, 467–474.
| Dickkopf-1 expression during early bovine placentation and its down-regulation in somatic cell nuclear transfer (SCNT) pregnancies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFahsL%2FF&md5=670433dcbb34640823d0b5b2288eb469CAS | 19444005PubMed |
Ledgard, A. M., Lee, R. S., and Peterson, A. J. (2009b). Bovine endometrial legumain and TIMP-2 regulation in response to presence of a conceptus. Mol. Reprod. Dev. 76, 65–74.
| Bovine endometrial legumain and TIMP-2 regulation in response to presence of a conceptus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFemtL3P&md5=8452b757597fee33c0d4cd3d8d8afbb5CAS | 18449874PubMed |
Leslie, M. V., and Hansen, P. J. (1991). Progesterone-regulated secretion of the serpin-like proteins of the ovine and bovine uterus. Steroids 56, 589–597.
| Progesterone-regulated secretion of the serpin-like proteins of the ovine and bovine uterus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XltFyhtQ%3D%3D&md5=9b777a044af26b1028993b87e6047edeCAS | 1819870PubMed |
Madore, E., Harvey, N., Parent, J., Chapdelaine, P., Arosh, J. A., and Fortier, M. A. (2003). An aldose reductase with 20alpha HSD activity is most likely the enzyme responsible for the production of PGF2{alpha} in the bovine endometrium. J. Biol. Chem. 278, 11 205–11 212.
| An aldose reductase with 20alpha HSD activity is most likely the enzyme responsible for the production of PGF2{alpha} in the bovine endometrium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXit1KgsLk%3D&md5=e96fb3a1d623661f9a204239b77a9c66CAS |
Mann, G. E., Lamming, G. E., Robinson, R. S., and Wathes, D. C. (1999). The regulation of interferon-tau production and uterine hormone receptors during early pregnancy. J. Reprod. Fertil. 54, 317–328.
| 1:CAS:528:DyaK1MXnslSitrc%3D&md5=24f8bc95aa618b4e3c3efad1f75a7188CAS |
Mann, G. E., Fray, M. D., and Lamming, G. E. (2006). Effects of time of progesterone supplementation on embryo development and interferon-tau production in the cow. Vet. J. 171, 500–503.
| Effects of time of progesterone supplementation on embryo development and interferon-tau production in the cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjs1Kis70%3D&md5=5ade20756b42e28a3a3b28ae3ba636a8CAS | 16624716PubMed |
McMillan, W. H., and Donnison, M. J. (1999). Understanding maternal contributions to fertility in recipient cattle: development of herds with contrasting pregnancy rates. Anim. Reprod. Sci. 57, 127–140.
| Understanding maternal contributions to fertility in recipient cattle: development of herds with contrasting pregnancy rates.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2Fns1Wmtw%3D%3D&md5=fb63a056618c674ba24a5490a3a7aa64CAS | 10610033PubMed |
McMillan, W. H., Peterson, A. J., Cox, S. F., Pearson, S. J., and Donnison, M. J. (1999). Reproductive performance during early pregnancy in cattle with contrasting early pregnancy rates. BSAS occ. Publ. 26, 283–288.
McNeill, R. E., Sreenan, J. M., Diskin, M. G., Cairns, M. T., Fitzpatrick, R., Smith, T. J., and Morris, D. G. (2006). Effect of systemic progesterone concentration on the expression of progesterone-responsive genes in the bovine endometrium during the early luteal phase. Reprod. Fertil. Dev. 18, 573–583.
| Effect of systemic progesterone concentration on the expression of progesterone-responsive genes in the bovine endometrium during the early luteal phase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltVWnt7o%3D&md5=171b790ba54582454f766cfe8fb72972CAS | 16836964PubMed |
Mitchell, M. D., Flint, A. P. F., and Turnbull, A. C. (1976). Plasma concentrations of 13,14-dihydro-15-keto-prostaglandin F during pregnancy in sheep. Prostaglandins 11, 319–329.
| Plasma concentrations of 13,14-dihydro-15-keto-prostaglandin F during pregnancy in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE28XhsVOhtrk%3D&md5=68ce1fd3e5eb0e8ea0578fbfc87b9aeaCAS | 944462PubMed |
Parent, M., Madore, E., MacLaren, L. A., and Fortier, M. A. (2006). Expression and regulation of 15-hydroxyprostaglandin dehydrogenase in the bovine endometrium during the oestrous cycle. Reproduction 131, 573–582.
| Expression and regulation of 15-hydroxyprostaglandin dehydrogenase in the bovine endometrium during the oestrous cycle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xjs12jsLc%3D&md5=986b3fa9e361edd17e96dc9299b88fd8CAS | 16514200PubMed |
Parkinson, T. J., Jenner, L. J., and Lamming, G. E. (1990). Comparison of oxytocin/prostaglandin F2α interrelationships in cyclic and pregnant cows. J. Reprod. Fertil. 90, 337–345.
| Comparison of oxytocin/prostaglandin F2α interrelationships in cyclic and pregnant cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXls1amtg%3D%3D&md5=55875cc5b5d09cf9dd30cc7a0bc4a7bcCAS | 2172533PubMed |
Peterson, A. J., and Lee, R. S. (2003). Improving successful pregnancies after embryo transfer. Theriogenology 59, 687–697.
| Improving successful pregnancies after embryo transfer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38jjslKrug%3D%3D&md5=10845e331e079890431eed5d988101aeCAS | 12499012PubMed |
Roberts, R. M., Ealy, A. D., Alexenko, A. P., Hans, C. S., and Ezashi, T. (1999). Trophoblast interferons. Placenta 20, 259–264.
| Trophoblast interferons.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtF2rsro%3D&md5=0ea4d9138567778c026ec15c22eeacbbCAS | 10329345PubMed |
Robinson, R. S., Fray, M. D., Wathes, D. C., Lamming, G. E., and Mann, G. E. (2006). In vivo expression of interferon tau mRNA by the embryonic trophoblast and uterine concentrations of interferon-tau protein during early pregnancy in the cow. Mol. Reprod. Dev. 73, 470–474.
| In vivo expression of interferon tau mRNA by the embryonic trophoblast and uterine concentrations of interferon-tau protein during early pregnancy in the cow.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XisFals74%3D&md5=573c73f6ec4e73847ef68f43d54593aeCAS | 16435375PubMed |
Robinson, R. S., Hammond, A. J., Wathes, D. C., Hunter, M. G., and Mann, G. E. (2008). Corpus luteum–endometrium–embryo interactions in the dairy cow: underlying mechanisms and clinical relevance. Reprod. Domest. Anim. 43, 104–112.
| Corpus luteum–endometrium–embryo interactions in the dairy cow: underlying mechanisms and clinical relevance.Crossref | GoogleScholarGoogle Scholar | 18638111PubMed |
Salamonsen, L. A., and Nie, G. (2002). Proteases at the endometrial–trophoblast interface: their role in implantation. Rev. Endocrin. Metab. Disord. 3, 133–143.
| Proteases at the endometrial–trophoblast interface: their role in implantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xltlelur0%3D&md5=4056de8d5dc0c524a5f9209f2e8305f8CAS |
Segerson, E. C., and Bazer, F. W. (1989). High-molecular weight basic and acidic immunosuppressive protein components in uterine secretions of pregnant cows. Biol. Reprod. 41, 1014–1023.
| High-molecular weight basic and acidic immunosuppressive protein components in uterine secretions of pregnant cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXhsFKnsrw%3D&md5=0c60e52c39cada8fd95eadacb64a9e24CAS | 2624862PubMed |
Spencer, T. E., Gray, C. A., Johnson, G. A., Taylor, K. M., Gertler, A., Gootwine, E., Ott, T. L., and Bazer, F. W. (1999). Effects of recombinant ovine interferon-tau, placental lactogen and growth hormone on the ovine uterus. Biol. Reprod. 61, 1409–1418.
| Effects of recombinant ovine interferon-tau, placental lactogen and growth hormone on the ovine uterus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXns1ynsLs%3D&md5=8d449470a827de6a7efbc618f34d6f83CAS | 10569983PubMed |
Spencer, T. E., Johnson, G. A., Bazer, F. W., Burghardt, R. C., and Palmarini, M. (2007). Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod. Fertil. Dev. 19, 65–78.
| Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhtleitr%2FN&md5=32b9e8aa2ae697d444b5000e4137e57eCAS | 17389136PubMed |
Spencer, T. E., Sandre, O., and Eckhard, W. (2008). Genes involved in conceptus–endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction 135, 165–179.
| Genes involved in conceptus–endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXit1yrtL4%3D&md5=221ea9d1418b824202c32d4d377bf676CAS | 18239047PubMed |
Sreenan, J. M., and Diskin, M. G. (1986). The extent and timing of embryonic mortality in the cow. In ‘Embryonic mortality in farm animals’. (Eds J. M. Sreenan and M. G. Diskin.) pp. 1–12. (MTP Press: Lancaster, UK.)
Thatcher, W. W., Guzeloglu, A., Mattos, R., Binelli, M., Hansen, T. R., and Pru, J. K. (2001). Uterine–conceptus interactions and reproductive failure in cattle. Theriogenology 56, 1435–1450.
| Uterine–conceptus interactions and reproductive failure in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjslyqsQ%3D%3D&md5=90f711f302d4abf606c4690fff364a67CAS | 11768809PubMed |
Thompson, J. G., McNaughton, C., Gasparrini, B., McGowan, L. T., and Tervit, H. R. (2000). Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J. Reprod. Fertil. 118, 47–55.
| 1:CAS:528:DC%2BD3cXpsl2guw%3D%3D&md5=581776ff0915c452499da7475f3df72cCAS | 10793625PubMed |
Tithof, P. K., Roberts, M. P., Guan, W., Elgayyar, M., and Godkin, J. D. (2007). Distinct phospholipase A2 enzymes regulate prostaglandin E2 and F2alpha production by bovine endometrial epithelial cells. Reprod. Biol. Endocrinol. 5, 16.
| Distinct phospholipase A2 enzymes regulate prostaglandin E2 and F2alpha production by bovine endometrial epithelial cells.Crossref | GoogleScholarGoogle Scholar | 17459165PubMed |
Ulbrich, S. E., Frohlich, T., Schulke, K., Englberger, E., Waldschmitt, N., Arnold, G. J., Reichenbach, H.-D., Reichenbach, M., Wolf, E., Meyer, H. H. D., and Bauersachs, S. (2009). Evidence for oestrogen-dependent uterine serpin (SERPINA14) expression during oestrus in the bovine endometrial glandular epithelium and lumen. Biol. Reprod. 81, 795–805.
| Evidence for oestrogen-dependent uterine serpin (SERPINA14) expression during oestrus in the bovine endometrial glandular epithelium and lumen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyhsLbN&md5=1b9ebfd92d10b70bccb0032cfc543b5bCAS | 19494250PubMed |
Ushizawa, K., Herath, C. B., Kaneyama, K., Shiojima, S., Hirasawa, A., Takahashi, T., Imai, K., Ochiai, K., Tokunaga, T., Tsunoda, Y., Tsujimoto, G., and Hashizume, K. (2004). cDNA microarray analysis of bovine embryo gene expression profiles during the pre-implantation period. Reprod. Biol. Endocrinol. 2, 77.
| cDNA microarray analysis of bovine embryo gene expression profiles during the pre-implantation period.Crossref | GoogleScholarGoogle Scholar | 15560851PubMed |
Wathes, D. C., and Lamming, G. E. (1995). The oxytocin receptor, luteolysis and the maintenance of pregnancy. J. Reprod. Fertil. Suppl. 49, 53–67.
| 1:CAS:528:DyaK2MXmslWit7k%3D&md5=d307c2e6c89c3bba4a3dccdb2d5ffa81CAS | 7623343PubMed |
Wathes, D. C., Reynolds, T. S., Robinson, R. S., and Stevenson, K. R. (1998). Role of the insulin-like growth factor system in uterine function and placental development in ruminants. J. Dairy Sci. 81, 1778–1789.
| Role of the insulin-like growth factor system in uterine function and placental development in ruminants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkvVyqs78%3D&md5=7a62986c9d3af1d4a1fec4c2a8f53e98CAS | 9684184PubMed |
Wilkening, S., and Bader, A. (2004). Quantitative real-time polymerase chain reaction: methodical analysis and mathematical model. J. Biomol. Tech. 15, 107–111.
| 15190083PubMed |
Wooding, F. B. (1992). The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. 31, 31–39.
Yamada, O., Todoroki, J., Kizaki, K., Takahashi, T., Imai, K., Patel, O. V., Schuler, L. A., and Hashizume, K. (2002). Expression of prolactin-related protein 1 at the fetomaternal interface during the implantation period in cows. Reproduction 124, 427–437.
| Expression of prolactin-related protein 1 at the fetomaternal interface during the implantation period in cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsV2htL4%3D&md5=04edcd76e1554df3f0728277a6d3b493CAS | 12201816PubMed |




