Diagnostic and typing methods for investigating Legionella infection
Christopher C. Blyth A , D. Nicholas Adams A and Sharon C. A. Chen A BA Centre for Infectious Diseases and Microbiology Laboratory Services, Sydney West Area Health Service
B Corresponding author. Email: Sharon.chen@swahs.health.nsw.gov.au
NSW Public Health Bulletin 20(10) 157-161 https://doi.org/10.1071/NB08062
Published: 9 November 2009
Abstract
Legionella infection is an important cause of community-acquired pneumonia in Australia. Morbidity and mortality is significant. Diagnosis remains a challenge with infection often unrecognised, particularly early in the course of illness. An understanding of available diagnostic methods and their limitations is important to public health practitioners and clinicians alike.
Legionella infections are responsible for 2–15% of community-acquired pneumonia.1,2 Morbidity and mortality varies greatly depending on the underlying health of the patient, the promptness of specific therapy and whether the disease is sporadic, nosocomial or part of an outbreak.3 Outbreaks or case clusters occur in community-acquired and nosocomial settings with cooling towers, spas and contaminated hot and cold water plumbing commonly implicated.1 Legionella infections are notifiable throughout Australia, with approximately 300–350 cases reported each year (data from 2001 to 2007).4
Numerous diagnostic methods and the typing of isolates are available to assist with epidemiological investigations. This paper will review these methods and how they can be used by public health practitioners to manage potential cases and suspected outbreaks.
Microbiology and clinical spectrum
Legionella spp. are ubiquitous environmental Gram-negative bacteria. They are able to survive in moist environments for long periods of time and grow well at temperatures ranging from 20 to 42°C.5 They have an increased tolerance to chlorine and thus enter water-supply systems and proliferate in thermal habitats, including air-conditioning towers, hot water systems, shower heads, taps, spas and respiratory ventilators.6 There are currently more than 50 species described, including at least 16 serogroups of L. pneumophila.5
Infections range from a severe multisystem disease including pneumonia to an asymptomatic infection.1,5,7 Pneumonia due to L. pneumophila is termed Legionnaires’ disease. Worldwide, L. pneumophila serogroup 1 is the most common cause of Legionnaires’ disease. Pneumonia can be caused by other Legionella spp.; L. longbeachae, L. bozemanii, L. dumoffii and L. micdadei are the most frequently described.1,2,5,8,9 Pontiac fever, a self-limiting non-pneumonic febrile illness, is also described.
In the period 1991–2000 in Australia, L. pneumophila was responsible for 51% of cases of clinical disease, with L. pneumophila serogroup 1 the most frequently reported pathogen.10 L. longbeachae is another frequent pathogen in Australia, responsible for 42% of the total number of cases.10
Laboratory diagnosis from clinical specimens
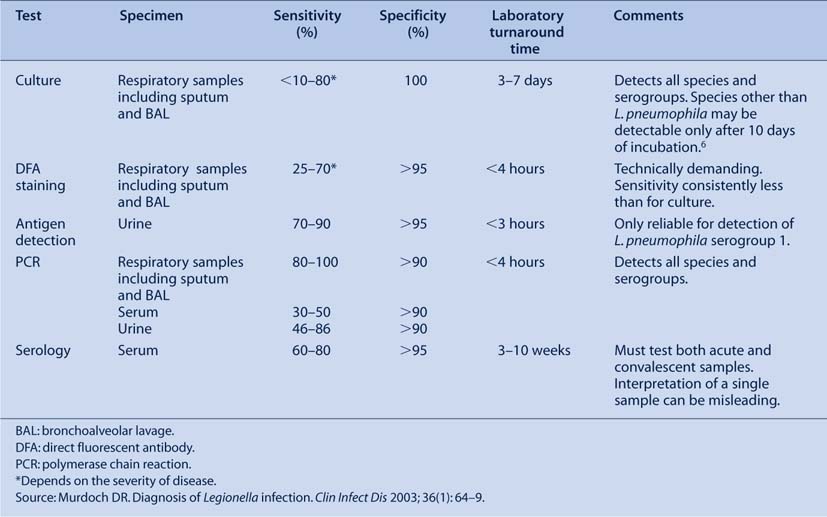
It is not possible to distinguish patients with Legionnaires’ disease from other forms of pneumonia by clinical or radiological means.11,12 As a result, laboratory confirmation is essential for diagnosis. Although diagnostic methods have improved, no currently available test is able to diagnose all Legionella infections in a timely fashion, with a high degree of sensitivity and specificity. The available methods are summarised in Table 1.
|
Definitive legionellosis is defined by the Public Health Laboratory Network as isolation of Legionella spp., detection of Legionella antigen in urine, seroconversion or significant increase in serum Legionella antibody levels.13 Suggestive legionellosis is defined as detection of Legionella antigen by direct fluorescent antigen (DFA), detection of Legionella DNA by polymerase chain reaction (PCR), or a single high antibody level to L. pneumophila or L. longbeachae.13 These laboratory definitions are used in combination with clinical parameters to identify, for public health purposes, confirmed and probable cases of Legionella infection.14
Culture
Isolation of Legionella spp. by culture is considered the ‘gold standard’ for diagnosis because of its superior specificity. Legionella spp. are most frequently isolated from respiratory tract specimens (e.g. sputa, bronchoalveolar lavage (BAL), lung). Lung biopsy specimens have the greatest yield but are rarely performed.5 Bronchoscopic samples have a greater diagnostic yield compared with expectorated sputum samples.15 In most laboratories, polyvalent or monoclonal antisera are used to identify presumptive L. pneumophila and L. longbeachae.13 These techniques are unreliable for other species, owing to a high degree of cross-reactivity between different species with molecular techniques preferred.
The major advantage of culture for diagnosis is that all Legionella spp. are able to be detected by this method. A culture isolate is also required for further epidemiological typing or for susceptibility testing.
There are, however, inherent problems with Legionella culture because the organism is fastidious and slow growing (often taking 5 days or more to grow).13 Specifically formulated media (most frequently buffered charcoal yeast-extract media) are required to enhance the growth of Legionella spp. and suppress other respiratory bacteria. Patients with Legionnaires’ disease are often non-productive of sputum and therefore require invasive procedures to obtain respiratory samples (e.g. BAL fluid). The yield from culture depends on the severity of the illness: 15–25% of mild pneumonia cases are culture positive compared with 95% in cases of severe pneumonia causing respiratory failure.15 Delays in sputa processing and prior specific antimicrobial therapy decrease the yield.5
Fluorescent microscopy
Direct fluorescent-antibody (DFA) staining is a rapid method of directly detecting Legionella spp. in respiratory secretions and tissue samples. Although rapid, it is insensitive, requiring large organism numbers for visualisation (i.e. severe disease). Reported sensitivity of fluorescent microscopy varies but is consistently less than that of culture.15 Furthermore, it is technically demanding, requiring experienced laboratory personnel. False positive results may occur because of cross-reactions with other bacteria and yeasts.5 Problems with both sensitivity and specificity have limited the use of DFA staining in most laboratories.
Legionella urinary antigen tests
Soon after L. pneumophila was identified as the cause of Legionnaires’ disease, it was noted that Legionella ‘antigen’ could be found in patients’ urine. The antigen detected is a component of the Legionella cell wall. Antigenuria can be detected as early as 1 day after the onset of symptoms and can persist for months despite therapy.1 Popular formats include the enzyme immunoassay (EIA) and immunochromogenic test (ICT).
The two most frequently used tests have excellent sensitivity and specificity for L. pneumophila serogroup 1. The Legionella Urinary Antigen EIA (Binax, Inverness Medical: Scarborough, Maine) has a sensitivity of 70–90% and specificity approaching 100% for L. pneumophila serogroup 1.2,15–17 The ICT membrane assay (NOW Legionella Urinary Antigen Test: Binax, Inverness Medical: Scarborough, Maine) is simple to perform, rapid and its sensitivity and specificity are similar to those of EIA.18 Similar to culture and fluorescent microscopy, an association between clinical severity and test sensitivity occurs.17 Results can be obtained in 3 hours with the Binax EIA and in 15 minutes with the Binax NOW kits.
Attempts to create a Legionella urinary antigen test to detect species and serogroups other than L. pneumophila serogroup 1 have been problematic (sensitivity 29–31% for species other than L. pneumophila serogroup 1).19 In particular, no commercial assay is available to reliably detect L. longbeachae in urine.
Polymerase chain reaction
PCR-based detection of Legionella DNA in sputum, urine and blood has been described.1,6,15 PCR amplifies minute amounts of Legionella DNA, providing results within a short time and enabling detection of infection caused by all Legionella species and serogroups. Molecular methods can be formulated to incorporate real-time or multiplex formats. Despite the availability of commercial assays (e.g. Chlamylege kit, Argene Inc, NY), Legionella PCR is available only in a limited number of laboratories in Australia.
When testing clinical samples from the lower respiratory tract, PCR has been shown to have sensitivity equal to or greater than culture.20–22 False positive results have been reported using both in-house and commercial assays.6 Legionella DNA can also be detected from other samples, but with reduced sensitivity (30–86%).15
Serology
Serological testing for Legionella infection is a valuable epidemiological tool but is of less immediate benefit to physicians because of delayed seroconversion. Indirect immunofluorescent assays (IFA) and enzyme-linked immunosorbent assays (ELISA or EIA) are the most frequently performed tests.13 IFA remains the standard reference test and is validated for L. pneumophila and L. longbeachae.15 ELISA assays are designed to provide a sensitive screen for legionellosis and detect IgM using L. pneumophila serogroup 1 or L. longbeachae sonicated whole cells as antigens.
Using IFA, a cut-off equal to or greater than 1 : 128 is recommended as evidence of recent or past infection. A single titre of 1 : 512 or higher for either L. pneumophila or L. longbeachae is a sensitive indicator of infection but may represent past infection or, on rare occasions, infection with another species.13 The demonstration of seroconversion or a four-fold rise in titre on a convalescent sample is required for diagnosis of definitive Legionella infection. In most cases, seroconversion is detected within 3–4 weeks; however, up to 10 weeks has been reported.23 A proportion of people with a proven Legionella infection do not develop detectable Legionella antibodies.15 Cross-reactive antibodies are occasionally found in patients with other infections or non-infectious conditions. Clinicians should be encouraged to obtain convalescent samples after a minimum of 3 weeks. If there is no seroconversion after this time and clinical suspicion remains high, an additional convalescent sample should be obtained. IgM measured by ELISA can become positive earlier in the course of illness compared with IFA, although it may remain elevated for years and numerous cross-reactions can occur.13
Identification of Legionella spp. from environmental specimens
Attempts to culture Legionella spp. from environmental sources may be undertaken to investigate a clinical case cluster or as a part of the regular surveillance. An environmental investigation is generally not required following individual cases; however, the decision to investigate should be made by individual public health units, taking local factors into consideration.14 A number of tools, including electronic maps of registered cooling towers, may be utilised to identify potential point sources (Vicky Sheppeard, pers. comm.).
A number of NATA-registered laboratories process environmental samples for Legionella. Culture methods are similar to those used in clinical laboratories. Following heat treatment to reduce growth of other bacteria, an aliquot of water is incubated on selective media. Following growth of suspicious colonies, antisera are used to identify presumptive L. pneumophila.
Typing of Legionella isolates
Approximately 4% of community-acquired and 37% of nosocomial Legionella infections constitute case clusters.1 Standard serotyping of isolates is inadequate in epidemiological investigations because L. pneumophila serogroup 1 is the predominant organism in outbreaks. Further methods are required for subtyping or differentiation between potentially related strains.
Serological typing to identify 12 ‘type’ strains within L. pneumophila serogroup 1 has been described.1 Not all of the monoclonal antibodies from this panel are available in Australia;13 thus, molecular methods are usually preferred.
Various molecular methods are available for genotyping of clinical and environmental Legionella isolates in suspected case clusters. These include amplified fragment length polymorphism (AFLP) analysis, pulsed-field gel electrophoresis (PFGE), restriction fragment length polymorphism (RFLP) analysis and multi-locus sequence typing (MLST). The choice of method depends on the preference of the laboratory performing the test. Compared with DNA fragment-based methods (e.g. AFLP, PFGE or RFLP), DNA sequencing (e.g. MLST) is robust, offers greater reproducibility and allows results to be shared and compared between laboratories.5,24
Subtyping of clinical and, if available, environmental isolates of Legionella is a powerful epidemiological tool to identify linked clinical cases and the possible common environmental source. Subtyping of Legionella spp. should be performed only if there is clear epidemiological evidence linking more than one case. Given the increasing use of non-culture-based methods, subtyping is limited by the infrequent isolation of Legionella spp. in culture. European data indicate that Legionella infections were diagnosed by culture in only 10% of cases.8
A rational approach to diagnosis
A rational approach to diagnosis is required because of the difficulty in distinguishing Legionella infection from other causes of community-acquired pneumonia. A diagnosis is necessary to enable identification and management of potential point sources. Testing algorithms may vary with different situations (e.g. a suspected outbreak compared with isolated cases). As each diagnostic method has limitations, a combination of tests is recommended.15
Based on the current evidence, it is our opinion that patients presenting with possible acute Legionella infection should have respiratory specimens cultured for Legionella, if available, combined with a Legionella urinary antigen test. Where available, a PCR-based assay to detect Legionella, together with a urinary antigen test, is a sensitive alternative; however, culture should still be attempted to obtain an isolate for identification and for genotyping if indicated. Reliance on urinary antigen tests will miss non-L. pneumophila serogroup 1 infections, including L. longbeachae. Fluorescent microscopy has little role, except in patients presenting with severe disease who have a negative Legionella urinary antigen. Serology remains the only method of documenting recent past infection. This may be of particular assistance where an alternative explanation for pneumonia has not been found or for epidemiological investigation of outbreaks where a point source is suspected. When a culture is available, molecular typing of clinical and environmental isolates is a powerful tool for identifying linked clinical cases and any possible common environmental sources.
Conclusion
Well-established methods such as culture for Legionella and urinary Legionella antigen detection remain the mainstay of diagnosis of Legionella infections. Newer methods, including PCR-based assays, are likely to become more widely available in the future. Given the current limitations of laboratory diagnosis, patients presenting with pneumonia will continue to receive empiric therapy against Legionella.
Acknowledgments
Vicky Sheppeard and Helen Ptolemy (Centre for Population Health) and Anne Smith (Division of Analytical Laboratories), Sydney West Area Health Service.
[1] Fields BS, Benson RF, Besser RE. Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev 2002; 15(3): 506–26.
| Crossref | GoogleScholarGoogle Scholar | PubMed | (Cited 26 June 2009.)
[5]
[6] Diederen BM. Legionella spp. and Legionnaires’ disease. J Infect 2008; 56(1): 1–12.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed | (Cited 26 June 2009.)
[14]
[15] Murdoch DR. Diagnosis of Legionella infection. Clin Infect Dis 2003; 36(1): 64–9.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[16] Kim MJ, Sohn JW, Park DW, Park SC, Chun BC. Characterization of a lipoprotein common to Legionella species as a urinary broad-spectrum antigen for diagnosis of Legionnaires’ disease. J Clin Microbiol 2003; 41(7): 2974–9.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[17] Yzerman EP, den Boer JW, Lettinga KD, Schellekens J, Dankert J, Peeters M. Sensitivity of three urinary antigen tests associated with clinical severity in a large outbreak of Legionnaires’ disease in The Netherlands. J Clin Microbiol 2002; 40(9): 3232–6.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[18] Dominguez JA, Gali N, Pedroso P, Fargas A, Padilla E, Manterola JM, et al. Comparison of the Binax Legionella urinary antigen enzyme immunoassay (EIA) with the Biotest Legionella Urin antigen EIA for detection of Legionella antigen in both concentrated and nonconcentrated urine samples. J Clin Microbiol 1998; 36(9): 2718–22.
| CAS | PubMed |

[19] Benson RF, Tang PW, Fields BS. Evaluation of the Binax and Biotest urinary antigen kits for detection of Legionnaires’ disease due to multiple serogroups and species of Legionella. J Clin Microbiol 2000; 38(7): 2763–5.
| CAS | PubMed |

[20] Cloud JL, Carroll KC, Pixton P, Erali M, Hillyard DR. Detection of Legionella species in respiratory specimens using PCR with sequencing confirmation. J Clin Microbiol 2000; 38(5): 1709–12.
| CAS | PubMed |

[21] Jaulhac B, Nowicki M, Bornstein N, Meunier O, Prevost G, Piemont Y, et al. Detection of Legionella spp. in bronchoalveolar lavage fluids by DNA amplification. J Clin Microbiol 1992; 30(4): 920–4.
| CAS | PubMed |

[22] Jonas D, Rosenbaum A, Weyrich S, Bhakdi S. Enzyme-linked immunoassay for detection of PCR-amplified DNA of legionellae in bronchoalveolar fluid. J Clin Microbiol 1995; 33(5): 1247–52.
| CAS | PubMed |

[23] Monforte R. Delayed seroconversion in Legionnaire’s disease. Lancet 1988; 332(8609): 513.
| Crossref | GoogleScholarGoogle Scholar |

[24] Scaturro M, Losardo M, De Ponte G, Ricci ML. Comparison of three molecular methods used for subtyping of Legionella pneumophila strains isolated during an epidemic of Legionellosis in Rome. J Clin Microbiol 2005; 43(10): 5348–50.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |


