Use of electronic medical records to monitor the safe and effective prescribing of medicinal cannabis: is it feasible?
Christine M. Hallinan A B C * , Jane M. Gunn
A B C * , Jane M. Gunn  B , Yining Qian C and Yvonne A. Bonomo
B , Yining Qian C and Yvonne A. Bonomo  B D
B D
A Department of General Practice, Melbourne Medical School, Faculty of Medicine, Dentistry and Health Sciences of the University of Melbourne, Level 2, 780 Elizabeth Street, Melbourne, Vic. 3004, Australia.
B Faculty of Medicine, Dentistry and Health Sciences of the University of Melbourne, Level 2, Alan Gilbert Building, Grattan Street, Parkville, Vic. 3010, Australia.
C Health and Biomedical Research Information Technology Unit (HaBIC R2), Faculty of Medicine, Dentistry and Health Sciences of the University of Melbourne, Level 2, 780 Elizabeth Street, Melbourne, Vic. 3004, Australia.
D Department of Addiction Medicine, St Vincent’s Hospital, Fitzroy, Vic. 3065, Australia.
Australian Journal of Primary Health 28(6) 564-572 https://doi.org/10.1071/PY22054
Submitted: 8 March 2022 Accepted: 17 June 2022 Published: 5 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of La Trobe University. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Background: General practitioners are well positioned to contribute to the pharmacovigilance of medical cannabis via the general practice electronic medical record (EMR). The aim of this research is to interrogate de-identified patient data from the Patron primary care data repository for reports of medicinal cannabis to ascertain the feasibility of using EMRs to monitor medicinal cannabis prescribing in Australia.
Methods: EMR rule-based digital phenotyping of 1 164 846 active patients from 109 practices was undertaken to investigate reports of medicinal cannabis use from September 2017 to September 2020.
Results: Eighty patients with 170 prescriptions of medicinal cannabis were identified in the Patron repository. Reasons for prescription included anxiety, multiple sclerosis, cancer, nausea, and Crohn’s disease. Nine patients showed symptoms of a possible adverse event, including depression, motor vehicle accident, gastrointestinal symptoms, and anxiety.
Conclusions: The recording of medicinal cannabis effects in the patient EMR provides potential for medicinal cannabis monitoring in the community. This is especially feasible if monitoring were to be embedded into general practitioner workflow.
Keywords: medication systems, electronic medical records, medicinal cannabis, patient safety, pharmacovigilance, physicians’ practice patterns, primary health care, public health: practice, safety management.
Introduction
Pharmacovigilance is defined as the detection, assessment, understanding, and prevention of adverse drug effects (WHO 2015). Pharmacovigilance also includes the surveillance of drug pharmacology, prescribing practices, and product quality including that of the supply chain (Dal Pan 2014). The practice of pharmacovigilance can be applied to both registered and unregistered (novel) therapeutics. Pharmacovigilance is particularly important in the context of novel therapeutics, given how approvals have been expedited and given the number of post-market adverse events associated with their use (Downing et al. 2017; Dhruva et al. 2018).
The Australian Government legislated the use of medicinal cannabis as a novel therapeutic for the management of specific indications, such as chemotherapy-induced nausea and vomiting, refractory paediatric epilepsy, palliative care indications, cancer pain, neuropathic pain, spasticity from neurological conditions, anorexia and wasting associated with chronic illness (such as cancer) on 1 November 2016 (TGA 2022b). Although legalised, most medicinal cannabis products are not listed on the Australian Register of Therapeutic Goods (ARTG) as they are still considered an unapproved therapeutic by the TGA (2022b). To prescribe medicinal cannabis, health practitioners must obtain Therapeutic Goods Administration (TGA) approval via the Authorised Prescriber Online System or the Special Access Scheme (SAS) (TGA 2022b).
There are two types of prescribers who can provide their patients medicinal cannabis in Australia, an Authorised Prescriber, and a Special Access Scheme (SAS) prescriber. Authorised prescribers are medical practitioners who can directly supply medicinal cannabis to their patients via an Authorised Prescriber script, SAS prescribers are practitioners who are required to submit either a SAS-A or SAS-B application to the TGA for approval. For SAS prescribers, the SAS-A application route provides a pathway for medical practitioners to prescribe medicinal cannabis to their patients who are classified as seriously ill, and the SAS-B route provides a pathway for medical and nurse practitioners to prescribe medicinal cannabis to their patients for multiple clinical indications of varied severity. Currently, it takes up to 48 h for SAS prescribers to receive approval for their patient’s scripts (TGA 2022b). From January 2017 to February 2022, 99.6% of SAS approved medicinal cannabis prescriptions were provided to patients via the SAS-B access scheme. Of these SAS-B applications, 217 030 were submitted to the TGA by 3929 prescribers on behalf of over 17 000 patients (TGA 2021a, 2022b). Fifty-two percent of the 217 030 applications were from prescribers based in Queensland (111 797), 20.5% (44 561) were from prescribers based in New South Wales and 20.0% (43 344) were from Victoria, 6.5% (14 096) were from prescribers based in Western Australia, and <1.5% (3148) of the applications came from prescribers based in South Australia, Australian Capital Territory, Tasmania, and Northern Territory (TGA 2021a). Across Australia, there is a large variation in prescribing rates between specialist physicians and general practitioners (GPs). Of self-reported practitioner specialty data for 118 798 applications provided by prescribers between January 2019 and May 2021, 59.2% (70 356) of the prescribers reported they were GPs, 8.3% (9884) reported they were specialist physicians, 7.4% (8743) reported they were cannabis clinic prescribers and 25.1% (29 803) did not report practitioner specialty to the TGA (2021a). GPs and specialist physicians reported they were specialists in the areas of general practice, psychiatry, pain management, neurology, anaesthesia, palliative care, oncology, rehabilitation, gastroenterology and in cannabis prescribing (TGA 2021a).
The pharmacovigilance of any therapeutic, including that of medicinal cannabis, incorporates the reporting of adverse events to the TGA’s database of adverse event notifications (DAEN). From January 2017 to February 2022, 53 adverse events associated with medicinal cannabis have been reported to the DAEN for eight products (TGA 2021b). Considering the total number of prescription approvals by 1 October 2021 was 172 162, the reports of serious adverse events are almost negligible (TGA 2021b). Yet, current research indicates the incidence of adverse events due to medicinal cannabis is higher than is found in TGA DAEN reports (MacCallum et al. 2021).
Medicinal cannabis products contain one or a combination of two main ingredients from the Cannabis sativa plant, cannabidiol (CBD) and tetrahydrocannabinol (THC). THC, a psychoactive cannabinoid is currently classified as a Schedule 8 (controlled) drug in Australia (TGA 2022b). Formulations of CBD and THC compounds that contain <2% THC and pure CBD are considered non-euphoric and are classified as a Schedule 4 (prescription) medicine. However, with changes to legislation enacted in February 2021, where low-dose formulations of CBD (<150 mg daily dose) were reclassified to a Schedule 3 (pharmacist only) over-the-counter product, the use of CBD in Australia is likely to increase (TGA 2020). Although CBD is considered to have an acceptable safety and tolerability profile at lower doses, there are known risks associated with drug-to-drug interactions, especially when CBD is used concomitantly with other commonly prescribed drugs metabolised via CYP450 pathways (Hallinan et al. 2022a). These interaction concerns are additional to the risks associated with all other cannabinoid products, as evidence for the safe and optimal use of medicinal cannabis is still emerging. To date, most of the evidence is from observational data and open label studies, rather than from randomised clinical controlled trials (Stockings et al. 2018; Sarris et al. 2020; Kurlyandchik et al. 2021; Pawliuk et al. 2021). Notable exceptions include medicinal cannabis efficacy trials for patients with treatment-resistant epilepsy (de Carvalho Reis et al. 2020).
Calls for alternative methods of generating data on the efficacy and safety of medications, including medicinal cannabis, are increasing globally and in Australia (Currow et al. 2012; Freeman et al. 2019; Hallinan et al. 2021). At the same time, approaches that use longitudinal real-life electronic medical record (EMR) data and consumer data via social media and smartphone software applications to augment efficacy data, are growing in importance and acceptability (Charles-Smith et al. 2015; Pierce et al. 2019; Hallinan et al. 2021).
GPs, both prescribers and non-prescribers of medicinal cannabis, are well positioned to contribute to pharmacovigilance of medicinal cannabis via the EMR (Hallinan et al. 2021). EMR systems have been widely adopted by Australian GPs in recent decades (Hunter et al. 2020). The aim of this research is to interrogate general practice EMR data that is de-identified at the source by using rule-based digital phenotyping (Banda et al. 2018) to: (1) establish how many patients have a record of medicinal cannabis use; (2) ascertain the reason for prescription; and (3) detect adverse events that are potentially related to medicinal cannabis prescribing to determine the feasibility of the use of EMRs to monitor medicinal cannabis prescribing in Australia.
Methods
Digital phenotyping is defined as the use of data that are automatically generated by technical platforms to measure (or offer robust proxies for) human behaviour. Digital phenotyping, where a clinical condition or characteristic is ascertained through the application of a computerised keyword query or a logical expression into an EMR database, is increasingly being used to monitor a breadth of conditions for screening, diagnosis, and the monitoring of treatment effects (Richesson et al. 2013; Huckvale et al. 2019). Using digital phenotyping, we examined EMR data from the Patron primary care data repository to identify the patient cohort (Boyle et al. 2019). Currently, the Patron database collects de-identified EMR data from over 144 general practice sites; of these, 91% are in Victoria, and the rest are in New South Wales and South Australia. The repository comprises around 721 GPs who work in general practices that use Best Practice™, Medical Director™, and ZedMed™ clinical software. With GP consent, patient EMRs are extracted from the practice clinical software, using the data extraction tool, GRHANITE™ (Boyle et al. 2019). The EMR data are de-identified locally and transmitted to the data repository via encrypted transmission. The GRHANITE™ tool de-identifies each patient by replacing the patients name with a unique patient identifier that links the patient to the individual visit in each patient table (Boyle et al. 2019). This unique identifier is applied before the data leaves the practice. Identifiers including patient address, date of birth, Medicare number, GP, and general practice names are either removed or de-identified prior to extraction to the data repository.
Records of patient visits between 1 September 2017 and 9 September 2020 were extracted from the Patron database in September 2020. This yielded structured and semi-structured EMR data contained in administrative, demographic, clinical, prescribing, and pathology tables relating to 1 164 846 active patients from around 561 GPs in 109 practices. Of these tables, the variables used for this analysis included reason for visit, current and past history, medication name (trade and generic), current medication history, past medication history, prescription repeat, reason for prescription, gender, age, socio-economic indexes for areas of relative disadvantage (SEIFA), and Aboriginal and Torres Strait Islander (ATSI) status. The prescription table included information on dose, frequency, route, generic and trade name, and formulation. The data in the prescription tables were used to validate the structured/semi-structured information in the reason for visit/clinical table. Data from free text notes within the EMR were not included in this analysis.
A list of terms and key words used to describe medicinal cannabis was established using the knowledge base of the clinical researchers (CH and YB) and was validated following literature and internet searches (Table 1). Using these terms, Patron data were examined using structured query language (SQL) rule-based phenotyping and temporal abstractions to select entries related to medicinal cannabis (Agarwal et al. 2016) (Fig. 1). Rule-base phenotyping was used to capture key terms, as at the time of extraction, standardised coding terminologies had not yet been fully established in the Patron database. The EMR allows for the entry of structured data that are bounded by standardised parameters. In the case where fields are not available, such as in products that are not listed in the PBS, entries are written into the prescription fields by the GP and analysed as semi-structured data.

|
A literature review of peer-reviewed research, manufacturer product information, United States Food and Drug Administration (FDA) and TGA websites was conducted to search for the top five most recently reported medicinal cannabis-related adverse events. Adverse events that were outside a 90-day window following the medicinal cannabis prescription date were not included in the adverse event count, as it was deemed not possible to relate the event to medicinal cannabis use. The data in the past history, current history, and reason for visit tables were searched to exclude patient records with indicators of potential adverse events that could clearly be attributed to causes outside of medicinal cannabis use, such as a prior diagnosis or other clinical event.
Ethics approval
Ethics project approval was granted by the University of Melbourne and registered with the University of Melbourne Human Research Ethics Committee (Ethics ID: 2057596.1).
Results
Patron data trends were compared to SAS-B monthly approvals for medicinal cannabis from September 2017 to September 2020 (Figs 2, 3) (TGA 2022b). Using this process, 80 patients and 170 validated prescriptions were identified from EMRs, based on the medicinal cannabis entries made by 26 GPs from 27 general practices. Of the 170 scripts, four had an anatomical therapeutic code (ATC) and were registered on the Pharmaceutical Benefits Scheme (PBS) (Sativex-ATC N02BG10 and Epidiolex-ATC NO3AX24). The other scripts required manual entry into the EMR fields by the GP. Of these semi-structured entries, GPs entered the prescription medication trade names for nearly all visits (169/170, 99.4%); dose and/or strength were entered into the patient medication field on 61.2% (104/170) of the visits.
|
|
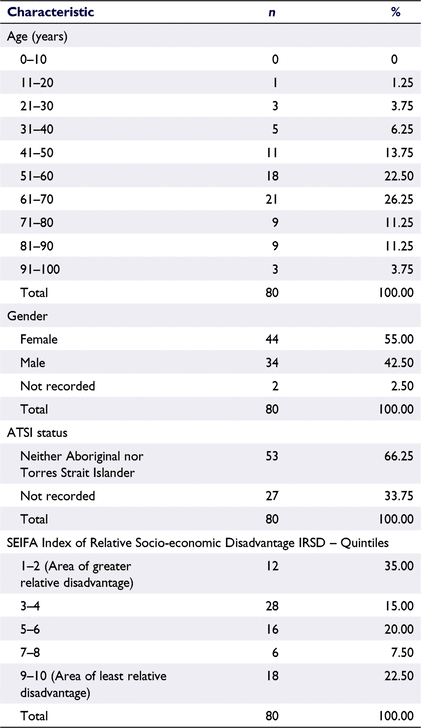
Most of the patients prescribed medicinal cannabis were female (55%), aged between 41 and 70 years (mean 57.3, range 19–96) and from socioeconomic areas of greater relative disadvantage (Table 2).
|
Forty-five of the 80 patients had a single prescription medicinal cannabis, and of the prescriptions with repeats, 18 patients had two repeats, seven patients had three repeats on the prescription, four patients had four repeats, four individual patients each had five, six, seven and eight repeats respectively, and one patient had 11 repeats on their prescription, and another had a prescription with 17 repeats.
The medical history most associated with medicinal cannabis prescribing were depression and anxiety, neurologic and other chronic pain, epilepsy, arthritis, cancer, insomnia, encephalopathy, gout, sleep disorder, stroke, and lastly, opioid dependence. The reason for prescription was poorly recorded, with only 29 reasons provided for 170 prescriptions. Of these reasons, 14 were for pain, four were for a mental health condition, three each were for multiple sclerosis and cancer, and two reports each were for nausea and Crohn’s disease.
Regarding formulations, most were a combination of tetrahydrocannabinol (THC) and cannabinol (CBD) (55.3%, 94/170), a lower number of prescriptions were for CBD only (35.9%, 61/170), and a minimal number were for THC only (8.8%, 15/170). Of the prescriptions, 84.1% (143/170) had a recording of cannabis strength and 40.6% (69/170) had recordings of patient dose (Table 3).

|
Of the 80 patients prescribed medicinal cannabis, nine showed symptoms of a possible adverse event within 90 days of a medicinal cannabis prescription. Depression was the most common symptom reported, with three patients (who did not have a record of a prior clinical history of depression) reporting depression. Two patients had a motor vehicle accident following a medicinal cannabis prescription, two patients reported gastrointestinal symptoms, nausea for one, and abdominal pain for the other patient. There was one patient record of anxiety with no prior record of anxiety in their history, and one record of an adverse events with no detail.
Discussion
The Patron database sourced for this research represents 10.3% of general practices in Victoria, and 2.6% of general practices in Australia (Health Direct Australia 2022). Our analysis shows the demographic data for medicinal cannabis prescribing is consistent with that of Victoria and Australia at the time; namely, a greater association with middle-to-older aged females, from more disadvantaged locations (TGA 2022b). This research also shows prescribing trends for Patron general practices are very similar to trends in Victoria (Figs 2, 3). Using publicly available TGA data, we found from September 2017 to September 2020, there were just over 11 000 approvals for medicinal cannabis prescriptions in Victoria (TGA 2021a). Across the same period, there were 170 records of medicinal cannabis in the Patron database, which amounts to 1.6% of approvals in Victoria; this demonstrates the number of records of medicinal cannabis prescriptions in general practice EMRs is relatively low, especially considering the Patron database represents 10.30% of all Victorian general practices, and the large number of approvals in Victoria overall (Figs 2, 3).
There are several possible reasons for this. First, the recording of medicinal cannabis prescriptions by prescribing and non-prescribing GPs on the EMR is discretionary, as most medicinal cannabis is not on the PBS and thus needs to be manually entered into the prescription field. Second, the Patron repository currently collects only structured and semi-structured data that are entered into discrete data fields, which are bounded by automated responses, the unstructured EMR data, such as free-text narrative in clinical notes, were not accessible as a data source. Furthermore, as medicinal cannabis is not registered with the TGA, and is not universally considered as a medicine by all GPs (Hallinan et al. 2021), some clinicians may consider it a complimentary therapeutic or even a herbal product and, as a result, may not enter the information into the EMR. Finally, for medicinal cannabis that is not prescribed by a patient’s usual GP, it is up to the patient to disclose medicinal cannabis use. It is well understood there is a stigma associated with ‘cannabis as a medicine’ (Hallinan et al. 2022b); hence, some patients may not disclose to their GP that they have been accessing medicinal cannabis. This would also apply to patients who have been accessing ‘artisanal medicinal cannabis’ (cannabis product that is not recommended by the TGA and prescribed outside of TGA guidelines) (Sulak et al. 2017; Lintzeris et al. 2020).
Of the 170 prescriptions, 26 unique reasons for visit were entered into the EMR. This is surprising, especially considering the TGA do not recommend medicinal cannabis as a first line treatment and the limited number of permitted indications that have TGA approval (TGA 2022b). Of the reasons reported, pain had the highest frequency with 33 reports of pain for conditions such as a motor bike accident (17), fibromyalgia (5), chronic pain (4), cancer pain (2), arachnoiditis (1), neuropathy (1), back pain (1), facial pain (1), and pain management (1). Prescribing for the treatment of PTSD (12), anxiety (3), Crohn’s disease (3), insomnia (3), multiple sclerosis (3), sarcoma (3), osteoarthritis (2), breast cancer (2) and nausea (2) were reported 33 times. Progressive supra-nuclear palsy and medullary sponge kidney were each reported once in the EMR, initiation of medicinal cannabis and/or medicinal cannabis was listed 17 times. For all patients, an initial reason for prescription was entered into the EMR. The reason-for-visit field was not always completed when a patient had a repeat prescription; this may be due to medicinal cannabis not being on the PBS as discussed above.
There were potentially nine patients who possibly had an adverse event based on what is known about adverse events and cannabis (Abuhasira et al. 2018; Allan et al. 2018). Yet, it is difficult to ascribe the suggested effects with certainty as these associations are based on assumptions such as patient recall, the appointment date, and the default date set by the EMR rather than a valid adverse event ‘signal’. This is an important area that requires further investigation.
Medicinal cannabis prescribing in Australia is on a trajectory that is clearly rising (Figs 2, 3). Best practice for the pharmacological management of clinical conditions is based on evidence from randomised controlled trials (RCTs), yet RCT evidence for medicinal cannabis is limited. Given this, novel approaches using real-world evidence, from non-interventional studies, registries, EMRs, and health service data, provides opportunities to gather information on effectiveness and adverse events, and can be also used to inform the design of RCTs (Banerjee et al. 2022).
Our analysis that uses real-world evidence from EMRs demonstrates it is certainly feasible to track usage with digital phenotyping methodology. With this feasibility comes an understanding that the EMR provides opportunities to detect, measure, and monitor medicinal cannabis use. These opportunities include the provision of levers to GPs to facilitate the recording of quality data that includes the embedding of reporting mechanisms into GP workflow via automated prompts and drop-down menus. For example, the EMR could provide a drop-down list of medicinal cannabis formulation, indication, effectiveness, side-effects, and adverse events. Other EMR functionalities could include the installation of ‘Point of Care’ applications onto the EMR, where on-screen recommendations open automatically for patients on medical cannabis, which provide suggestions and alerts to the GP for enhanced vigilance through real-time monitoring.
The delivery of educational resources that are embedded within the EMR to guide GPs could also provide another valuable lever. GP guidance resources would deliver information on aspects of medicinal cannabis that are important to track in a patient, such as patient reports of: a reduction of symptoms for which the medicinal cannabis was prescribed, improvements in daily functioning, the presence of side-effects (i.e. fatigue, nausea, dizziness) or adverse events (i.e. hallucinations), and drug-to-drug interactions. Other strategies shown to be of benefit in other health domains include an option for financial (i.e. immunisation) and non-financial incentives (i.e. continuing professional development – CPD points) to promote the recording and monitoring of medicinal cannabis prescribing by GPs (Fernholm et al. 2019; Hallinan 2019).
Other countries are increasingly engaging consumers in the reporting of medicinal cannabis effects through social media and software applications, including from smartwatches and smartphones. The use of this data is growing in importance and acceptability, especially with improvement in the app-literacy of patients (Charles-Smith et al. 2015; Pierce et al. 2019). This is worth considering in the Australian context given the rising prevalence of medicinal cannabis prescribing with each year.
This research was limited by the low number of medicinal cannabis prescribing approvals in Victoria (and Australia) prior to August 2020 (TGA 2022a), and by the number of Victorian general practices that were represented in the Patron database at the time. Notwithstanding, from mid-2020 onwards, there has been a rapid rise in the number of medicinal cannabis approvals, and an additional 40 general practices, including practices from NSW and Victoria, that are currently contributing to the Patron repository. With the growth in TGA prescription approvals, and an expanding number of general practices held in the Patron repository, this rapidly changing environment provides opportunities for, and the impetus to, re-test the feasibility of monitoring medicinal cannabis in the general practice EMR. Given this, we plan to repeat this analysis in October 2022.
For this research, we captured prescriptions that were manually entered as either structured or semi-structured data into EMR medication fields. Although this delivers valuable information for the monitoring of medications, the use of unstructured clinical free-text notes also provides many opportunities (Hong et al. 2018; Rojas and Capurro 2019). The technology to enable the use of unstructured text as a data source (i.e. doctors free-text notes) is currently emerging. As these technologies develop, the utility of the EMR as a data source will be optimised, and with this, the general practice EMR will become more widely used for the surveillance and monitoring of all types of therapeutics.
Conclusions
As novel therapeutics continue to emerge, real-time adverse event reporting is becoming increasingly important. However, such reporting can be a time- and resource-consuming activity for clinicians, regulatory authorities, and drug manufacturers. Our proof of concept has demonstrated the recording of the effects of medicinal cannabis in GP EMRs provides a feasible option for the monitoring and surveillance of medicinal cannabis use in the community, especially if it can be embedded into a GP’s workflow.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate. Data were analysed using Stata 16 software; coding is available on request.
Conflicts of interest
YB is a principal investigator for an industry-sponsored clinical trial of the pharmacokinetics of medicinal cannabis for Zelira Therapeutics Ltd.
Declaration of funding
The Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE) is established through the National Health and Medical Research Council (NHMRC) Centre of Research Excellence scheme. It draws together >20 Australian research leaders and clinicians from major national universities and research institutions to establish a research evidence base to inform safe clinical use of medicinal cannabinoids and to guide policy as cannabinoids are introduced into therapeutic practice in Australia.
References
Abuhasira R, Schleider LB-L, Mechoulam R, Novack V (2018) Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. European Journal of Internal Medicine 49, 44–50.| Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly.Crossref | GoogleScholarGoogle Scholar |
Agarwal V, Podchiyska T, Banda JM, Goel V, Leung TI, Minty EP, Sweeney TE, Gyang E, Shah NH (2016) Learning statistical models of phenotypes using noisy labeled training data. Journal of the American Medical Informatics Association 23, 1166–1173.
| Learning statistical models of phenotypes using noisy labeled training data.Crossref | GoogleScholarGoogle Scholar |
Allan GM, Finley CR, Ton J, Perry D, Ramji J, Crawford K, Lindblad AJ, Korownyk C, Kolber MR (2018) Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity, and harms. Canadian Family Physician Medecin de Famille Canadien 64, e78–e94.
Banda JM, Seneviratne M, Hernandez-Boussard T, Shah NH (2018) Advances in electronic phenotyping: from rule-based definitions to machine learning models. Annual Review of Biomedical Data Science 1, 53–68.
| Advances in electronic phenotyping: from rule-based definitions to machine learning models.Crossref | GoogleScholarGoogle Scholar |
Banerjee R, Erridge S, Salazar O, Mangal N, Couch D, Pacchetti B, Sodergren MH (2022) Real world evidence in medical cannabis research. Therapeutic Innovation & Regulatory Science 56, 8–14.
| Real world evidence in medical cannabis research.Crossref | GoogleScholarGoogle Scholar |
Boyle D, Sanci L, Emery J, Gunn J, Hocking JS, Manski-Nankervis J-A, Canaway R (2019) Patron primary care research data repository. (University of Melbourne). Dataset. Dataset. Available at https://medicine.unimelb.edu.au/school-structure/general-practice/engagement/data-for-decisions [Accessed 15 July 2022]
Charles-Smith LE, Reynolds TL, Cameron MA, Conway M, Lau EHY, Olsen JM, Pavlin JA, Shigematsu M, Streichert LC, Suda KJ, Corley CD (2015) Using social media for actionable disease surveillance and outbreak management: a systematic literature review. PLoS ONE 10, e0139701
| Using social media for actionable disease surveillance and outbreak management: a systematic literature review.Crossref | GoogleScholarGoogle Scholar |
Currow DC, Rowett D, Doogue M, To THM, Abernethy AP (2012) An international initiative to create a collaborative for pharmacovigilance in hospice and palliative care clinical practice. Journal of Palliative Medicine 15, 282–286.
| An international initiative to create a collaborative for pharmacovigilance in hospice and palliative care clinical practice.Crossref | GoogleScholarGoogle Scholar |
Dal Pan GJ (2014) Ongoing challenges in pharmacovigilance. Drug Safety 37, 1–8.
| Ongoing challenges in pharmacovigilance.Crossref | GoogleScholarGoogle Scholar |
de Carvalho Reis R, Almeida KJ, da Silva Lopes L, de Melo Mendes CM, Bor-Seng-Shu E (2020) Efficacy and adverse event profile of cannabidiol and medicinal cannabis for treatment-resistant epilepsy: systematic review and meta-analysis. Epilepsy & Behavior 102, 106635
| Efficacy and adverse event profile of cannabidiol and medicinal cannabis for treatment-resistant epilepsy: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Dhruva SS, Ross JS, Desai NR (2018) Real-world evidence: promise and peril for medical product evaluation. P & T: A Peer-Reviewed Journal for Formulary Management 43, 464–472.
Downing NS, Shah ND, Aminawung JA, Pease AM, Zeitoun J-D, Krumholz HM, Ross JS (2017) Postmarket safety events among novel therapeutics approved by the US food and drug administration between 2001 and 2010. JAMA 317, 1854–1863.
| Postmarket safety events among novel therapeutics approved by the US food and drug administration between 2001 and 2010.Crossref | GoogleScholarGoogle Scholar |
Fernholm R, Arvidsson E, Wettermark B (2019) Financial incentives linked to quality improvement projects in Swedish primary care: a model for improving quality of care. BMJ Open Quality 8, e000402
| Financial incentives linked to quality improvement projects in Swedish primary care: a model for improving quality of care.Crossref | GoogleScholarGoogle Scholar |
Freeman TP, Hindocha C, Green SF, Bloomfield MAP (2019) Medicinal use of cannabis based products and cannabinoids. BMJ 365, l1141
| Medicinal use of cannabis based products and cannabinoids.Crossref | GoogleScholarGoogle Scholar |
Hallinan (2019) Does the removal of financial incentives from Australian general practices affect immunisation rates? PhD thesis, Minerva Access, Medicine, Dentistry & Health Science, Melbourne Medical School, The University of Melbourne. Available at http://hdl.handle.net/11343/233864 [Accessed 15 July 2022]
Hallinan CM, Gunn JM, Bonomo YA (2021) Implementation of medicinal cannabis in Australia: innovation or upheaval? Perspectives from physicians as key informants, a qualitative analysis. BMJ Open 11, e054044
| Implementation of medicinal cannabis in Australia: innovation or upheaval? Perspectives from physicians as key informants, a qualitative analysis.Crossref | GoogleScholarGoogle Scholar |
Hallinan CM, Eden E, Graham M, Greenwood L-M, Mills J, Popat A, Truong L, Bonomo Y (2022a) Over the counter low-dose cannabidiol: a viewpoint from the ACRE Capacity Building Group. Journal of Psychopharmacology 36, 661–665.
| Over the counter low-dose cannabidiol: a viewpoint from the ACRE Capacity Building Group.Crossref | GoogleScholarGoogle Scholar |
Hallinan CM, Habibabadi SK, Bonomo YA, Conway M (2022b) Social media discourse and search engine queries on cannabis as a medicine cannabis as a medicine: a systematic approach. PLOS-One
Health Direct Australia (2022) National Health Services Directory. (Health Direct Australia). Available at https://about.healthdirect.gov.au/nhsd [Accessed 10 April 2022]
Hong N, Wen A, Shen F, Sohn S, Liu S, Liu H, Jiang G (2018) Integrating structured and unstructured EHR data using an FHIR-based type system: a case study with medication data. AMIA Summits on Translational Science Proceedings. pp. 74–83.
Huckvale K, Venkatesh S, Christensen H (2019) Toward clinical digital phenotyping: a timely opportunity to consider purpose, quality, and safety. npj Digital Medicine 2, 88
| Toward clinical digital phenotyping: a timely opportunity to consider purpose, quality, and safety.Crossref | GoogleScholarGoogle Scholar |
Hunter B, Biezen R, Alexander K, Lumsden N, Hallinan C, Wood A, McMorrow R, Jones J, Nelson C, Manski-Nankervis J-A (2020) Future Health Today: codesign of an electronic chronic disease quality improvement tool for use in general practice using a service design approach. BMJ Open 10, e040228
| Future Health Today: codesign of an electronic chronic disease quality improvement tool for use in general practice using a service design approach.Crossref | GoogleScholarGoogle Scholar |
Kurlyandchik I, Tiralongo E, Schloss J (2021) Safety and efficacy of medicinal Cannabis in the treatment of fibromyalgia: a systematic review. The Journal of Alternative and Complementary Medicine 27, 198–213.
| Safety and efficacy of medicinal Cannabis in the treatment of fibromyalgia: a systematic review.Crossref | GoogleScholarGoogle Scholar |
Lintzeris N, Mills L, Suraev A, Bravo M, Arkell T, Arnold J, Benson M, Mcgregor I (2020) Medical cannabis use in the Australian community following introduction of legal access: the 2018–2019 Online Cross-Sectional Cannabis as Medicine Survey (CAMS-18). Harm Reduction Journal 17, 37
| Medical cannabis use in the Australian community following introduction of legal access: the 2018–2019 Online Cross-Sectional Cannabis as Medicine Survey (CAMS-18).Crossref | GoogleScholarGoogle Scholar |
MacCallum CA, Lo LA, Boivin M (2021) “Is medical cannabis safe for my patients?” A practical review of cannabis safety considerations. European Journal of Internal Medicine 89, 10–18.
| “Is medical cannabis safe for my patients?” A practical review of cannabis safety considerations.Crossref | GoogleScholarGoogle Scholar |
Pawliuk C, Chau B, Rassekh SR, McKellar T, Siden HH (2021) Efficacy and safety of paediatric medicinal cannabis use: a scoping review. Paediatrics & Child Health 26, 228–233.
| Efficacy and safety of paediatric medicinal cannabis use: a scoping review.Crossref | GoogleScholarGoogle Scholar |
Pierce CE, de Vries ST, Bodin-Parssinen S, Härmark L, Tregunno P, Lewis DJ, Maskell S, Van Eemeren R, Ptaszynska-Neophytou A, Newbould V, Dasgupta N, Wisniewski AFZ, Gama S, Mol PGM (2019) Recommendations on the use of mobile applications for the collection and communication of pharmaceutical product safety information: lessons from IMI WEB-RADR. Drug Safety 42, 477–489.
| Recommendations on the use of mobile applications for the collection and communication of pharmaceutical product safety information: lessons from IMI WEB-RADR.Crossref | GoogleScholarGoogle Scholar |
Richesson RL, Hammond WE, Nahm M, Wixted D, Simon GE, Robinson JG, Bauck AE, Cifelli D, Smerek MM, Dickerson J, Laws RL, Madigan RA, Rusincovitch SA, Kluchar C, Califf RM (2013) Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. Journal of the American Medical Informatics Association 20, e226–e231.
| Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory.Crossref | GoogleScholarGoogle Scholar |
Rojas E, Capurro D (2019) ‘Characterization of drug use patterns using process mining and temporal abstraction digital phenotyping.’ (Springer: Cham, Switzerland)
Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J (2020) Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry 20, 24
| Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review.Crossref | GoogleScholarGoogle Scholar |
Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L (2018) Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. PAIN 159, 1932–1954.
| Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies.Crossref | GoogleScholarGoogle Scholar |
Sulak D, Saneto R, Goldstein B (2017) The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy & Behavior 70, 328–333.
| The current status of artisanal cannabis for the treatment of epilepsy in the United States.Crossref | GoogleScholarGoogle Scholar |
TGA (2020) Over-the-counter access to low dose cannabidiol. (Therapeutic Goods Administration: Canberra, ACT, Australia) Available at https://www.tga.gov.au/media-release/over-counter-access-low-dose-cannabidiol [Accessed 14 January 2020]
TGA (2021a) About the TGA freedom of information. (Therapeutic Goods Administration: Canberra, ACT, Australia) Available at https://www.tga.gov.au/freedom-information [Accessed 2 October 2021]
TGA (2021b) Database of adverse event notifications - medicines. (Therapeutic Goods Administration: Canberra, ACT, Australia) Available at https://www.tga.gov.au/database-adverse-event-notifications-daen [Accessed 2 October 2021]
TGA (2022a) Medicinal cannabis access data dashboard. (Australian Government Department of Health: Canberra, ACT, Australia) Available at https://www.tga.gov.au/medicinal-cannabis-access-data-dashboard [Accessed 20 August 2021]
TGA (2022b) Medicinal cannabis: role of the TGA. (Therapeutic Goods Administration: Canberra, ACT, Australia) Available at https://www.tga.gov.au/medicinal-cannabis-role-tga [Accessed 8 February 2022]
WHO (2015) ‘WHO pharmacovigilance indicators: a practical manual for the assessment of pharmacovigilance systems.’ (World Health Organisation (WHO): Geneva, Switzerland)


