Patterns of real-world opioid prescribing in Australian general practice (2013–18)
Doreen Busingye A C , Benjamin Daniels A B , Jonathan Brett B , Allan Pollack A , Josephine Belcher A , Kendal Chidwick
A C , Benjamin Daniels A B , Jonathan Brett B , Allan Pollack A , Josephine Belcher A , Kendal Chidwick  A and Suzanne Blogg A
A and Suzanne Blogg A
A NPS MedicineWise, Level 7/418A Elizabeth Street, Surry Hills, NSW 2010, Australia.
B Medicines Policy Research Unit, Centre for Big Data Research in Health, Level 2, AGSM Building (G27), UNSW, Sydney, NSW 2052, Australia.
C Corresponding author. Email: dbusingye@nps.org.au
Australian Journal of Primary Health 27(5) 416-424 https://doi.org/10.1071/PY20270
Submitted: 12 November 2020 Accepted: 25 May 2021 Published: 15 September 2021
Journal Compilation © La Trobe University 2021 Open Access CC BY
Abstract
Little is known about private-market opioid prescribing and how Australian opioid policies impact prescribing across public and private markets in Australia. We aimed to investigate publicly subsidised and private-market opioid prescribing from 2013 to 2018. We used prescribing records from MedicineInsight, an Australian primary care database, to examine trends in prescriptions for non-injectable opioid formulations from October 2013 to September 2018. We examined annual opioid prescribing trends overall, by opioid agent, and by market (public and private). We further examined patterns of fentanyl patch prescribing focusing on co-prescribed medicines and use in opioid-naïve patients. Opioids accounted for 8% of all prescriptions over the study period and 468 893 patients were prescribed at least one opioid of interest. Prescribing rates for oxycodone/naloxone and tapentadol increased, whereas those for fentanyl patches, morphine and single-agent oxycodone decreased over the study period. Private-market prescribing rates of codeine (schedule 4) increased notably following its up-scheduling to prescription-only status. Among patients prescribed fentanyl patches, 29% were potentially opioid-naïve and 49% were prescribed another opioid on the same day. The private-medicines market is a small but growing component of opioid use in Australia and one way in which prescribers and patients can avoid access restrictions in the public market for these medicines. Although fentanyl patch prescribing declined, there is room for improvement in prescribing fentanyl patches among opioid-naïve patients, and co-prescribing of fentanyl patches with other sedatives.
Keywords: community, codeine, fentanyl, oxycodone, prescribing trends, tapentadol, co-prescribing, MedicineInsight.
Introduction
The use of prescription opioids to treat pain has dramatically increased internationally during the past 20 years (Berterame et al. 2016; Islam et al. 2016; Karanges et al. 2016; Karanges et al. 2018). In Australia, dispensing of opioids increased 15-fold between 1992 and 2012 (Blanch et al. 2014) and, although the rate of this growth in use appears to have slowed in recent years, opioid dispensing has remained elevated since 2012 (Karanges et al. 2018; Lalic et al. 2019). The global growth and widespread use of opioids have been accompanied by increases in opioid-related poisonings, overdoses, misuse, and deaths (Dart et al. 2015; Martins et al. 2015), and there remains a great deal of concern around the quality use of these medicines in the community (Larance et al. 2018).
Opioids are commonly used to treat acute and malignant pain, as well as in palliative care and for treatment of opioid use disorder (Caraceni et al. 2012; Royal Australian College of General Practitioners 2017). Much of the growth in opioid use during the past two decades is attributed to the treatment of chronic non-cancer pain (CNCP) (Gisev et al. 2018a). Recent evidence has shown that the number of people initiated on opioid treatment in the community since 2013 has declined slightly, but the prevalence of opioid treatment has increased (Lalic et al. 2019), suggesting that people are using opioids for longer periods, despite the limited evidence of effectiveness of long-term treatment (Chou et al. 2015; Shaheed et al. 2016; Royal Australian College of General Practitioners 2017). Up to 80% of people receiving extended treatment with opioids develop opioid-related adverse effects (Therapeutic Guidelines 2019), and opioid-related harms, including death, have been increasing during the past 10 years (Roxburgh et al. 2017; Lam et al. 2020). There is particular concern around the use of fentanyl in the community due to concerns around misuse, as well as escalating deaths related to overdose (Roxburgh et al. 2019).
To date, the majority of research on the use of opioids in Australia has relied on dispensing claims from Australia’s national medicines subsidy provider, the Pharmaceutical Benefits Scheme (PBS) (Blanch et al. 2014; Karanges et al. 2016; Karanges et al. 2018; Lalic et al. 2019). Australia maintains universal, subsidised health care for all eligible citizens and permanent residents. General practitioner (GP) consultations are subsidised through the Medicare Benefits Schedule (MBS) (Australian Government Department of Health 2019a) and prescription medicines are subsidised through the PBS (Grove 2016). Medicines not listed on the PBS but approved for use in Australia are accessed via the non-subsidised, private medicines market (Grove 2016; Australian Government Department of Health 2019b). PBS-listed medicines can be dispensed privately if prescribed outside their subsidised indication.
Most opioids used in the community are subsidised by the PBS; however, there are substantial private prescription and, until 2018, over-the-counter (OTC) markets in Australia (estimated to account for 12% to 17% of opioid use in the country) that are not captured in PBS claims data (Islam et al. 2016; Gisev et al. 2018b). The PBS places numerous restrictions on indications and repeats (number of re-fills allowed for each issued prescription) for subsidised access to opioid agents (Table S1, available as Supplementary material to this paper). Although these PBS restrictions do not apply to private-market access, the private-market prescriptions are subject to individual State or Territory restrictions on opioid prescribing. Similarly, Australia’s medicines regulatory agency, the Therapeutic Goods Administration (TGA), also restricts access to these agents via the scheduling of medicines in the Poisons Standard (Australian Therapeutic Goods Administration 2017). For instance, in February 2018, the TGA up-scheduled OTC codeine-containing medicines to prescription-only (Schedule 4), thus there is no longer an OTC market for codeine (Australian Therapeutic Goods Administration 2018). Monitoring private-market opioid use is, therefore, essential, not only to gain a more comprehensive picture of opioid utilisation in Australia, but also to understand the impact of access restrictions on community opioid utilisation.
In this study, we used data from MedicineInsight, a sample of general practices data across Australia, to investigate recent trends in community opioid prescribing, including both publicly subsidised and private-market prescriptions. Specifically, we detail the prescribing trends for different opioid agents between 2013 and 2018, and explore how public access restrictions and scheduling changes the impact on the private prescription market. Given the widespread concern around its use, we further focus on how transdermal fentanyl patches are being prescribed in the community – examining characteristics of patients, the proportion that were opioid-naïve at the time of prescribing, and medicines co-prescribed with fentanyl patches.
Methods
Study setting and data source
We used records from the MedicineInsight database for our study. MedicineInsight has been described in detail elsewhere (Busingye et al. 2019). Briefly, MedicineInsight is a primary care database of longitudinal electronic health records (EHRs) collected from over 700 general practices across Australia, representing over 5000 GPs and ~9% of all general practices in Australia. The data captured in MedicineInsight include patient details (sex, year of birth, and postcode of residence) and prescribed medicines history (medicine name, date prescribed, number of repeats, route of administration, and whether the prescription was for a publicly subsided or private-market medicine). Prescriptions data in MedicineInsight represent prescribed but not dispensed medicines. We used records for the 5 years from 1 October 2013 to 30 September 2018.
As the number of prescriptions recorded in the data during each year is affected by the number of patients who visited a practice as well as differences in data recording such as more prescriptions being recorded electronically in later years, we report opioid prescribing rates and trends as proportions of all issued prescriptions for all medicines in each study year.
Baseline study population and opioid-treated cohort
We defined the baseline study population as those patients with valid, non-missing data for age and sex, who visited a MedicineInsight-participating general practice site at least three times during the 5-year study period. MedicineInsight is an open cohort and patients in Australia can visit multiple general practices (i.e. they are not registered with a single practice). To improve data quality, we restricted our study population to a cohort of regularly attending patients, who are more likely to be receiving most of their care at the MedicineInsight practice, thereby enabling sufficient opportunities for prescriptions, diagnoses and tests to be recorded. From this baseline population, we defined the opioid-treated cohort as those patients prescribed at least one opioid medicine of interest.
Opioids of interest
We examined non-injectable formulations of buprenorphine, codeine, fentanyl, hydromorphone, methadone, morphine, oxycodone (with and without naloxone), tramadol, and tapentadol, including their derivatives where applicable (Table S1). We excluded those buprenorphine, methadone, and opioid combination products whose sole indication in Australia is for the treatment of opioid dependence, but did not exclude the Aspen methadone product, which has a dual indication for pain and opioid dependence. We excluded codeine products used as antitussives, and injectable formulations based on the medicine brand name or route of administration. Codeine was classified as Schedule 4 (S4; prescription-only medicine) and schedule 8 (S8; controlled drugs). Codeine S4 includes mostly medicine formulations containing ≤30 mg of codeine per dosage unit compounded with one or more other therapeutically active substance (e.g. paracetamol or aspirin). We included relevant prescriptions where the route of administration was either missing or indeterminate.
Statistical analysis
We used descriptive statistics to summarise characteristics of the study cohort. We determined socioeconomic status according to the Australian Bureau of Statistics’ Socioeconomic Indexes for Areas (SEIFA) (Australian Bureau of Statistics 2018). To examine annual trends in opioid prescribing, repeat prescribing, and public- and private-market prescribing, we summed the number of prescriptions for each opioid within 12-month periods from 1 October to 30 September of the following calendar year, from October 2013 through September 2018. We considered a patient to be opioid-naïve at the time of a fentanyl patch prescription if they had at least one GP visit or other encounter recorded at the practice ≥6 months before the first fentanyl patch prescription with no evidence of prescriptions for any other opioids of interest or evidence of injectable opioid formulations and opioids indicated for opioid-dependence therapy, during the 6-month period before the first fentanyl patch prescription. We examined prescribing of fentanyl patches with: the other opioids of interest; pregabalin (ATC code: N03AX16) and gabapentin (N03AX12); antidepressants (N06A); benzodiazepines (N05BA, N05CD, N03AE), zolpidem (N05CF02) and zopiclone (N05CF01); and antipsychotic medicines (N05A). We defined co-prescribing as at least one record of a fentanyl patch prescription occurring on the same day as a prescription for at least one of the medicines listed above. To indicate the reliability of the estimates of proportions, 95% confidence intervals (CIs) adjusted for clustering by practice site were included and non-overlap of 95% CIs was used to determine significant differences between time periods or groups where appropriate (Krzywinski and Altman 2013).
Ethics approval
Approval to conduct this study was granted on 12 December 2018 by the MedicineInsight Independent Data Governance Committee (DG 2018–044). NPS MedicineWise has RACGP National Research and Evaluation Ethics Committee (NREEC) ethics approval (NREEC 17–017) for the standard operation and use of the MedicineInsight program by NPS MedicineWise.
Results
We identified 3 351 958 patients who met our inclusion criteria for the baseline study population. Of these, 14% (468 893) were prescribed at least one opioid of interest over the study period and had a median (Q1–Q3) age of 58 (42–73) years (Table 1). In the baseline study population, patients from the most socioeconomically disadvantaged areas (20%) were more likely to be prescribed opioids than those from the least disadvantaged areas (10%).
There were just over 4 million opioid prescriptions recorded during the 5-year study period, comprising ~8% of all prescriptions recorded during this time (Table S2). Oxycodone, with or without naloxone, was the most prescribed opioid during the study period, accounting for 38% of prescribed opioids, followed by tramadol, buprenorphine and codeine S4 (14% each; Table S3). Overall, the proportion of all medicine prescriptions accounted for by opioids increased by ~7% over the study period – from 7.5% in Year 1 to 8.0% in Year 5 (Table S4). The prescribing rates for individual opioid agents were relatively stable, with the exception of oxycodone, tapentadol, fentanyl patches and morphine (Fig. 1, Table S4). The proportion of all prescriptions accounted for by the fixed-dose combination of oxycodone/naloxone more than doubled over the study period (from 0.47% (95% CI: 0.41, 0.54) to 0.97% (0.86, 1.08)), whereas the proportion of prescriptions accounted for by tapentadol quadrupled between the first full year of the medicine’s availability (Year 2 of the study period) and the end of the study period (from 0.18% (0.15, 0.20) to 0.73% (0.67, 0.80)). Prescribing rates for fentanyl patches, morphine and single-agent oxycodone (without naloxone) decreased between the first and last year of the study (Fig. 1, Table S4).
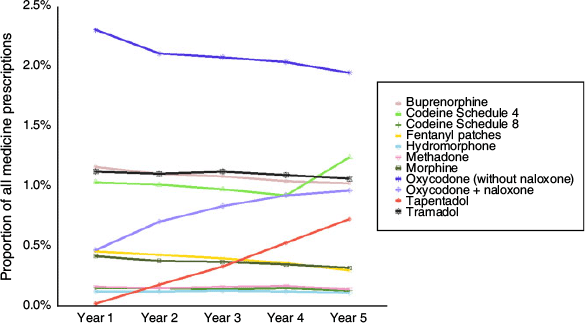
|
Most opioid prescriptions (94%) were written for publicly subsidised opioids (Table S5), but there was variation in publicly subsidised and private-market prescribing between opioid agents. Over the entire study period, methadone had the largest proportion of prescriptions written for the private market (39%; Table S5). Codeine S4 accounted for more than half (52%) of all opioid private-market prescriptions (Table S3). Notably, codeine S4, as a proportion of all private prescriptions for all medicines, more than doubled from Year 4 to Year 5 of the study period; however, this was not observed for the publicly subsidised prescriptions (Fig. 2, Table S6). The prescribing rate for both the publicly subsidised and private-market prescriptions for tapentadol increased significantly over the study period.

|
Overall, 11% of opioid prescriptions included at least one repeat (Table S7). Repeat prescriptions were more common for private-market prescriptions, where they comprised 23% of all private-market opioid prescriptions, compared with 11% of publicly subsidised opioid prescriptions. The opioids most likely to be prescribed with at least one repeat included tramadol (44%), codeine S4 (15%), and methadone (12%; Fig. 3, Table S7). Trends in repeat prescribing over the study period were relatively stable across public and private markets for most opioid agents, with the exception of tapentadol (Table S8). From Year 2 to Year 3, the proportion of private-market tapentadol prescriptions that included a repeat fell from 38% to 15%, and further declined to 11% by Year 5 (Fig. S1, Table S8).
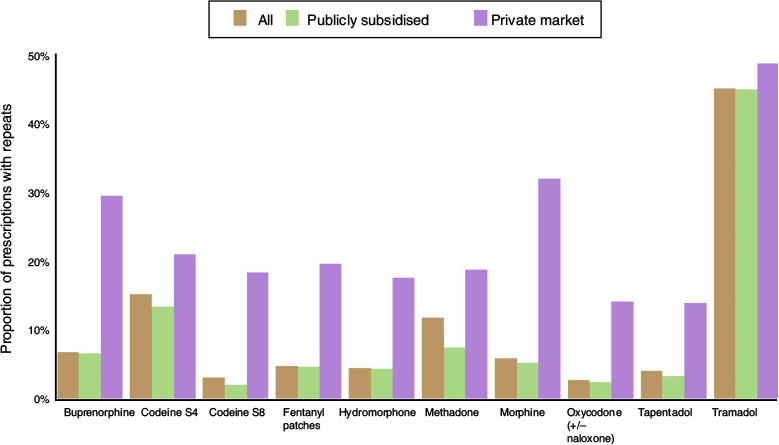
|
The proportion of all medicine prescriptions accounted for by fentanyl patches declined from 0.46% to 0.30% during the study period (Fig. 1, Table S4). Declines were also observed for both public and private markets, though <1% of fentanyl patch prescriptions were private (Tables S3, S6 and S5). Approximately 5% of fentanyl patch prescriptions included at least one repeat (Fig. 3, Table S7).
There were 15 295 patients with at least one recorded prescription for fentanyl patches in the 5-year study period, with a median age (Q1–Q3) of 75 (60–85) years (Table 2). In the baseline study population, females were more likely to have been prescribed fentanyl patches (0.5%) than males (0.4%), and the most socioeconomically disadvantaged patients (0.7%) were more likely than the least disadvantaged (0.2%) (Table 2). Among the 10 251 patients identified as being prescribed fentanyl patches for the first time during the 5-year study period, 29% were potentially opioid-naïve according to their GP record (data not shown). On at least one occasion over the 5-year study period, 49% of all patients prescribed fentanyl patches were also prescribed another opioid of interest on the same day (i.e. ‘co-prescription’). Similarly, of all patients prescribed fentanyl patches, the proportion with any co-prescription with each of ‘benzodiazepines/zolpidem/zopiclone’, ‘antidepressants’, ‘pregabalin/gabapentin’, and ‘antipsychotics’ was 28%, 28%, 18%, and 11%, respectively (Table 3).
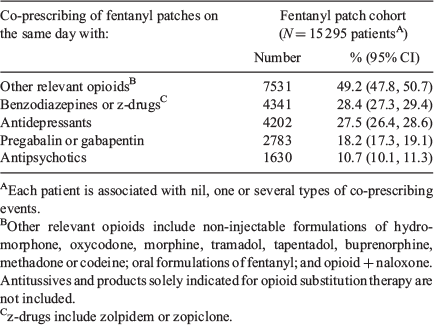
|
Discussion
Our study found that community opioid prescribing is common in Australia, with more than one in seven patients visiting a GP being prescribed an opioid medicine during the study period. Our findings highlight that prescribing behaviour has changed since 2013. Although prescribing rates for many opioid agents remained relatively constant, GP prescriptions for single-agent oxycodone, morphine, and fentanyl patches declined over the study period; and those for oxycodone/naloxone and tapentadol increased steadily.
These changes in prescribing are likely driven by several factors. The introduction and public subsidy of the fixed-dose oxycodone/naloxone combination in Australia during December 2011 resulted in increased initiations of this combination and decreasing single-agent oxycodone use (Schaffer et al. 2019). Although the combination product does not appear to have entirely replaced single-agent oxycodone, people beginning treatment with oxycodone are likely to do so with the combination product. The observed increases in prescriptions for oxycodone/naloxone and tapentadol may represent expansion of the overall opioids market in Australia. Previous research found that the introduction of the oxycodone/naloxone combination grew the oxycodone market (Schaffer et al. 2019) and is likely associated with the marketing campaigns that accompanied the introduction of each agent (Hadland et al. 2019; Woodley 2019). Earlier Australian studies using a data series that ended in 2015 reported declining or slowing rates of use for several opioid agents including tramadol and fentanyl, and our findings suggest these downward trends have continued (Karanges et al. 2016; Karanges et al. 2018). The decline in fentanyl patch prescribing we observed may be due to increasing GP awareness around the harms associated with its use (Roxburgh et al. 2017).
Our study highlights the importance of using data from the private market to fully understand how GPs prescribe opioids. Previous research has estimated that between 12% and 17% of opioid medicine utilisation (including OTC) is not publicly subsidised and, therefore, not captured in PBS data (Islam et al. 2016; Gisev et al. 2018b). Currently, private-market and OTC medicines data are not widely available in Australia, making post-market surveillance of private-market opioid use challenging. In 2012, the Pharmacy Guild Survey, which collected medicines dispensing data from a representative sample of Australian pharmacies, including private-market dispensing, was discontinued (Australian Government Department of Health 2012), and since that time, studies have used national sales data to explore private market use. Sales data have been a valuable research tool when paired with PBS dispensing data (Gisev et al. 2018b; Cairns et al. 2020); however, these data are aggregate and include opioids used in treating public hospital inpatients, obscuring private-market opioid use in the community. In light of these data gaps, our study using MedicineInsight data provides valuable information around community opioid use and how it shifts between markets in Australia.
Our results suggest that the private opioid medicines market in Australia may be growing. In our study, private-market opioid scripts accounted for just 6% of all opioid prescriptions, but increased considerably during the last year of the study period. Low-strength, OTC codeine-containing medicines were up-scheduled to prescription-only in 2018 and we observed a rapid increase in codeine prescribing in the private market during that year (Australian Government Department of Health 2016). This likely relates to people who previously bought low-dose codeine over the counter, wished to continue after the up-scheduling, and are now doing so under the care of their GPs. Prior to the scheduling change, there was a fear that people previously taking low-dose codeine may change to high-dose codeine following the up-scheduling; however, early evaluations of the impact of the change have found decreased codeine-related harms (Cairns et al. 2020).
Although the PBS restrictions do not allow repeats on issued prescriptions for most opioids, the finding that tramadol prescriptions were most likely to have at least one repeat may reflect the former PBS allowance of two repeat prescriptions for the 50 mg capsule (formerly PBS item code – 8611F) (Australian Government Department of Health 2020), which was also the most prescribed strength of tramadol in this study. However, following the June 2020 update of the PBS restrictions, this PBS item no longer exists.
Our results showed that fentanyl patch prescribing declined between 2013 and 2018, but there were possible concerning findings around fentanyl patch prescribing in our study. First, nearly one-third of fentanyl patch prescriptions were potentially for opioid-naïve patients when they began fentanyl treatment. Due to the potency of fentanyl and risk of overdose in opioid-naïve individuals, Australian guidelines recommend against using fentanyl as a first opioid treatment (NPS MedicineWise 2006). Polypharmacy in chronic non-cancer pain has been previously documented, and although multimodal analgesia, including gabapentinoids, may play a role in care, there is limited evidence for benzodiazepines or multiple opioid analgesia and any benefits must be balanced against increasing risk of harm (Giummarra et al. 2015). Same-day fentanyl patch co-prescribing with other opioids, benzodiazepines, and gabapentanoids appeared to be fairly common. This may indicate room for improvement, as a recent Australian study found that one-quarter of fentanyl-related deaths involved benzodiazepines and two-thirds of deaths involving multiple opioids involved benzodiazepines (Roxburgh et al. 2019). Some patients using fentanyl patches initially may require supplemental doses of a short-acting opioid for ‘breakthrough’ pain (Therapeutic Guidelines 2019).
The variation in prescribing of opioids by patient characteristics, such as socioeconomic status and age, is consistent with evidence from Australia (Degenhardt et al. 2016; Islam et al. 2018; Islam and Wollersheim 2018). The high prescribing rate of opioids among older patients may reflect high prevalence of chronic pain and pain-related conditions such as arthritis or malignancy in older age groups. The finding that prescribing of opioids was high among socioeconomically disadvantaged groups may suggest that opioid prescribing is a surrogate for inadequate pain management due to limited access to multimodal and multidisciplinary pain interventions, including health professionals such as GPs, nurses, psychologists, physiotherapists, osteopaths and pharmacists (Finestone et al. 2016). Also, it has been shown that the prevalence of chronic pain conditions and mental disorders is high in individuals with low socioeconomic status (Blyth et al. 2001; Poleshuck and Green 2008).
Strengths and limitations
Our study comprised a sample of data from general practices across Australia that allowed for the examination of private-market prescribing, which is often lacking in Australian opioid utilisation studies. This analysis has limitations inherent in EHRs, as previously described (Busingye et al. 2019). Information on medicines prescribed in hospitals and/or by specialists and OTC is not captured in our data and our estimates of the number of opioid-naïve patients may be higher than the true value. Defining co-prescribing as prescriptions prescribed on the same day may have underestimated the true level of co-prescribing, but provides an indication of the minimum level of co-prescribing.
Conclusions
Our study highlights that opioid treatment is dynamic and complex. As the use of some agents declines, use of others increases. The private-medicines market is a small but growing component of opioid use in Australia and one way in which prescribers and patients can avoid access restrictions in the public market for these medicines. Finally, although fentanyl patch prescribing has declined in recent years, there is room for improvement in prescribing fentanyl patches among opioid-naïve patients, as well as co-prescribing of fentanyl patches with other sedatives.
Conflicts of interest
The authors DB, AP, JBe, KC and SB are employees of NPS MedicineWise, the data custodian of MedicineInsight. BD and JBr have no conflicts of interest to declare.
Declaration of funding
This study was funded by the Australian Government Department of Health.
Acknowledgements
We are grateful to the general practices, general practitioners and patients who allow the use of de‐identified information for MedicineInsight. We thank NPS MedicineWise staff who have provided valuable input.
References
Australian Bureau of Statistics (ABS) (2018) Socio-Economic Indexes for Areas. (ABS: Canberra, ACT, Australia) Available at http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa [Verified 7 November 2019]Australian Government Department of Health (2012) Australian Statistics on Medicines 2012. Available at http://www.pbs.gov.au/info/statistics/asm/asm-2012 [Verified 25 November 2019]
Australian Government Department of Health (2016) Final decisions and reasons for decisions by delegates of the Secretary to the Department of Health Canberra.
Australian Government Department of Health (2019a) MBS Online: Medicare Benefits Schedule. Available at http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home [Verified 28 November 2019]
Australian Government Department of Health (2019b) PBS Frequently Asked Questions. Available at http://www.pbs.gov.au/info/general/faq [Verified 28 November 2019]
Australian Government Department of Health (2020) Pharmacist claiming of tramadol 50mg capsule prescriptions from 1 June 2020. Available at https://www.pbs.gov.au/pbs/news/2020/06/pharmacist-claiming-of-tramadol-50mg [Verified 19 May 2021]
Australian Therapeutic Goods Administration (2017) Scheduling Basics. Available at https://www.tga.gov.au/scheduling-basics [Verified 6 December 2019]
Australian Therapeutic Goods Administration (2018) Codeine information hub. Available at https://www.tga.gov.au/codeine-info-hub [Verified 6 December 2019]
Berterame S, Erthal J, Thomas J, Fellner S, Vosse B, Clare P, Hao W, Johnson DT, Mohar A, Pavadia J, Samak AKE, Sipp W, Sumyai V, Suryawati S, Toufiq J, Yans R, Mattick RP (2016) Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet 387, 1644–1656.
| Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study.Crossref | GoogleScholarGoogle Scholar | 26852264PubMed |
Blanch B, Pearson SA, Haber PS (2014) An overview of the patterns of prescription opioid use, costs and related harms in Australia. British Journal of Clinical Pharmacology 78, 1159–1166.
| An overview of the patterns of prescription opioid use, costs and related harms in Australia.Crossref | GoogleScholarGoogle Scholar | 24962372PubMed |
Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ (2001) Chronic pain in Australia: a prevalence study. Pain 89, 127–134.
| Chronic pain in Australia: a prevalence study.Crossref | GoogleScholarGoogle Scholar | 11166468PubMed |
Busingye D, Gianacas C, Pollack A, Chidwick K, Merrifield A, Norman S, Mullin B, Hayhurst R, Blogg S, Havard A, Stocks N (2019) Data Resource Profile: MedicineInsight, an Australian national primary health care database. International Journal of Epidemiology 48, 1741–1741h.
| Data Resource Profile: MedicineInsight, an Australian national primary health care database.Crossref | GoogleScholarGoogle Scholar | 31292616PubMed |
Cairns R, Schaffer AL, Brown JA, Pearson S-A, Buckley NA (2020) Codeine use and harms in Australia: evaluating the effects of re-scheduling. Addiction (Abingdon, England) 115, 451–459.
| Codeine use and harms in Australia: evaluating the effects of re-scheduling.Crossref | GoogleScholarGoogle Scholar |
Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, Dale O, De Conno F, Fallon M, Hanna M, Haugen DF, Juhl G, King S, Klepstad P, Laugsand EA, Maltoni M, Mercadante S, Nabal M, Pigni A, Radbruch L, Reid C, Sjogren P, Stone PC, Tassinari D, Zeppetella G (2012) Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. The Lancet. Oncology 13, e58–e68.
| Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC.Crossref | GoogleScholarGoogle Scholar | 22300860PubMed |
Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA (2015) The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Annals of Internal Medicine 162, 276–286.
| The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop.Crossref | GoogleScholarGoogle Scholar | 25581257PubMed |
Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL (2015) Trends in Opioid Analgesic Abuse and Mortality in the United States. The New England Journal of Medicine 372, 241–248.
| Trends in Opioid Analgesic Abuse and Mortality in the United States.Crossref | GoogleScholarGoogle Scholar | 25587948PubMed |
Degenhardt L, Gisev N, Cama E, Nielsen S, Larance B, Bruno R (2016) The extent and correlates of community-based pharmaceutical opioid utilisation in Australia. Pharmacoepidemiology and Drug Safety 25, 521–538.
| The extent and correlates of community-based pharmaceutical opioid utilisation in Australia.Crossref | GoogleScholarGoogle Scholar | 26781123PubMed |
Finestone HM, Juurlink DN, Power B, Gomes T, Pimlott N (2016) Opioid prescribing is a surrogate for inadequate pain management resources. Canadian Family Physician Medecin de Famille Canadien 62, 465–468.
Gisev N, Pearson S-A, Dobbins T, Currow DC, Blyth F, Larney S, Dunlop A, Mattick RP, Wilson A, Degenhardt L (2018a) Combating escalating harms associated with pharmaceutical opioid use in Australia: the POPPY II study protocol. BMJ Open 8, e025840
| Combating escalating harms associated with pharmaceutical opioid use in Australia: the POPPY II study protocol.Crossref | GoogleScholarGoogle Scholar | 30518593PubMed |
Gisev N, Pearson SA, Karanges EA, Larance B, Buckley NA, Larney S, Dobbins T, Blanch B, Degenhardt L (2018b) To what extent do data from pharmaceutical claims under-estimate opioid analgesic utilisation in Australia? Pharmacoepidemiology and Drug Safety 27, 550–555.
| To what extent do data from pharmaceutical claims under-estimate opioid analgesic utilisation in Australia?Crossref | GoogleScholarGoogle Scholar | 29047196PubMed |
Giummarra MJ, Gibson SJ, Allen AR, Pichler AS, Arnold CA (2015) Polypharmacy and chronic pain: harm exposure is not all about the opioids. Pain Medicine 16, 472–479.
| Polypharmacy and chronic pain: harm exposure is not all about the opioids.Crossref | GoogleScholarGoogle Scholar | 25280054PubMed |
Grove A (2016) The Pharmaceutical Benefits Scheme: a quick guide. Available at https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/pubs/rp/rp1516/Quick_Guides/PBS [Verified 28 November 2019]
Hadland SE, Rivera-Aguirre A, Marshall BDL, Cerdá M (2019) Association of pharmaceutical industry marketing of opioid products with mortality from opioid-related overdoses. JAMA Network Open 2, e186007
| Association of pharmaceutical industry marketing of opioid products with mortality from opioid-related overdoses.Crossref | GoogleScholarGoogle Scholar | 30657529PubMed |
Islam MM, Wollersheim D (2018) Variation in prescription opioid dispensing across neighborhoods of diverse socio-economic disadvantages in Victoria, Australia. Pharmaceuticals (Basel, Switzerland) 11, 116
| Variation in prescription opioid dispensing across neighborhoods of diverse socio-economic disadvantages in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Islam MM, McRae IS, Mazumdar S, Taplin S, McKetin R (2016) Prescription opioid analgesics for pain management in Australia: 20 years of dispensing. Internal Medicine Journal 46, 955–963.
| Prescription opioid analgesics for pain management in Australia: 20 years of dispensing.Crossref | GoogleScholarGoogle Scholar | 26602489PubMed |
Islam MM, McRae IS, Mazumdar S, Simpson P, Wollersheim D, Fatema K, Butler T (2018) Prescription opioid dispensing in New South Wales, Australia: spatial and temporal variation. BMC Pharmacology & Toxicology 19, 30
| Prescription opioid dispensing in New South Wales, Australia: spatial and temporal variation.Crossref | GoogleScholarGoogle Scholar |
Karanges EA, Blanch B, Buckley NA, Pearson S-A (2016) Twenty-five years of prescription opioid use in Australia: a whole-of-population analysis using pharmaceutical claims. British Journal of Clinical Pharmacology 82, 255–267.
| Twenty-five years of prescription opioid use in Australia: a whole-of-population analysis using pharmaceutical claims.Crossref | GoogleScholarGoogle Scholar | 26991673PubMed |
Karanges EA, Buckley NA, Brett J, Blanch B, Litchfield M, Degenhardt L, Pearson S-A (2018) Trends in opioid utilisation in Australia, 2006–2015: insights from multiple metrics. Pharmacoepidemiology and Drug Safety 27, 504–512.
| Trends in opioid utilisation in Australia, 2006–2015: insights from multiple metrics.Crossref | GoogleScholarGoogle Scholar | 29280224PubMed |
Krzywinski M, Altman N (2013) Points of significance: error bars. Nature Methods 10, 921–922.
| Points of significance: error bars.Crossref | GoogleScholarGoogle Scholar | 24161969PubMed |
Lalic S, Ilomäki J, Bell JS, Korhonen MJ, Gisev N (2019) Prevalence and incidence of prescription opioid analgesic use in Australia. British Journal of Clinical Pharmacology 85, 202–215.
| Prevalence and incidence of prescription opioid analgesic use in Australia.Crossref | GoogleScholarGoogle Scholar | 30338545PubMed |
Lam T, Kuhn L, Hayman J, Middleton M, Wilson J, Scott D, Lubman DI, Smith K, Nielsen S (2020) Recent trends in heroin and pharmaceutical opioid-related harms in Victoria, Australia up to 2018. Addiction (Abingdon, England) 115, 261–269.
| Recent trends in heroin and pharmaceutical opioid-related harms in Victoria, Australia up to 2018.Crossref | GoogleScholarGoogle Scholar |
Larance B, Degenhardt L, Peacock A, Gisev N, Mattick R, Colledge S, Campbell G (2018) Pharmaceutical opioid use and harm in Australia: The need for proactive and preventative responses. Drug and Alcohol Review 37, S203–S205.
| Pharmaceutical opioid use and harm in Australia: The need for proactive and preventative responses.Crossref | GoogleScholarGoogle Scholar | 29024092PubMed |
Martins SS, Sampson L, Cerda M, Galea S (2015) Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature. American Journal of Public Health 105, e29–e49.
| Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature.Crossref | GoogleScholarGoogle Scholar | 26451760PubMed |
NPS MedicineWise (2006) Fentanyl patches (Durogesic) for chronic pain. Available at https://www.nps.org.au/radar/articles/fentanyl-patches-durogesic-for-chronic-pain [Verified 9 March 2020]
Poleshuck EL, Green CR (2008) Socioeconomic disadvantage and pain. Pain 136, 235–238.
| Socioeconomic disadvantage and pain.Crossref | GoogleScholarGoogle Scholar | 18440703PubMed |
Roxburgh A, Hall WD, Dobbins T, Gisev N, Burns L, Pearson S, Degenhardt L (2017) Trends in heroin and pharmaceutical opioid overdose deaths in Australia. Drug and Alcohol Dependence 179, 291–298.
| Trends in heroin and pharmaceutical opioid overdose deaths in Australia.Crossref | GoogleScholarGoogle Scholar | 28826104PubMed |
Roxburgh A, Hall WD, Gisev N, Degenhardt L (2019) Characteristics and circumstances of heroin and pharmaceutical opioid overdose deaths: Comparison across opioids. Drug and Alcohol Dependence 205, 107533
| Characteristics and circumstances of heroin and pharmaceutical opioid overdose deaths: Comparison across opioids.Crossref | GoogleScholarGoogle Scholar | 31704378PubMed |
Royal Australian College of General Practitioners (RACGP) (2017) Prescribing drugs of dependence in general practice, Part C2: The role of opioids in pain management. (RACGP: Melbourne, Vic., Australia) Available at https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Guidelines/Drugs%20of%20dependence/Part-C2-The-role-of-opioids-in-pain-management.PDF [Verified 6 February 2019]
Schaffer AL, Karanges EA, Buckley NA, Wilson A, Degenhardt L, Larance B, Pearson SA (2019) Increases in controlled-release oxycodone utilisation following the subsidy of oxycodone with naloxone formulations: An Australian population-based study. Pharmacoepidemiology and Drug Safety 28, 97–105.
| Increases in controlled-release oxycodone utilisation following the subsidy of oxycodone with naloxone formulations: An Australian population-based study.Crossref | GoogleScholarGoogle Scholar | 30421838PubMed |
Shaheed CA, Maher CG, McLachlan A (2016) Investigating the efficacy and safety of over-the-counter codeine containing combination analgesics for pain and codeine based antitussives. Available at https://www.tga.gov.au/alert/review-efficacy-and-safety-over-counter-codeine-combination-medicines [Verified 22 July 2020]
Therapeutic Guidelines (2019) Chronic pain: pharmacological management. Available at https://tgldcdp.tg.org.au/viewTopic?topicfile=chronic-pain-pharmacological-management [Verified 29 November 2019]
Woodley M (2019) Mundipharma fined for misleading advertising of opioids. Available at https://www1.racgp.org.au/newsgp/professional/mundipharma-fined-for-misleading-advertising-of-op [Verified 23 March 2020]




