Apparent absence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) in frogs in Malaita Province, Solomon Islands
Maasafi Alabai A B , Tommy Esau A B , Esau Kekeubata A B , Dorothy Esau A B , Jackson Waneagea A , Lamanai’a Lobotalau A , James Alick A , John Silas A , Ledison Solome A , Jimson Waneagea A , Kwai’ikwala Mousisi A , Timothy P. Cutajar C , Christopher D. Portway C , David J. MacLaren D and Jodi J. L. Rowley C E F
C E F
A Kwainaa’isi Cultural Centre, Atoifi Postal Agency, East Kwaio, Malaita Province, Solomon Islands.
B Baru Conservation Alliance, Atoifi Postal Agency, East Kwaio, Malaita Province, Solomon Islands.
C Australian Museum Research Institute, Australian Museum, 1 William Street, Sydney, NSW 2010, Australia.
D College of Medicine and Dentistry, James Cook University, Cairns, Qld 4870, Australia.
E Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, UNSW Sydney, Biological Sciences Building (D26), Randwick, NSW 2052, Australia.
F Corresponding author. Email: jodi.rowley@unsw.edu.au
Pacific Conservation Biology - https://doi.org/10.1071/PC20047
Submitted: 28 May 2020 Accepted: 19 October 2020 Published online: 13 November 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
A major driver of global biodiversity loss is disease. One of the most devastating wildlife diseases known is chytridiomycosis, which is caused by the amphibian chytrid fungus Batrachochytrium dendrobatidis, and is implicated in population declines in over 500 frog species. Thought to originate in Asia, B. dendrobatidis now has a global distribution, likely due to human movement and trade. The pathogen has yet to be detected in Melanesia, but there have been few surveys for B. dendrobatidis in the region, and none in the Solomon Islands archipelago, a biogeographic region with a unique and culturally important frog fauna. We swabbed 200 frogs of eight species in three genera in lowland and highland sites in East Kwaio on the island of Malaita in the Solomon Islands. All frogs tested negative for the pathogen but it is possible that the pathogen is present despite non-detection, so further surveys for the pathogen are needed throughout the country. Despite this, it is safest to take a precautionary approach and assume that B. dendrobatidis has not yet been introduced to the Solomon Islands, and that naïve native amphibian populations may be at risk of decline if the pathogen is introduced. Protocols are needed to prevent the accidental import of infected frogs via tourism or in logging or mining equipment. Monitoring of frog populations near areas of high risk such as ports is also recommended. The frogs of the Solomon Islands archipelago are biologically unique and culturally significant, and protecting them from the potentially devastating impacts of B. dendrobatidis is vital.
keywords: amphibian, bacteria, biodiversity loss, biosecurity, chytridiomycosis, Cornufer guentheri, Cornufer guppyi, Cornufer hedigeri, Cornufer solomonis, Cornufer vertebralis, East Kwaio, frog, fungus, Litoria lutea, Litoria thesaurensis, Papurana krefftii, pathogen, Solomon Islands, wildlife disease.
Introduction
A significant driver of global biodiversity loss is disease, and the most devastating wildlife disease known to date is chytridiomycosis (Fisher et al. 2012; Scheele et al. 2019). Caused by the amphibian chytrid fungus (Batrachochytrium dendrobatidis) (Berger et al. 1998; Longcore et al. 1999), chytridiomycosis is responsible for the greatest recorded loss of biodiversity attributable to a disease, driving declines in over 500 species (Scheele et al. 2019). Batrachochytrium dendrobatidis is thought to have originated in Asia (O’hanlon et al. 2018) but is now distributed across the globe (Scheele et al. 2019). Human movements and commercial trade have been linked to international movement of B. dendrobatidis (Fisher and Garner 2007; Schloegel et al. 2009; Farrer et al. 2011). Despite its near-ubiquitous distribution, B. dendrobatidis has yet to be detected in Melanesia, with surveys of frogs in Papua New Guinea (Swei et al. 2011; Dahl et al. 2012; Bower et al. 2020) and Fiji (Narayan et al. 2011) failing to detect the pathogen.
The Solomon Islands is a country comprised of six major islands and over 900 smaller islands east of Papua New Guinea. The Solomon Islands and the biogeographically aligned Bougainville and Buka Islands of Papua New Guinea (collectively known as the Solomon Islands archipelago) has a unique frog fauna, with 16 of the 19 known species endemic to them (Pikacha et al. 2008). The second largest (and most heavily populated) island in the country is Malaita, covering 4300 km2. Frogs are known to be culturally significant on Malaita, and different species may be used as food, traditional medicine and as totem animals (Pollard et al. 2015). For the Kwaio community, particularly those living away from the coast, frogs are an important source of protein and are strongly incorporated into cultural beliefs. As part of collaborative biodiversity research led by the Kwaio community (Alabai et al. 2019; Callaghan et al. 2019; Lavery et al. 2018), we conducted a survey for B. dendrobatidis in East Kwaio on Malaita, the first published survey for the pathogen in the Solomon Islands and in the Solomon Islands archipelago.
Methods
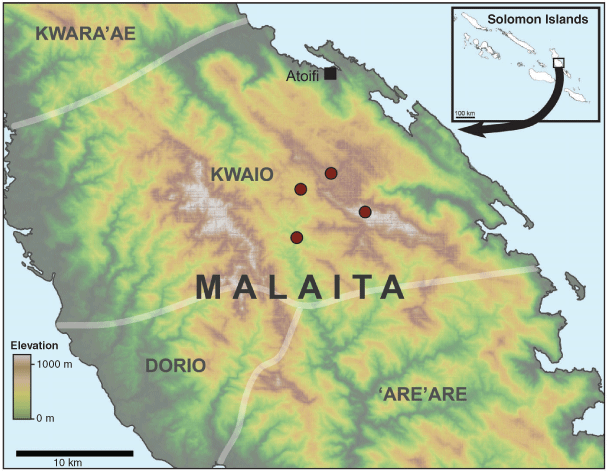
From 20 to 25 July 2019 we surveyed for frogs along streams and in rainforest at four sites from 285 to 1155 m elevation in East Kwaio, Malaita (Fig. 1); Kwainaa’asi (−8.946, 161.011, 920 m elevation), Alalau Fulanitofe (−8.976, 161.037, 1155 m elevation), Aifasu (−8.995, 160.984, 285 m elevation) and Kafurumu (−8.958, 160.987, 460 m elevation). Lowland sites were a mosaic of gardens and rainforest, while highland sites were rainforest and montane cloud forest. The region has a wet equatorial tropical environment, with high rainfall and relatively constant temperatures. The average temperature is 27°C near the coast (Bradbury et al. 2017).
|
During nocturnal fieldwork, each frog was captured by hand and placed in plastic bags, before swabbing a total of 30 times (five strokes along each side of the abdominal area, five strokes along the underside of each thigh, and five strokes along the underside of each foot including the digits) using a MW100 swab (Medical Wire and Equipment, Corsham, UK). Samples were transferred to −20°C within 14 days before testing with diagnostic qPCR using Taqman chemistry (Boyle et al. 2004; Hyatt et al. 2007).
The tip of each swab was removed and added to a 2-mL Sarstedt screw-capped tube containing 40 mg of 0.5 mm diameter Zirconium/silica beads (Qiagen) and 100-µL PrepMan™ Ultra Sample Preparation Reagent (Applied Biosystems). Samples were vortexed for 10 s then homogenised in a Qiagen Tissuelyser 11 (2 × 45 s @ 30 cycles/s). Samples were then centrifuged (1 min @ 18407g) and incubated at 100°C for 10 min, then 20 µL of supernatant was extracted from each sample tube and placed into a new sterile 1.5 mL microcentrifuge tube. Samples were diluted 1/10 and the aliquots stored at −20°C before thermal cycling (modified from Hyatt et al. 2007).
Each swab was analysed in singlicate. The qPCR reaction contained 1 × SensiFAST Probe Lo-ROX Mix (Bioline Australia), 200 nM ITS1–3 Fwd primer (CCT TGA TAT AAT ACA GTG TGC CAT ATG TC), 200 nM 5.8S Rev. primer (AGC CAA GAG ATC CGT TGT CAA A) (Boyle et al. 2004), 1 unit of TaqMan™ ChytrMGB2 FAM probe (Applied Biosystems), 1 × TaqMan™ Exogenous Internal Positive Control DNA (IPC-Vic. probe) and 1 × Exogenous IPC reagent mix (Applied Biosystems). The Exogenous IPC control was used to monitor PCR reaction inhibition. Triplicate No Amplification Controls (NAC) were included using 1 × TaqMan™ IPC PCR blocking reagent. Triplicate No Template Controls (NTC) were used to monitor probe degradation.
A synthetic ITS-1 gBlocks® gene fragment from Integrated DNA Technologies Inc. (IDT) was used to generate the qPCR standards (Rebollar et al. 2017; J. Kerby, unpubl. data). Five log10 dilutions ranging from 109 to 101 copies were set up in triplicate for generation of the ITS-1 Standard curve used for quantification of ITS-1 copy numbers. The qPCR reactions were run on an ABI Quantstudio3 qPCR Machine and analysed using QuantStudio Design and Analysis Software (ver. 1.4.1), with a sample considered Bd positive if the number of ITS-1 copies amplified was greater than zero (Briggs et al. 2010; DiRenzo et al. 2018).
A subset of specimens swabbed were taken as voucher specimens and identified to species via morphological and molecular analysis. For swabs from individuals that were not collected, 38 were identified to genus only as the Kwaio names used on some swabs encompassed several species. For example, frogs in the genus Litoria are not differentiated in Kwaio language, and so for samples of Litoria lutea and Litoria thesaurensis taken from non-vouchered individuals, they were recorded simply as Litoria spp.
Exact binomial 95% confidence intervals for infection prevalence were computed using the method of Clopper and Pearson (1934).
Results
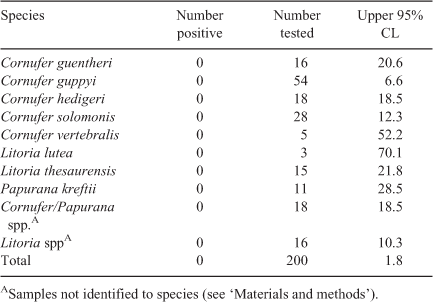
We swabbed 200 individuals of eight species belonging to three genera (Table 1), representing all native frog species observed during surveys and 36% of the known number of species in the archipelago (22 including the introduced Cane Toad Rhinella marina; Pikacha et al. 2008). All samples tested negative for B. dendrobatidis, giving an overall upper 95% confidence limit for prevalence of 1.8% at the time of sampling (Table 1).
|
Discussion
This study reports the results of the first survey for the amphibian chytrid fungus B. dendrobatidis in the Solomon Islands, and is one of few published studies that has attempted to document the pathogen elsewhere in Melanesia. To date, there is no evidence of B. dendrobatidis infecting frog species anywhere in Melanesia.
Although we cannot rule out the presence of B. dendrobatidis at the survey sites in East Kwaio, Malaita, if we assume that all species are equally likely to carry the infection, the theoretical upper 95% confidence limit for prevalence is 1.8% at the time of sampling. Although susceptibility is likely to differ among species, the theoretical upper 95% confidence limits for prevalence are still very low in the most sampled species (i.e. 6.6% in Cornufer guppyi). In addition, our surveys included rainforest streams at high elevations (>900 m elevation), potentially suitable environmental conditions for B. dendrobatidis (Woodhams and Alford 2005; Bielby et al. 2008).
The majority of frogs swabbed were in the genus Cornufer (family Ceratobatrachidae). Species in this family of direct-developing frogs have yet to be reported as infected by B. dendrobatidis. Despite the inherently lower likelihood of exposure to B. dendrobatidis within direct-developing frogs, population declines and extinctions attributed to the pathogen are documented in frogs with similar life-histories elsewhere, and direct-developing species may actually be more susceptible to the pathogen (Mesquita et al. 2017).
We also swabbed frogs in the genera Litoria and Papurana. In Australia, many species in the genus Litoria have been reported with B. dendrobatidis infection. Twenty-five have experienced population declines attributed to the pathogen, with declines in three species being greater than 90% (Scheele et al. 2019). As a result, if B. dendrobatidis is truly absent from the Solomon Islands archipelago, its arrival may pose a particular threat to species in the genus Litoria. However, the true susceptibility of the frog fauna of the Solomon Islands to B. dendrobatidis remains unknown, and is likely to be influenced by various factors including evolutionary history, and differences in life-histories, behaviour, innate immunity (including skin microbiome antimicrobial peptides), and environmental conditions (Rowley and Alford 2009).
Our failure to detect B. dendrobatidis during our surveys may have several explanations. The pathogen may be truly absent from the Solomon Islands archipelago, it may be patchily distributed or otherwise absent from our study sites, yet present elsewhere, or it may be present at our study sites, but at such low infection intensities and/or prevalence that it remained undetected. Further surveys for the pathogen are necessary across the Solomon Islands archipelago, but a precautionary approach should be taken – management strategies and disease surveillance protocols that assume B. dendrobatidis has not yet been introduced to the Solomon Islands archipelago and that native amphibians may be at risk of impact if the fungus is introduced, should be implemented.
We echo recent calls for action for an international, multi-disciplinary approach to reduce the chances of the pathogen being imported into Melanesia, and limit its impact if it is (Bower et al. 2019, 2020). Strategies should focus on preventing its importation, including via tourism, logging, and mining activities. The development of protocols to prevent escape or release of any imported frogs into the wild, including any stowaways on imported cargo (sensu Pili et al. 2019), and testing frog populations near areas of high risk (i.e. ports) for B. dendrobatidis is recommended.
The Solomon Islands archipelago is home to a unique frog fauna. Although there is limited western scientific knowledge of this fauna, there is extensive traditional knowledge that demonstrates the importance amphibians have in traditional livelihoods and customs. Protecting this fauna from the devastating impacts of disease is vital.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
For their hospitality and hard work in the field we are indebted to Bukele, Taawa’i, Etaalamo, Faaisia, Fifanabe’u, Fotageni, Fou’asua, Jimson, John, Kotoringi, Laete’esafi, Lamanai’a, Lengari’i, Loni, Lo’ubata, Maageni, Naata, Nabe, Rubeamae, Sale, Susufia, Tagwa, Telegeni and Wedi. Funding for our fieldwork was provided by the Australian Museum Foundation (AMF), to whom we are extremely grateful. The AMF had no role in the preparation of the data or manuscript or the decision to submit for publication. An Animal Research Authority (approval 17-01) was provided by the Australian Museum Animal Ethics Committee in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes (NHMRC 2013). A research permit (RP/2018/003) and export permit (EX2019/077) were granted by the Solomon Islands Government.
References
Alabai, M., Esau, T., Kekeubata, E., Waneagea, J., MacLaren, D., Major, R., and Callaghan, C. (2019). First record of Solomons Nightjar Eurostopodus nigripennis for Malaita, with a description of its nest site. Bulletin of the British Ornithologists’ Club 139, 325–327.| First record of Solomons Nightjar Eurostopodus nigripennis for Malaita, with a description of its nest site.Crossref | GoogleScholarGoogle Scholar |
Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., Slocombe, R., Ragan, M. A., Hyatt, A. D., McDonald, K. R., and Hines, H. B. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences 95, 9031–9036.
| Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America.Crossref | GoogleScholarGoogle Scholar |
Bielby, J., Cooper, N., Cunningham, A., Garner, T. W., and Purvis, A. (2008). Predicting susceptibility to future declines in the world’s frogs. Conservation Letters 1, 82–90.
| Predicting susceptibility to future declines in the world’s frogs.Crossref | GoogleScholarGoogle Scholar |
Bower, D. S., Lips, K. R., Amepou, Y., Richards, S., Dahl, C., Nagombi, E., Supuma, M., Dabek, L., Alford, R. A., Schwarzkopf, L., and Ziembicki, M. (2019). Island of opportunity: can New Guinea protect amphibians from a globally emerging pathogen? Frontiers in Ecology and the Environment 17, 348–354.
| Island of opportunity: can New Guinea protect amphibians from a globally emerging pathogen?Crossref | GoogleScholarGoogle Scholar |
Bower, D. S., Jennings, C. K., Webb, R. J., Amepou, Y., Schwarzkopf, L., Berger, L., Alford, R. A., Georges, A., McKnight, D. T., Carr, L., and Nason, D. (2020). Disease surveillance of the amphibian chytrid fungus Batrachochytrium dendrobatidis in Papua New Guinea. Conservation Science and Practice 2020, e256.
| Disease surveillance of the amphibian chytrid fungus Batrachochytrium dendrobatidis in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Boyle, D. G., Boyle, D. B., Olsen, V., Morgan, J. A. T., and Hyatt, A. D. (2004). Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms 60, 141–148.
| Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay.Crossref | GoogleScholarGoogle Scholar | 15460858PubMed |
Bradbury, R. S., Hii, S. F., Harrington, H., Speare, R., and Traub, R. (2017). Ancylostoma ceylanicum hookworm in the Solomon Islands. Emerging Infectious Diseases 23, 252.
| Ancylostoma ceylanicum hookworm in the Solomon Islands.Crossref | GoogleScholarGoogle Scholar | 28098526PubMed |
Briggs, C. J., Knapp, R. A., and Vredenburg, V. T. (2010). Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proceedings of the National Academy of Sciences 107, 9695–9700.
| Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians.Crossref | GoogleScholarGoogle Scholar |
Callaghan, C. T., Kekeubata, E., Waneagea, J., Alabai, M., Esau, T., MacLaren, D., and Major, R. E. (2019). A collaborative bird survey of East Kwaio, Malaita, Solomon Islands. Check List 15, 1119–1136.
| A collaborative bird survey of East Kwaio, Malaita, Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Clopper, C. J., and Pearson, E. S. (1934). The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26, 404–413.
| The use of confidence or fiducial limits illustrated in the case of the binomial.Crossref | GoogleScholarGoogle Scholar |
Dahl, C., Kiatik, I., Baisen, I., Bronikowski, E., Fleischer, R. C., Rotzel, N. C., Lock, J., Novotny, V., Narayan, E., and Hero, J. M. (2012). Batrachochytrium dendrobatidis not found in rainforest frogs along an altitudinal gradient of Papua New Guinea. The Herpetological Journal 22, 183–186.
DiRenzo, G. V., Grant, E., Longo, A., Che-Castaldo, C., Zamudio, K., and Lips, K. (2018). Imperfect pathogen detection from non‐invasive skin swabs biases disease inference. Methods in Ecology and Evolution 9, 380–289.
| Imperfect pathogen detection from non‐invasive skin swabs biases disease inference.Crossref | GoogleScholarGoogle Scholar |
Farrer, R. A., Weinert, L. A., Bielby, J., Garner, T. W., Balloux, F., Clare, F., Bosch, J., Cunningham, A. A., Weldon, C., du Preez, L. H., and Anderson, L. (2011). Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proceedings of the National Academy of Sciences 108, 18732–18736.
| Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage.Crossref | GoogleScholarGoogle Scholar |
Fisher, M. C., and Garner, T. W. J. (2007). The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biology Reviews 21, 2–9.
| The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species.Crossref | GoogleScholarGoogle Scholar |
Fisher, M. C., Henk, D. A., Briggs, C. J., Brownstein, J. S., Madoff, L. C., McCraw, S. L., and Gurr, S. J. (2012). Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186.
| Emerging fungal threats to animal, plant and ecosystem health.Crossref | GoogleScholarGoogle Scholar | 22498624PubMed |
Hyatt, A. D., Boyle, D. G., Olsen, V., Boyle, D. B., Berger, L., Obendorf, D., Dalton, A., Kriger, K., Heros, M., Hines, H., Phillott, R., Campbell, R., Marantelli, G., Gleason, F., and Coiling, A. (2007). Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 73, 175–192.
| Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis.Crossref | GoogleScholarGoogle Scholar | 17330737PubMed |
Lavery, T., Kekeubata, E., Esau, T., Flannery, T., MacLaren, D., and Waneagea, J. (2018). Rat and bat hunt helped heal rift from colonial cruelty. Nature 563, 626.
| Rat and bat hunt helped heal rift from colonial cruelty.Crossref | GoogleScholarGoogle Scholar | 30487624PubMed |
Longcore, J. E., Pessier, A. P., and Nichols, D. K. (1999). Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91, 219–227.
| Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians.Crossref | GoogleScholarGoogle Scholar |
Mesquita, A. F., Lambertini, C., Lyra, M., Malagoli, L. R., James, T. Y., Toledo, L. F., Haddad, C. F., and Becker, C. G. (2017). Low resistance to chytridiomycosis in direct-developing amphibians. Scientific Reports 7, 1–7.
| Low resistance to chytridiomycosis in direct-developing amphibians.Crossref | GoogleScholarGoogle Scholar |
Narayan, E., Molinia, F., and Hero, J. M. (2011). Absence of invasive Chytrid fungus (Batrachochytrium dendrobatidis) in native Fijian ground frog (Platymantis vitiana) populations on Viwa-Tailevu, Fiji Islands. Acta Herpetologica 6, 261–266.
NHMRC (2013). Australian code for the care and use of animals for scientific purposes, 8th edn. (Canberra: National Health and Medical Research Council.)
O’hanlon, S. J., Rieux, A., Farrer, R. A., Rosa, G. M., Waldman, B., Bataille, A., Kosch, T. A., Murray, K. A., Brankovics, B., Fumagalli, M., and Martin, M. D. (2018). Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360, 621–627.
| Recent Asian origin of chytrid fungi causing global amphibian declines.Crossref | GoogleScholarGoogle Scholar | 29748278PubMed |
Pili, A. N., Sy, E. Y., Diesmos, M. L. L., and Diesmos, A. C. (2019). Island hopping in a biodiversity hotspot archipelago: reconstructed invasion history and updated status and distribution of alien frogs in the Philippines. Pacific Science 73, 321–343.
| Island hopping in a biodiversity hotspot archipelago: reconstructed invasion history and updated status and distribution of alien frogs in the Philippines.Crossref | GoogleScholarGoogle Scholar |
Pikacha, P., Morrison, C., and Richards, S. (2008). ‘Frogs of the Solomon Islands.’ (Institute of Applied Sciences, University of the South Pacific: Suva, Fiji).
Pollard, E. M., Thaman, R., Brodie, G., and Morrison, C. (2015). Threatened biodiversity and traditional ecological knowledge: associated beliefs, customs and uses of herpetofauna among the ‘Are’Are on Malaita Island, Solomon Islands. Ethnobiology Letters 6, 99–1.
| Threatened biodiversity and traditional ecological knowledge: associated beliefs, customs and uses of herpetofauna among the ‘Are’Are on Malaita Island, Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
Rebollar, E. A., Woodhams, D. C., LaBumbard, B., Kielgast, J. and Harris, R.N. (2017). Prevalence and pathogen load estimates for the fungus Batrachochytrium dendrobatidis are impacted by ITS DNA copy number variation. Diseases of Aquatic Organisms 123, 213–226.
Rowley, J. J. L. and Alford, R. A. (2009). Factors affecting interspecific variation in susceptibility to disease in amphibians. In ‘Amphibian biology. Vol. 8: Amphibian decline: diseases, parasites, maladies and pollution’. (Eds. H. Heatwole and J. W. Wilkinson.) Chapter 3, pp. 3053–3066. (Surrey Beatty & Sons: Chipping Norton, NSW, Australia.)
Scheele, B. C., Pasmans, F., Skerratt, L. F., Berger, L., Martel, A., Beukema, W., Acevedo, A. A., Burrowes, P. A., Carvalho, T., Catenazzi, A., and De la Riva, I. (2019). Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363, 1459–1463.
| Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity.Crossref | GoogleScholarGoogle Scholar | 30923224PubMed |
Schloegel, L. M., Picco, A. M., Kilpatrick, A. M., Davies, A. J., Hyatt, A. D., and Daszak, P. (2009). Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biological Conservation 142, 1420–1426.
| Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana).Crossref | GoogleScholarGoogle Scholar |
Swei, A., Rowley, J. J. L., Rödder, D., Diesmos, M. L., Diesmos, A. C., Briggs, C. J., Brown, R., Cao, T. T., Cheng, T. L., Chong, R. A., and Han, B. (2011). Is chytridiomycosis an emerging infectious disease in Asia? PloS One 6, e23179.
| Is chytridiomycosis an emerging infectious disease in Asia?Crossref | GoogleScholarGoogle Scholar | 21887238PubMed |
Woodhams, D. C., and Alford, R. A. (2005). Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conservation Biology 19, 1449–1459.
| Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland.Crossref | GoogleScholarGoogle Scholar |


