The development of Future Health Today: piloting a new platform for identification and management of chronic disease in general practice
Barbara Hunter A * , Karyn Alexander A , Ruby Biezen A , Christine Mary Hallinan A , Anna Wood A , Craig Nelson B C and Jo-Anne Manski-Nankervis
A , Anna Wood A , Craig Nelson B C and Jo-Anne Manski-Nankervis  A D
A D
A Department of General Practice, University of Melbourne, Melbourne, Vic. 3000, Australia.
B Western Health Chronic Disease Alliance, Department of Nephrology, Western Health, Footscray, Vic. 3000, Australia.
C Department of Medicine Western Health, University of Melbourne, Melbourne, Vic. 3000, Australia.
D NHMRC Centre for Research Excellence in Digital Technology to Transform Chronic Disease Outcomes, University of Melbourne, Melbourne, Vic. 3000, Australia.
Australian Journal of Primary Health 29(1) 8-15 https://doi.org/10.1071/PY22022
Submitted: 8 February 2022 Accepted: 13 October 2022 Published: 2 November 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of La Trobe University. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Chronic disease identification and management is a significant issue in Australia, with general practice being the primary contact point for those at risk of, or living with, chronic disease. However, there is a well-described gap between guideline recommendations for chronic disease management and translation in the general practice setting. In 2018, a group of researchers, clinicians and software developers collaborated to develop a tool to support the identification and management of chronic disease in general practice, with the aim to create a platform that met the needs of general practice. The co-design process drew together core principles and expectations for the establishment of a technological platform, called Future Health Today (FHT), which would sit alongside the electronic medical record (EMR) management system within general practice. FHT used algorithms applied to EMR data to identify patients with, or at risk of, chronic disease and requiring review. Using chronic kidney disease as a clinical focus, the FHT prototype was piloted in a large, metropolitan general practice, and a large regional general practice. Based on user feedback, the prototype was further developed and improved. This paper provides a report on the key features and functionalities that participants identified and implemented in practice.
Keywords: chronic disease, chronic disease management, chronic kidney disease, co-design, general practice, health informatics, innovation, primary care.
Chronic disease management in primary care
More than 11 million Australians are estimated to have at least one chronic disease, such as type 2 diabetes, chronic kidney disease (CKD) or heart disease (AIHW 2020a). Most people who are at risk of or have a chronic disease will receive at least some health care in general practice. Successful chronic disease management has been linked to the use of ‘Chronic Care Models’ (Coleman et al. 2009). Key elements of ‘Chronic Care Models’ include decision support and clinical information systems, often facilitated through technology. The challenge is to develop and implement health technologies that avoid the pitfalls of the past, such as the failure to adopt, scale up and/or sustain use (Greenhalgh 2018), retrofitted non-purpose built solutions and minimal use due to resource-intensive implementation (Culler et al. 2013; Pefanis et al. 2018).
Research design and the team
‘Co-design’ is a research strategy and design process (Dimopoulos-Bick et al. 2019) that includes end-users and stakeholders, making the acceptance and adoption of technology more likely (Altman et al. 2018). In the initial co-design, we used tools such as patient journey maps, storyboards, service prototypes and conducted ‘Think Aloud’ interviews with end-users to develop the minimum viable product (described elsewhere, Hunter et al. 2020). This paper describes the subsequent co-design process undertaken to test and refine the prototype by gathering end-user, stakeholder and expert feedback. Our prototype focused on CKD, a condition that is both common and under-diagnosed. Nearly 2 million Australians are estimated to have biomedical markers of CKD; and many of those with the condition are not aware of their diagnosis (AIHW 2020b).
The research team for this project included disease specialists, academic and clinical general practitioners (GPs), academic lawyers, non-clinician primary care researchers, health informaticians and information technology experts.
Ethics approval
Ethics approval was granted by the Melbourne Health Human Research Ethics Committee (HREC/47394/MH-2018). The research was undertaken with appropriate informed consent of participants.
The intervention
FHT is an audit and feedback intervention, comprising a software platform that encompasses a web-based ‘dashboard’ and a ‘point-of-care’ (POC) clinical prompt to facilitate the management of chronic disease in general practice. The dashboard organises patient populations according to their kidney health status using sophisticated algorithms. This population can then be recalled to the general practice for medical review. The dashboard contains links to education, guidelines and consumer resources, and can also be used to facilitate quality improvement (QI) programs. The program can ‘drill down’ into information within the electronic medical record (EMR) database to display, in graphical format, a patient’s anthropometric measures (i.e. height, weight and body mass index (BMI)), summaries of medications, pathology results, medical conditions and Medicare Benefit Schedule items billed. The POC tool deploys when a patient’s EMR is opened, facilitating opportunistic chronic disease identification and management through the prompts for clinicians to consider specific clinical actions, and links directly to the platform that provides the GP with the patient-specific data (as described above) and evidence-based guidelines. Fig. 1 provides a visualisation of how FHT interacts with the practice EMR. A data governance document for the program was developed and made publicly available on our website and is available in Supplementary File S1.
The first stage of implementation involved installing FHT server-side components (CKD algorithms, web application programming interface (API), and database). Participants were provided with a link and login to the FHT-dashboard and the POC was installed on individual computers. Evaluation was undertaken during the implementation process where key components of the technology, elements that were desirable (but not yet incorporated), and factors impacting implementation within the clinical setting were explored.
Participants
FHT was first piloted at a large metropolitan general practice in the western suburbs of Melbourne (Site A), who use Zedmed EMR (Zedmed). One GP and one practice nurse (PN) at that site had contributed to the co-design of FHT. Six staff (three GPs, one PN, one chronic disease health assistant (CDHA), one practice manager (PM)) agreed to test and implement FHT. The CDHA is a non-clinical role, responsible for facilitating and managing large-scale patient recall for chronic disease management and assisting with care planning.
After the initial pilot activity at Site A, FHT was introduced to a large regional general practice in northern Victoria (Site B) who use Best Practice EMR (Best Practice Software Pty Ltd), where the PM and one PN tested the initial installation and implementation of FHT.
Data collection
Data collection took place at Site A in July–October 2019 and included:
-
Ten development/troubleshooting interviews (30–60 min duration) were conducted over 4 weeks with pilot testers (two GPs, PN, CDHA) using the FHT dashboard and/or POC, focusing on functionality and issues associated with use (e.g. system or algorithm problems, user errors).
-
Five implementation interviews were conducted with FHT users (one GP, PN, CDHA) over 6 weeks, focusing on how FHT was used, changes required and the impact of FHT on clinical practice.
-
A single focus group (60 min) at the end of the implementation period (three Researchers, three GPs, PN, CDHA, PM) was conducted where barriers and facilitators to implementation, health professionals’ engagement with FHT, attributed improvements and recommendations to improve the program were examined.
-
Eight interviews were conducted with patients who had been identified by the FHT algorithms, where their perceptions of the use of FHT in general practice were explored.
An additional two informal interviews were conducted with the PM and PN at Site B in late 2019 to determine if the technology was working and broadly acceptable to them.
Issues raised during interviews were discussed with the implementation and technical team to facilitate timely resolution. Researchers also asked participants to document any troubleshooting queries and to provide this to the research or technical team.
Analysis
Interview transcripts and field notes were analysed using content and thematic techniques. Analyses applied deductive and inductive coding schema, encompassing a provisional list of codes including components of the theoretical framework underpinning the project (Clinician Performance Feedback Intervention Theory; CP-FIT; Brown et al. 2019) and implementation research outcomes (acceptability, adoption, appropriateness, feasibility, implementation cost), supplemented by codes generated from the data.
What was learnt from the initiative?
The PMs at both sites were strong proponents for its implementation, enabling staff time to test the FHT prototype over the 6 months of implementation. Site A facilitated implementation through the CDHA and PN who coordinated chronic disease management and were familiar with using clinical audit tools for filtering and recall processes. They used the FHT platform and supported GPs in using the POC component of FHT. In contrast, the PM and PN at Site B primarily used FHT in a light touch, minimal-use manner over a shorter time period (4 weeks). At both sites, participants attempted to use FHT as part of their usual workflow and were hoping to streamline processes and facilitate clinical care using FHT.
Initial troubleshooting
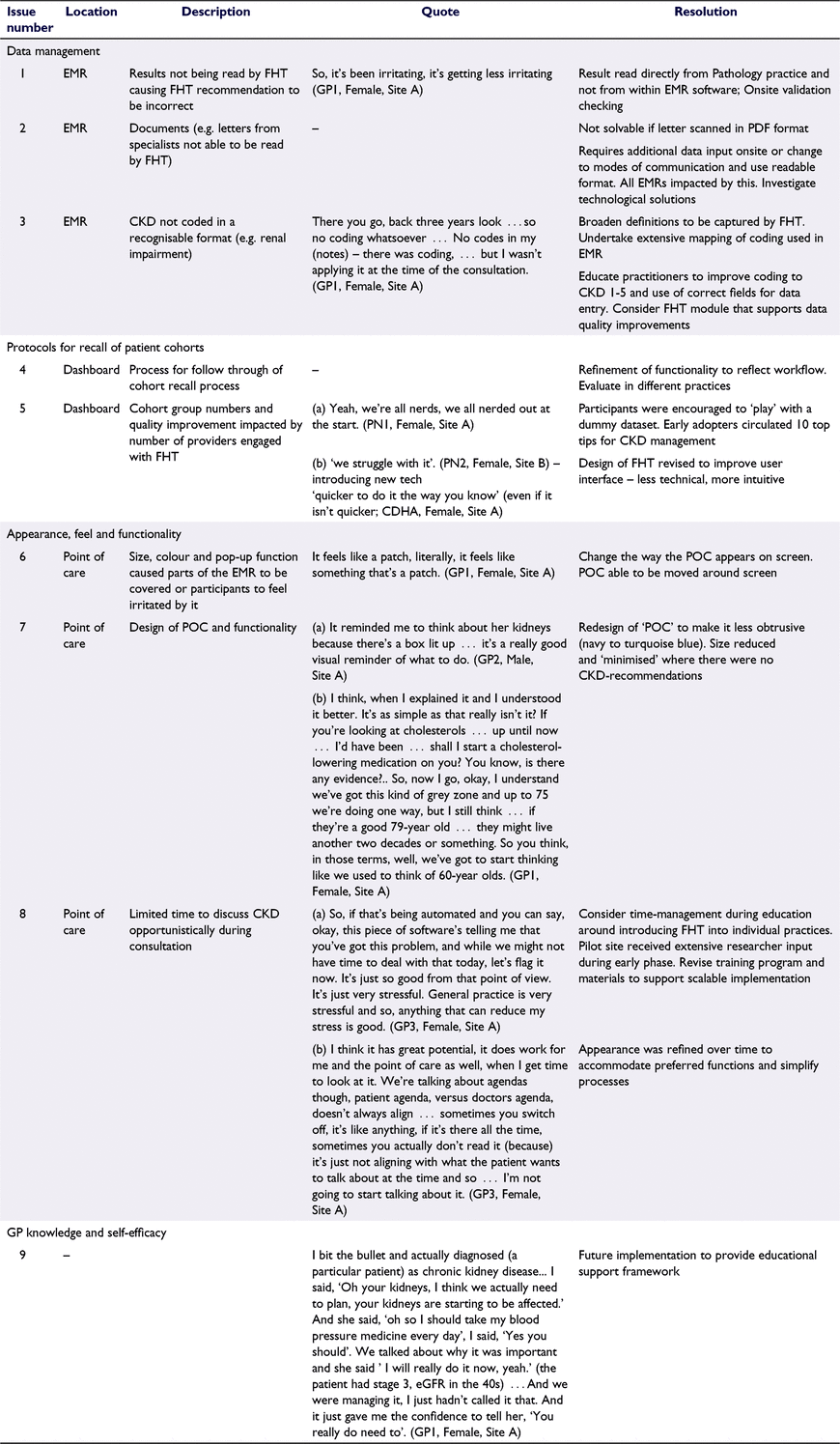
The first ‘real-world’ test of FHT at Site A was designed to explore if FHT could function as intended, and to iteratively improve the platform as issues or improvements were identified. Participants were encouraged to report significant issues as they occurred and to record minor issues/improvements for further discussion. Issues were logged with the technical team, prioritised and progressively reviewed until resolved during this ‘troubleshooting phase’ (Table 1).
|
We revised algorithms and functions to reflect technical requirements, clinical guidelines and pragmatic decisions adopted by the clinical team. For example, Australian guidelines recommend a statin for patients with CKD aged >50 years (Kidney Health Australia 2020); however, we revised the clinical prompt recommendations to also reflect US preventive guidelines that did not recommend statin use in people aged >75 years without a history of CVD (Hager et al. 2017). This revision was made in response to clinician feedback and in consultation with nephrology colleagues. Other issues required revision of algorithms to allow for specific issues related to the EMR database to be addressed (e.g. information not displayed/captured as expected).
At the end of the first month of testing, participants reported that the system was working well and that many ‘teething’ issues had been resolved. However, time saved using the system was instead used to check accuracy as participants did not yet ‘trust the information it was giving’ (PN1). In looking for errors, they discovered more issues related to their EMR rather than the FHT platform, prompting several internal actions, including an EMR software-update to correctly extract pathology data; corrections to CKD-coding and archiving of inactive patients (Table 1, Quotes 1, 3).
Fit with workflow
Site A actively worked to embed FHT into clinical workflow, using the FHT prompts to initiate kidney health checks, review medications and instigate referrals.
The PN and CDHA used the dashboard to generate patient recall lists. The PN identified two categories of patients (diagnosed CKD requires initiation/intensification of statin or blood pressure medications) and generated a list of names (n = 46), which was checked using patient files to determine the accuracy and appropriateness for recall. The patient’s usual GP was asked to review and authorise recall if appropriate. From this process, 17 patients were recalled (Alexander et al. 2019). The PN reported that although the FHT recall function worked, they were unsure about what ‘would happen next’ if they marked a patient as ‘recalled’ and were unsure how to indicate in the EMR the action taken. A ‘recall-authorisation’ feature was inserted into FHT because of PN (and co-design) feedback. The PN and CDHA also noted that the search and filter functionality of the dashboard could be difficult for users less familiar with existing audit software. Site B participants, self-described as ‘less technically savvy’ reported difficulties in understanding and using these functions. These statements align with feedback from participants in the ‘think-aloud’ co-design sessions and suggest that the user interface required work.
After extensive use, Site A participants flagged items that they would like to see added to future iterations of FHT, including the ability to label and save lists of patients. Navigation of FHT depended upon participants’ familiarity with similar features in other databases (including clinical audit software, spreadsheets like Excel and other data management software), with three participants confidently using filtering and sort functions (CDHA, PN and one GP) whereas one GP required support to use this feature.
Experience facilitates use
GPs were more likely to use the POC tool prior to consultation to review patient information. This tool sits permanently at the bottom corner of the computer screen and updates as new patient records are opened in the EMR. Although participants initially expressed annoyance at its presence, over time, it became less intrusive as the technological team reduced the size of the POC-icon and automatically minimised it when FHT did not have any recommendations.
GPs reported using POC frequently, not switching it off, and addressing the recommendations provided by FHT directly, or after further interrogation of patient data. They also found it easy to move between the POC and the patient record, and reported opportunistic interventions with patients as FHT became part of routine practice (Table 1, Quotes 8a, 8b).
Accuracy
Irrespective of the content of the alert, GPs were checking patient records to confirm that the recommendation (or lack of recommendation) was correct. In some instances, the GP used their knowledge of the patient to initiate a kidney health check even when FHT indicated that no action was required. For these participants, the presence of the POC, rather than the detail contained within it, prompted them to reconsider patient risk factors and preventative health strategies (Table 1, Quote 7a).
Actionability
GPs sometimes experienced patient resistance to FHT recommendations, especially where medications needed to be enhanced. However, one GP described how the FHT recommendation both validated her decision to initiate a statin with a reluctant patient, and increased her confidence explaining it (Table 1, Quote 7b). Nevertheless, for FHT recommendations to be discussed, they needed to align with the patient’s agenda, and they may require more consultation time (Table 1, Quotes 8a, 8b). This was also foreseen in the co-design session.
Links to guidelines
Participants supported the inclusion of resources and guidelines, accessible from both the FHT dashboard and POC, but reported not revisiting them following implementation, despite occasionally wanting to refresh their knowledge (e.g. initiating a statin). Participants had no explanation for this, aside from ‘forgetting’ that resources were available.
Clinical knowledge
Self-reported awareness of kidney health increased among participants, following CKD and FHT education and training sessions, direct use of FHT and the recall process. As knowledge increased, participants’ confidence with diagnosis and management of CKD also increased (Table 1, Quote 9).
Patient perspective
Patient feedback is reported elsewhere (Alexander et al. 2019); however, all eight patient interview participants reported no concerns with GPs using a program like FHT as they saw it to be an extension of the systems GPs currently use, and in many instances, preferred that their GPs were using relevant available technology.
Improvements in implementation
The installation of FHT at Site B was more streamlined and quicker than the initial installation at Site A, despite using a different EMR infrastructure. This was the result of many refinements of the tool, growing sophistication of the platform and experience in installation of the FHT team.
Participants at Site B reported that the system was not disruptive to their usual systems; however, they found the user interface of the dashboard to be inaccessible due to its technical look and feel, mirroring comments made from Site A.
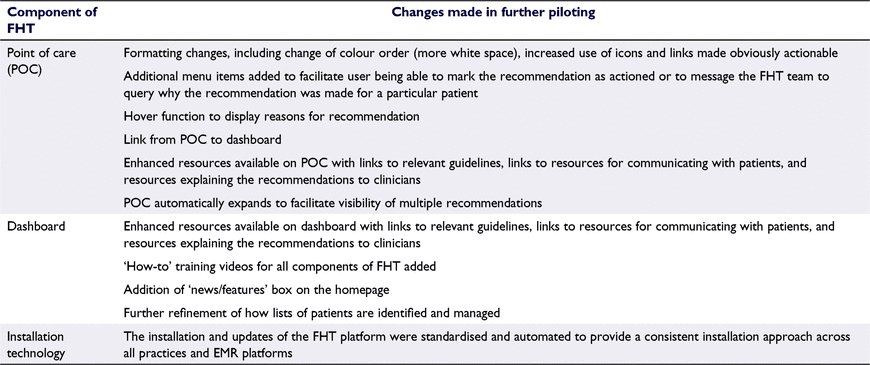
Significant improvements were made to the platform after the implementation period, including a new ‘landing page’ where users could create, view and manage a list of patients through recall and management processes and record QI activities. This was intended to increase the accessibility of the platform and avoid the need to export patient lists to obtain recall permissions from individual GPs. QI resources were added and FHT information and support was strengthened. The POC pop-up was revised to only emerge (and turn orange) when a recommendation was active and to auto-minimise (and turn green) when not required. Exemplar screenshots of the components of the FHT platform and POC are available in Supplementary File S2.
FHT was further refined during a larger pilot comprising 12 practices in 2020–21 (see Table 2 for summary of refinements) and was expanded to include recommendations relating to type 2 diabetes, cardiovascular disease and identification of cancer risk. The revised version has been implemented in a randomised controlled trial involving 41 practices (due to conclude in late 2022).
|
Conclusion
This evaluation demonstrated that implementation of co-designed technology into general practice that involves continued iterative development is acceptable to participants. Although participants desired the sophisticated functionality of the platform, they preferred the interface to look unobtrusive, be easy to use, and fit within general practice context and workflow. The prototype required several workarounds to align with clinical practice, but over time, significant improvements were made to the platform: QI resources were added, and FHT information and support was strengthened. Further research is underway to evaluate ongoing use of FHT, and additional modules are currently being developed as a result of additional grant funding being obtained. Options for sustainability of the program beyond the initial funding period are being explored.
Supplementary material
Supplementary material is available online.
Data availability
Qualitative data used to generate the results in the paper are not available, given the small sample of participants.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Future Health Today (FHT) is a collaboration between The University of Melbourne and Western Health (https://futurehealthtoday.com.au). This research is supported by the Medical Research Future Fund’s Rapid Applied Research Translation program in conjunction with the Melbourne Academic Centre for Health and the Paul Ramsay Foundation.
Acknowledgements
The authors acknowledge the GPs, general practice nurses (PNs) and practice managers (PMs) who contributed to the design and piloting of FHT and serve on our advisory groups. The authors also acknowledge the technical team for their work in translating the co-designed concepts into the FHT software platform.
References
AIHW (2020a) Chronic conditions and multimorbidity. Available at https://www.aihw.gov.au/reports/australias-health/chronic-conditions-and-multimorbidity [Accessed 18 January 2022]AIHW (2020b) Chronic kidney disease. Available at https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/contents/how-many-australians-have-chronic-kidney-disease [Accessed 18 January 2022]
Alexander K, Hunter B, Biezen R, Boyle D, Gunn J, Haikerwal M, Jones J, Janus E, Lumsden N, Manski-Nankervis J (2019) Co-designing technology to improve chronic kidney disease in general practice (University of Melbourne: Melbourne, Vic., Australia) In ‘Poster presentation at 8th annual NHMRC symposium on research translation’. Available at https://futurehealthtodaycomau.files.wordpress.com/2022/01/karyn-alexander-poster-nov-2019.pdf [Accessed 18 January 2022]
Altman M, Huang TTK, Breland JY (2018) Design thinking in health care: a systematic review. Preventing Chronic Disease 15, 180128
| Design thinking in health care: a systematic review.Crossref | GoogleScholarGoogle Scholar |
Brown B, Gude WT, Blakeman T, van der Veer SN, Ivers N, Francis JJ, Lorencatto F, Presseau J, Peek N, Daker-White G (2019) Clinical Performance Feedback Intervention Theory (CP-FIT): a new theory for designing, implementing, and evaluating feedback in health care based on a systematic review and meta-synthesis of qualitative research. Implementation Science 14, 40
| Clinical Performance Feedback Intervention Theory (CP-FIT): a new theory for designing, implementing, and evaluating feedback in health care based on a systematic review and meta-synthesis of qualitative research.Crossref | GoogleScholarGoogle Scholar |
Coleman K, Austin BT, Brach C, Wagner EH (2009) Evidence on the Chronic Care Model in the new millennium. Health Affairs 28, 75–85.
| Evidence on the Chronic Care Model in the new millennium.Crossref | GoogleScholarGoogle Scholar |
Culler SD, Parchman ML, Lozano-Romero R, Noel PH, Lanham HJ, Leykum LK, Zeber JE (2013) Cost estimates for operating a primary care practice facilitation program. The Annals of Family Medicine 11, 207–211.
| Cost estimates for operating a primary care practice facilitation program.Crossref | GoogleScholarGoogle Scholar |
Dimopoulos-Bick TL, O’Connor C, Montgomery J, Szanto T, Fisher M, Sutherland V, Baines H, Orcher P, Stubbs J, Maher L, Verma R, Palmer VJ (2019) “Anyone can co-design?”: a case study synthesis of six experience-based co-design (EBCD) projects for healthcare systems improvement in New South Wales, Australia. Patient Experience Journal 6, 93–104.
| “Anyone can co-design?”: a case study synthesis of six experience-based co-design (EBCD) projects for healthcare systems improvement in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Greenhalgh T (2018) How to improve success of technology projects in health and social care. Public Health Research & Practice 28, e2831815
| How to improve success of technology projects in health and social care.Crossref | GoogleScholarGoogle Scholar |
Hager MR, Narla AD, Tannock LR (2017) Dyslipidemia in patients with chronic kidney disease. Reviews in Endocrine and Metabolic Disorders 18, 29–40.
| Dyslipidemia in patients with chronic kidney disease.Crossref | GoogleScholarGoogle Scholar |
Hunter B, Biezen R, Alexander K, Lumsden N, Hallinan C, Wood A, McMorrow R, Jones J, Nelson C, Manski-Nankervis J-A (2020) Future Health Today: codesign of an electronic chronic disease quality improvement tool for use in general practice using a service design approach. BMJ Open 10, e040228
| Future Health Today: codesign of an electronic chronic disease quality improvement tool for use in general practice using a service design approach.Crossref | GoogleScholarGoogle Scholar |
Kidney Health Australia (2020) ‘Chronic Kidney Disease (CKD) management in primary care.’ 4th edn. (Kidney Health Australia: Melbourne, Vic., Australia) Available at https://kidney.org.au/health-professionals/ckd-management-handbook [Accessed 18 January 2022]
Pefanis A, Botlero R, Langham RG, Nelson CL (2018) eMAP:CKD: electronic diagnosis and management assistance to primary care in chronic kidney disease. Nephrology Dialysis Transplantation 33, 121–128.
| eMAP:CKD: electronic diagnosis and management assistance to primary care in chronic kidney disease.Crossref | GoogleScholarGoogle Scholar |


