Spatial and temporal variation in the breeding of Masked Lapwings (Vanellus miles) in Australia
Lynda E. Chambers A D , Heather Gibbs B , Michael A. Weston B C and Glenn C. Ehmke CA Centre for Australian Weather and Climate Research, Bureau of Meteorology, GPO Box 1289, Melbourne, Vic. 3001, Australia.
B School of Life and Environmental Sciences, Deakin University, 221 Burwood Highway, Burwood, Vic. 3125, Australia.
C Research and Conservation Department, Birds Australia, Suite 2-05, Green Building, 60 Leicester Street, Carlton, Vic. 3052, Australia.
D Corresponding author. Email: L.Chambers@bom.gov.au
Emu 108(2) 115-124 https://doi.org/10.1071/MU07064
Submitted: 22 October 2007 Accepted: 26 February 2008 Published: 1 May 2008
Abstract
Spatial and temporal variation in the breeding of Masked Lapwings (Vanellus miles) in Australia were examined using data from Birds Australia’s Nest Record Scheme (NRS; 1957–2002), the Atlas of Australian Birds (1998–2006), and climatic data (1952–2006). Breeding in north-western Australia was concentrated in summer, while in other regions the peak of breeding occurred during spring. Breeding success varied between regions and years but was generally highest in Tasmania. Clutch-size (mean 3.57 eggs ± 0.033 s.e., n = 549 clutches) did not vary regionally or temporally. In the north-east, breeding became earlier over time (~1.9 days per year, NRS), while in the south-east, breeding became later (~0.9 days per year); in other regions temporal trends were not evident. Only Tasmania showed a significant temporal change in breeding success (decrease of ~1.5% per year). All regions experienced warming climates, and annual rainfall increased in north-western regions and decreased in eastern regions. There were weak or no relationships between the amount or success of breeding, clutch-size and the climatic variables considered (with the possible exception of Tasmania), suggesting either that data limitations precluded us from detecting subtle effects or that Masked Lapwings have been little influenced or are resilient to changes in climate over most of their range.
Introduction
Breeding phenology (or timing) can vary markedly within a species (L’Hyver and Miller 1991). Breeding tends to occur later at higher, more polar, latitudes and higher altitudes (Valencia et al. 2002; Summers and Nicoll 2004), reflecting the harsher environmental conditions in these regions. For example, Azure-winged Magpies (Cyanopica cyanus) laid their first eggs on average 4 days later for every 100 m increase in elevation (Valencia et al. 2002). Welcome Swallows (Hirundo neoxena) in eastern Australia began breeding later at higher latitudes (southward) and higher altitudes (Marchant and Fullagar 1983). Several Australian raptors laid eggs 2–3 days later for every degree of latitude southward (Olsen and Marples 1993). Much of the difference in breeding between locations can be explained by differences in climate, with climate affecting breeding in several key ways, including thermoregulation of eggs and young, and availability of food for breeding adults and chicks (Elkins 1983; Dunn 2004; Visser et al. 2004).
Fluctuations of climate can influence the timing and success of breeding (Dunn 2004; Chambers et al. 2005). Many studies have examined how changes in climate have altered the phenology of avian breeding (Sparks et al. 2002; Parmesan and Yohe 2003; Dunn 2004). To date, most evidence of changes in breeding phenology in relation to a warming climate is from the northern hemisphere where warmer temperatures in the months preceding the breeding season correspond to an earlier start to laying (Parmesan and Yohe 2003; Dunn 2004; but see Visser et al. 2004). Earlier breeding among Welcome Swallows in New Zealand coincided with rapid local climatic warming (Evans et al. 2003). Some northern hemisphere species have experienced increased hatching and fledging success in association with warmer spring temperatures (Sparks et al. 2002; Crick 2004; Dunn 2004). Variation in clutch-size has also been linked to climatic variations or climate proxies, such as latitude or altitude (Olsen and Marples 1993; Winkler et al. 2002; Sanz 2003), though this association is not universal (Marchant and Fullagar 1983; L’Hyver and Miller 1991; Summers and Nicoll 2004).
This paper examines the temporal and spatial patterns in breeding of a common, socially monogamous bird in relation to climatic variables. The Masked Lapwing (Vanellus miles) is a ground-nesting shorebird (Charadriiformes) with two subspecies. The nominate subspecies occurs in tropical northern Australia (and outside Australia in the Moluccas and southern New Guinea) and subspecies novaehollandiae occurs in eastern and southern Australia (Marchant and Higgins 1993). This species is particularly well suited to an examination of spatial and temporal variation in avian breeding in Australia because it occurs throughout most of Australia (Barrett et al. 2003) and its nests and young are fairly obvious (Marchant and Higgins 1993). Masked Lapwings are the fourth-most recorded species in the Birds Australia Nest Record Scheme, and have been recorded on ~19% of Atlas of Australian Birds surveys (Birds Australia, unpubl. data). While there have been several studies of the breeding of this species (Dann 1981; Giese and Jones 1996), resident Australian shorebirds are poorly known with respect to their breeding biology, and their breeding phenology is often complex and poorly understood (Tomkovich and Weston 2007). We are unaware of any studies of the influence of climate on shorebird breeding in Australia.
Methods
Climate data
Monthly rainfall, and maximum and minimum temperature data were obtained at a resolution of 1° × 1° for the years 1952–2006. These data were used to calculate annual totals (rainfall) or annual averages (temperature) as well as seasonal totals and averages for the two seasons preceding breeding in eastern Australia (autumn, March–May; winter, June–August). The Southern Oscillation Index (SOI), a measure of broad-scale atmospheric variation, was calculated from monthly fluctuations in air-pressure difference between Tahiti and Darwin, Australia (Troup 1967). Negative or positive values of the SOI are often associated with reduced or increased rainfall respectively over eastern and northern Australia. All climatic data were obtained from the National Climate Centre (Australian Bureau of Meteorology, Melbourne).
Breeding data
Two sources of breeding information were used: the Atlas of Australian Birds (Birds Australia, Melbourne), which provided broad spatial coverage, and the Nest Record Scheme (Birds Australia), which provided a longer temporal series and more detail on breeding.
Atlas of Australian Birds
The Atlas is a volunteer-based dataset that constitutes the most comprehensive set of observations on the distribution and occurrence of birds at the continental scale (see Barrett et al. 2003). The main period of data collection was 1998–2002, but collection of data has continued and is ongoing. A total of 45 614 records of Masked Lapwings was available for analysis, and the years with sufficient data for analysis were 1998–2006 inclusive.
The Atlas data were used to calculate the ‘amount’ of breeding in any given month (Br%) within predefined regions (see below). To calculate Br%, the total number of breeding records of Masked Lapwings during each month of each year was divided by the total number of records with sightings of Masked Lapwings in that month and year for each region. The amount of breeding was calculated relative to Masked Lapwing sightings to minimise the influence of changes in the abundance of Masked Lapwings on our relative measure of how likely Masked Lapwings were to breed. We restricted our data to surveys that were completed within a single day because: (1) we wished to tie breeding to the time of year as precisely as possible and (2) because preliminary analysis suggested that Br% varied with the duration of surveys. Typically, the presence or absence but not the stage of breeding was recorded, so it was not possible to use the Atlas data to estimate the timing of breeding at time-scales finer than 1 month.
Nest Record Scheme
Nest Record Scheme (NRS) data for Masked Lapwings covered the period 1957–2002, though few records were available in the 1950s and 2000s and records were concentrated in the central and eastern Australia (Fig. 1). NRS contributors report details of breeding events, including the number of eggs and young (see http://www.birdsaustralia.com.au/our-projects/nest-record-scheme.html, accessed 15 April 2008). NRS data provide an estimate of the timing of breeding based on an estimate of hatching date; laying was rarely observed (see Griffioen 2001). Hatching date was estimated using the midpoint between the last record of eggs and the first record of chicks for each breeding attempt. Where chicks were not recorded the last record of eggs was used.

|
NRS data also provide measures of yearly breeding success and clutch-size. Nesting success was calculated using three methods to fully explore the trade-off between quality of derived variables and quantity of available data. ‘Apparent overall success’ was derived from records where observers classified each nesting attempt as a success, failure or unknown. Nests were considered successful if at least one young fledged. Because young are mobile and cryptic soon after hatching, it is possible that successful nests were incorrectly regarded as unsuccessful. We thus used another measure, ‘inferred success’, where successful nests were defined as those where at least one young hatched and the nest was not known to have failed. Fates of nests were considered unknown where nesting failure during the incubation stage was not reported. ‘Mayfield hatching success’ was based on a daily survival rate (DSR), a method which avoids a bias whereby unsuccessful nests are often not sampled (Mayfield 1961, 1975; Klett and Johnson 1982). We limited our use of DSRs to incubation only, to avoid violating assumptions of constant survivorship (see Jehle et al. 2004). Nests with unknown fates were excluded from DSR analyses and failures were assumed to occur at the 40% point between the last active and first inactive check of a nest (Miller and Johnson 1978). The South-east Region was the only region that had sufficient data for temporal analysis of DSRs (i.e. ≥50 nest records; Hensler and Nichols 1981; Klett and Johnson 1982; Crick and Baillie 1996).
Clutch-size was estimated where the maximum number of eggs was recorded on two different days (laying interval is generally 24 h; Marchant and Higgins 1993); 549 records of clutch-size were available.
Regions
Too few breeding records were available for any given place to enable temporal trends to be determined so breeding data were pooled within six predetermined regions bounded by latitude and longitude. The regions were constructed to balance the need for enough data to calculate robust temporal trends in the breeding variables while maintaining the ability to assess latitudinal and longitudinal differences in breeding (Table 1; Fig. 1).

|
Analysis
Relationships between breeding and climatic variables were examined using correlation analysis and simple linear regression, as well as trends over time in the breeding (mean date of breeding and breeding success) and climatic variables (rainfall, mean maximum and minimum temperatures and the SOI).
Results
Temporal trends in regional climate
All regions experienced significant warming trends in maximum and minimum annual temperatures and June–August and March–May maximum temperatures. Minimum temperatures tended to increase in all regions for the June–August and March–May periods, with many increases being statistically significant (Table 2). In southern and eastern Australia (South-central, South-east, Tasmania and North-east Regions) rainfall declined over time (1952–2006), though this was not statistically significant in the South-central Region (except during March–May) or when considering the June–August period. There was a significant increase in annual rainfall in the North-west Region of 4.42 mm per year (P = 0.025, R2 = 7.4%). There was no significant trend in the annual or March–May SOI, and a weak negative trend in June–August SOI, from 1952 to 2006.

|
Regional variation in breeding
Most breeding records came from the more southerly regions, with very few observations from the Central Region (Table 1). Breeding (estimated hatching dates) in the North-west Region predominantly occurred during the summer months (December–February, with a peak during January), while in the other five regions breeding tended to occur in spring (peaking during August and September). The timing of the peak of reported breeding for the Eastern and Central Regions was similar. In the South-central Region the distribution of timing of breeding had an unexpected (assuming timing of breeding is normally distributed) proportion of breeding attempts in the latter part of the calendar year and early in the following year, whereas in the North-east Region there was a higher than expected proportion of breeding attempts several months before the peak of breeding. The timing of breeding in Tasmania appeared to be more constrained (Table 3).

|
Atlas data revealed a September peak in the amount of breeding in Tasmania, the South-east and North-east Regions (Fig. 2a). This was broadly consistent with the pattern derived from NRS data. In the South-central Region breeding was rarely recorded before July, peaked during August and then gradually declined through to December. In the Central Region there was no clear peak in breeding records, with breeding recorded May–January. Timing of the greatest amount of breeding was ill-defined in the North-west Region, with breeding recorded in most months of the year.

|
The percentage of Atlas records recording breeding activity (Br%) was much higher in the Tasmania Region than elsewhere, with ~6–8% of all Tasmanian records involving breeding (Fig. 2). The South-east Region also had a higher Br% (~2–4%) than other regions (generally ~1–2%). On an annual basis, the Br% in any one region was not significantly related to Br% in any other region (results not shown; P > 0.05 in all cases).
Breeding success, as measured by apparent overall success and inferred success varied with region and year (Table 4). Breeding success was generally highest in the Tasmania Region, though this varied annually. DSRs were also higher in Tasmania than in the southern mainland regions. Mayfield estimates of hatching success were substantially lower than inferred success (Table 4). There were no significant correlations between timing of breeding and breeding success in the regions where there were sufficient data (North-east, South-central, South-east or Tasmania Regions).

|
The average clutch-size of Masked Lapwings was 3.57 eggs (±0.03 s.e., n = 549 clutches). There was no evidence of regional differences in clutch-size, although more data would be required to detect any subtle variation between regions.
Trends in breeding
Temporal trends in the timing of breeding were calculated for regions with sufficient data (see Table 5 for the range of years used in the analyses). Trends were calculated using all years for which data were available and verified against trend calculations that used only those years with at least five breeding observations (a condition resulting in a more robust estimate of mean timing of breeding). For the South-central and Tasmania Regions there were no significant trends in the timing of breeding (Table 5). In the North-east Region breeding became earlier over time (~1.9 days per year) while in the South-east Region breeding was delayed (~0.9 days per year). The North-west and Central Regions did not have sufficient data for analysis (Table 5).

|
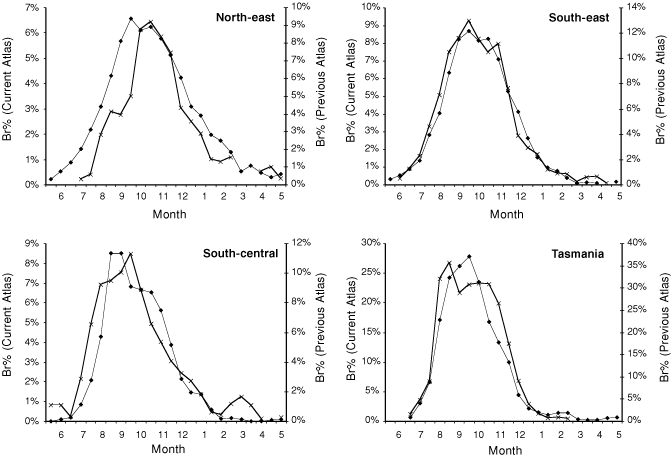
A limited comparison of seasonal changes in the amount of breeding (Br%) between the Atlas data used in this study (1998–2006) and the previous Atlas of Australian birds (1977–81) was conducted (Fig. 3). The data presented represents smoothed values, that is a monthly Br% value was calculated for each month and for each month offset by 15 days. Only area searches of 32 days duration or less were used, as these were least affected by changes in survey methodology between atlases; even so, the peak Br% was higher in all regions in the original atlas, possibly because the criteria used to define breeding were less stringent. As changes in survey methodology should be consistent across all regions, different patterns of change between the regions probably reflect real differences in the amount of breeding. In particular, the shift in the peak of the breeding season in the North-east Region from October to September provides independent support for the substantial change in timing of breeding detected using NRS data (Fig. 3), whereas in the other three regions breeding seasons appear similar during both Atlas periods.
Breeding varied between years in most regions (Fig. 2), though there were no significant linear trends in Br% over time in any region (results not shown; P > 0.05; too few records were available for the Central and North-west Regions for analysis).
Only the North-east, South-central, South-east and Tasmania Regions had sufficient data to examine temporal trends in breeding success (apparent overall success and inferred success). Only the Tasmania Region showed a significant change in breeding success over time, with a decrease of ~1.5% per year for inferred success (P = 0.001, R2 = 66.7%) and ~2% per year for apparent overall success (P = 0.022, R2 = 48.4%). However, the initial years (1964–69) of data in this region are dominated by nearly 500 records from a single observer, with very high levels of reported breeding success (over 90%). These data overlap those used in Thomas (1969) in which relatively low success figures are quoted. DSRs could only be calculated for the South-east Region for three decades and this suggested an increase over time (1960s including 1959, 23% success; 1970s, 25%; 1980s, 53%). There were no significant temporal trends in clutch-size (P = 0.27–0.99, R2 <0.1–2.8%) for the regions with sufficient data for analysis (North-east, South-central, South-east and Tasmania Regions) or for all regions combined.
Relationships between breeding and climate
There appeared to be little or no relationship between climate and timing of breeding (Table 6). The few significant correlations between climate and the timing of breeding generally disappeared when the analysis was restricted to at least five breeding records per year. There were no significant correlations between climate and breeding success or clutch-size, except in Tasmania where breeding success (apparent overall and inferred success) was negatively correlated with maximum and minimum temperatures (particularly annually and during March–May and into September–November; results not shown). Correlations do not necessarily indicate a causal link; both the temperature data and breeding success variables had significant trends for Tasmania. To explore this further, correlations were calculated for the detrended values (residual values of the observed variable minus that fitted using the trend equation with year as the explanatory variable). The correlations were no longer statistically significant, indicating that the previously obtained correlations were a result of the strong trends in both the temperature and breeding success variables.

|
Although the analysis was restricted by the availability of only a few years of data for the proportion of records involving breeding (Br%) by region, there appeared to be few significant relationships between the amount of breeding and the climatic variables (Table 7). Annual rainfall was negatively related to the amount of breeding in all regions, but this was significant only in Tasmania. June–August rainfall was negatively related to Br% in all but the South-east Region (significantly so in the South-central Region). Maximum temperatures during March–May (MAM) had the strongest correlation with the amount of regional breeding (r = –0.879 for Tasmania, r = –0.701 for South-central Region; P < 0.05 for both) and was negatively correlated with Br% in three of the four regions tested. Minimum temperatures during MAM, particularly in Tasmania, were also negatively correlated, again suggesting that temperatures during March–May could be important in determining the amount of breeding by Masked Lapwings during the following spring.

|
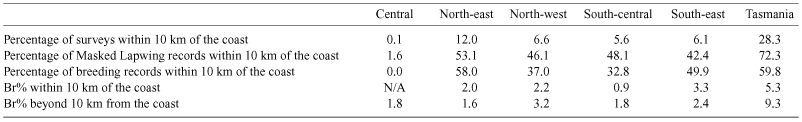
We also considered the possibility that coastal climates influence lapwing breeding (Table 8). Although Br% was not higher in near-coastal regions, lapwings were recorded more often near the coast.
Discussion
Although Masked Lapwings are common, widespread, and obvious when breeding, no long-term, Australia-wide, analysis of breeding data for this species is available (Dann 1981; Giese and Jones 1996; Marchant and Higgins 1993). The climatic trends experienced by Masked Lapwings are consistent in direction and magnitude with previous studies (e.g. Nicholls 2006), with trends towards warmer temperatures, increased rainfall in the North-west and Central Regions and decreased rainfall elsewhere. However, their breeding distribution spans a wide range of climates (Marchant and Higgins 1993), the extremes of which are greater than the climatic changes documented over the study period. Thus Masked Lapwings may have ‘ample intraspecific variation in response mechanisms, so that dispersal across latitudes may allow rapid adaptation through changes in existing frequencies of response mechanisms’ (Visser et al. 2004). There is anecdotal evidence that Masked Lapwings, while generally resident, move in response to climatic variability, such as avoiding areas affected by drought and using ephemeral wetlands (Marchant and Higgins 1993).
Temporal trends in breeding
In the North-east Region, breeding became earlier over time. This is as predicted when warming occurs where breeding was previously limited by cool non-breeding conditions, and matches changes seen in many other species following climate warming (Dunn 2004; Chambers et al. 2005). In the South-east Region, Masked Lapwings tended to begin breeding later in the year, perhaps as a result of reduced rainfall delaying peak prey availability (see below).
Although temperatures increased in all regions, changes in breeding phenology were only detected in two regions. This suggests that the breeding phenology of Masked Lapwings is unrelated to climate, or that changes in breeding phenology vary by region and habitat, or that changes so far are too small to be detected given the data available. Warming could also extend breeding seasons, and potentially promote multiple clutching, but there was insufficient data to examine this.
There was no temporal trend in the amount of breeding (Br%) in any region over the seven years of available data. Perhaps Br% was more influenced by more local factors, such as habitat condition, predation, demography and environmental change, than climate change. Population size may also influence the amount of breeding recorded, but populations of Masked Lapwings have probably remained stable at the continental scale (Barrett et al. 2003). Our results suggest that the amount of breeding has remained stable over time. Clutch-size did not vary regionally or over time, and seems to be a conservative trait among shorebirds, especially lapwings (Tomkovich and Weston 2007).
Actual breeding success could not be measured with the available data, so three surrogates were used. Apparent overall success and inferred success both indicated a significant increase in breeding success in Tasmania over time, but it was not possible to verify this using the DSRs as there were too few data. Where comparable, all three measures changed in similar ways over time, providing evidence that they are consistent, at least when sample sizes were adequate.
Regional variation in breeding
Two regions seem to differ from other regions with respect to breeding: Tasmania, where there was more breeding and higher breeding success than elsewhere, and the North-west, where the breeding season was ill-defined and apparently later than elsewhere in Australia.
While there is no clear explanation for the relatively high amount of breeding (Br%) in Tasmania, it could reflect higher population density (owing to an abundance of productive pastures) or a greater probability of detecting nests (owing to a relative lack of feral predators, in particular Red Fox (Vulpes vulpes)). As an island, Tasmania also has relatively more coastline than other regions. This could reduce daily and seasonal temperature fluctuations (Crowder 1995) or provide more profitable foraging habitats, or both. Breeding in the South-east Region tended to be slightly more successful than in the regions with lower rainfall, suggesting that rainfall and associated habitat and prey suitability may play a role in breeding success. The wetter regions are also those where rainfall is predicted to decrease under climate-change projections (IPCC 2007), so it is possible that breeding success will decrease over time.
With the exception of the North-west (NRS and Atlas data) and possibly Central (Atlas data) Regions, the timing of breeding of Masked Lapwings was similar throughout Australia, generally occurring during the austral spring, or boreal dry season (August–November). Breeding is said to occur at almost any time of year in northern Australia and mainly June–December in the south (Marchant and Higgins 1993). The peak of laying in southern Victoria (Point Cook and Phillip Island) occurred in the same month as the peak for Tasmania and New Zealand, i.e. late August if replacement clutches are excluded, otherwise mid-September (Dann 1981). The similarity in the timing of breeding over much of Australia is remarkable given the substantial climatic variation across the continent, and the diverse array of habitats occupied by this species. Moreover, breeding phenology is thought to be a rather plastic trait, but, as a fairly large bird, lapwings may be less sensitive to challenges of extreme climates (Dunn 2004).
A key influence on breeding among birds is availability of prey. While biparentally incubating shorebirds, such as lapwings, rarely experience energetic stress during incubation, they might when attending a brood (Weston and Elgar 2005). Diet and energetic demands may vary with climate. At Phillip Island, young fed mainly on earthworms and beetle larvae, and less frequently on surface-dwelling insects (Dann 1981). Availability of earthworms was influenced by rainfall, which increases in August–September, just before the peak of breeding, and brings worms to the upper layers of soil, where they are available to lapwings (Dann 1981). Banded Lapwings (Vanellus tricolor) breed several weeks earlier than Masked Lapwings, possibly because their preferred prey increases in abundance earlier (Dann 1981). Timing can also be influenced by thermoregulatory constraints (Dunn 2004).
The variation in hatching success by location and temporally ranged from 28% to 80% in other studies and from 20% to 100% in this study, though mean clutch-sizes were similar throughout Australia and New Zealand (3.5–3.7 eggs) (Thomas 1969; Barlow et al. 1972; Dann 1981; Giese and Jones 1996; this study).
Several factors besides climate vary with geographical region and may represent potentially confounding covariables. First, there are two allopatric subspecies of Masked Lapwings. The subspecies limits were not sufficiently well defined, and data were too few, to permit analysis of breeding within subspecies. Second, habitat and relevant ecological factors (such as supply and availability of food) are also likely to vary both with (and within) regions and with climate. More information on the exact mechanism through which climate influences breeding is required to interpret fully the patterns we describe. Third, there were few observations in the Central and North-western Regions, either because the species was not abundant or there were few observers present.
Relationship between breeding and climatic variables
Timing of breeding
Even for this fairly common and conspicuous species, the quantity of data available was limiting. Of the four regions with sufficient data, two (North-east and South-east) had strong temporal trends in the timing of breeding but no significant relationship to the climatic variables, while in the other two regions (South-central and Tasmania), annual rainfall and, in Tasmania, March–May minimum temperatures, apparently influenced the timing of breeding. Despite many, mainly northern hemisphere, species showing an advance in laying date with increasing temperature, this is not a general rule (Crick et al. 1997; Crick and Sparks 1999) and was not the case for the Masked Lapwing over much of its range. Perhaps the change in temperature experienced was not large enough to trigger a response or the cue to breed was not related to temperature but to something else, such as photoperiod (Dunn 2004). Timing of breeding did not vary consistently with rainfall (but see Thomas 1969). Even where rainfall appeared to influence breeding, the direction of the relationship varied between regions, and the magnitude of the relationship varied dramatically depending on the timing of the rainfall. Without further research it is difficult to isolate what processes might operate among lapwings, though the temperature changes seen over much of Australia were generally lower than for northern hemisphere land masses (IPCC 2007). Also, rainfall can be vary greatly in both quantity and timing, and thus in its ecological effects.
Amount of breeding
There have been few Australian studies of the relationship between the amount of breeding in birds and changes in climate, despite the substantial effect that changes in the proportion of the population breeding can have on total reproductive output (McLean et al. 2005; Gibbs 2007). The amount of breeding among Masked Lapwings was related to climate in only two of the four regions investigated, the South-central and Tasmania Regions. Increases in temperature and decreases in rainfall over time may have opposing effects on the amount of breeding observed, one possible explanation for the lack of temporal trends we report. With data for only seven years we could not adequately test for interactions between multiple climatic and breeding variables, but it is possible that in some instances overall temporal trends may be masked by locally specific responses to climate. It is not clear why less rainfall or cooler temperatures are linked to more breeding, though ground-nesting species, such as lapwings, may avoid breeding when the ground is waterlogged (Williams 1967; Marchant and Higgins 1993). Heavy rain can suppress avian breeding in some circumstances (Marchant 1981).
Breeding success and clutch-size
Breeding success was not strongly influenced by climate, possibly because success is influenced by a wide range of non-climatic factors, such as changes in land-use, trampling from stock, predation or human interference (Thomas 1969; Barlow et al. 1972; Giese and Jones 1996).
Reproductive fitness of a species can be greatly influenced by changes in clutch-size (Winkler et al. 2002; Dunn 2004). Although in some species clutch-size has increased over time, this does not necessarily follow from increased temperatures or shifts to earlier laying dates (Dunn 2004) and was not detected for Masked Lapwings. Perhaps females advance their laying date to synchronise brood-rearing with the peak of food availability (often climate related) and laying date may be a more plastic trait than clutch-size (Dunn 2004). Rainfall changes did not appear to influence clutch-size (Barlow et al. 1972; this study).
Conclusions
Climate over the period of this study did not seem to influence breeding success or clutch-size substantially. Temporal trends were observed in the timing of breeding in two regions (North-east and South-east) and relationships with annual climatic variables (for both timing and Br%) were observed in the other two regions (South-central and Tasmania). These relationships were not always in line with predictions derived from the northern hemisphere. Breeding did occur earlier in warm years in the coldest region (Tasmania) but not elsewhere, and breeding phenology did not vary consistently with rainfall. There were also substantial regional differences in the amount of breeding (Br%). Complex interactions between Masked Lapwings and the climate they experience preclude simple summary and explanation at the continental scale, but we predict that climate change will have further and possibly significant impacts on this species over the longer term, particularly because temporal trends in timing of breeding have already occurred.
Acknowledgements
Thanks go to Birds Australia and the Bureau of Meteorology for providing access to the data used in this study. The NRS is funded by the Vera Moore Foundation (and formerly by the Norman Wettenhall Foundation), and we gratefully acknowledge the support of an Australian Government Natural Heritage Trust (NHT) Grant via the Port Phillip and Westernport Catchment Management Authority, which focuses on the conservation of beach-nesting shorebirds. The Atlas of Australian Birds (post-1998) has been funded by the Australian Government’s NHT and the Marsh Family Trust. Thanks also go to all the volunteers whose observations made this study possible. James O’Connor, Greg Roff, John McBride and two anonymous reviewers improved the manuscript.
Barlow, M. L. , Muller, P. M. , and Sutton, R. R. (1972). Breeding data on the spur-winged plover in Southland, New Zealand. Notornis 19, 212–249.
Chambers, L. E. , Hughes, L. , and Weston, M. A. (2005). Climate change and its impact on Australia’s avifauna. Emu 105, 1–20.
| Crossref | GoogleScholarGoogle Scholar |
Crick, H. Q. P. , and Sparks, T. H. (1999). Climate change related to egg-laying trends. Nature 399, 423–424.
| Crossref | GoogleScholarGoogle Scholar |
Dann, P. (1981). Breeding in the Banded and Masked Lapwings in southern Victoria. Emu 81, 121–127.
Evans, K. L. , Tyler, C. , Blackburn, T. M. , and Duncan, R. P. (2003). Changes in the breeding biology of the Welcome Swallow (Hirundo tahitica) in New Zealand since colonisation. Emu 103, 215–220.
| Crossref | GoogleScholarGoogle Scholar |
Hensler, G. L. , and Nichols, J. D. (1981). The Mayfield method of estimating nesting success: a model, estimators and simulation results. Wilson Bulletin 43, 42–53.
Jehle, G. , Yackel Adams, A. A. , Savidge, J. A. , and Skagen, S. K. (2004). Nest survival estimation: a review of alternatives to the Mayfield estimator. Condor 106, 472–484.
| Crossref | GoogleScholarGoogle Scholar |
Mayfield, H. R. (1961). Nesting success calculated from exposure. Wilson Bulletin 73, 255–261.
Troup, A. J. (1967). Opposition of anomalies in upper tropospheric winds at Singapore and Canton. Australian Meteorological Magazine 15, 32–37.
Weston, M. A. , and Elgar, M. A. (2005). Parental care in Hooded Plovers (Thinornis rubricollis). Emu 105, 283–292.
| Crossref | GoogleScholarGoogle Scholar |

Williams, G. R. (1967). The breeding biology of California Quail in New Zealand. Proceedings of the New Zealand Ecological Society 14, 88–99..

Winkler, D. W. , Dunn, P. O. , and McCulloch, C. E. (2002). Predicting the effects of climate change on avian life-history traits. Proceedings of the National Academy of Sciences, USA 99, 13 595–13 599.
| Crossref | GoogleScholarGoogle Scholar |