Evidence of philopatry and natal dispersal in Humboldt Penguins
Alejandro Simeone A C and Roberta S. Wallace BA Universidad Andres Bello, Facultad de Ecologia y Recursos Naturales, Departamento de Ecologia y Biodiversidad, Republica 470, Santiago, Chile.
B Milwaukee County Zoo, 10001 West Blue Mound Road, Milwaukee, WI 53226, USA.
C Corresponding author. Email: asimeone@unab.cl
Emu 114(1) 69-73 https://doi.org/10.1071/MU13021
Submitted: 10 July 2012 Accepted: 5 June 2013 Published: 24 September 2013
Abstract
We report evidence of both philopatry and natal dispersal in Humboldt Penguins (Spheniscus humboldti) from a colony in central Chile. Between 1994 and 2001, we tagged 241 Humboldt Penguin chicks with subcutaneous transponder chips. Seven birds (3%) were found as adults at their natal colony: five were breeding (philopatric birds) and two were prospecting for nest-sites. Another four birds (2%) were found breeding at other colonies up to 90 km from the natal colony. Philopatric birds bred at 3.6– 6.1 years old (mean ± s.d. = 4.8 ± 1 years) at nests located 5–80 m from their natal nests (30 ± 25 m). Most philopatric and the prospecting birds used the same types of nests as their natal ones and we suggest that birds breeding for the first time may use cues of structural aspects of their natal nest when choosing a nesting site for the first time.
Introduction
Seabirds are typically philopatric, that is individuals tend to return to breed in the colony in which they hatched (Hamer et al. 2002; Gaston 2004). Individuals of a wide variety of seabird families, including the Diomedeidae (Gauthier et al. 2010), Procellariidae (Ovenden et al. 1991; Bradley et al. 1999), Spheniscidae (Dugger et al. 2010), Phalacrocoracidae (Schjørring 2001), Sulidae (Osorio-Beristain and Drummond 1993), Alcidae (Halley et al. 1995; Ibarguchi et al. 2011) and Laridae (Coulson and Coulson 2008), return to breed where they were hatched. Young individuals of some species return to their natal colony for one or more years before breeding for the first time, apparently to examine local environmental conditions and conspecific breeding success (Halley et al. 1995; Danchin et al. 1998; Bradley et al. 1999). Despite the philopatric tendency of seabirds, young birds may disperse from their natal colony and breed for the first time at a colony elsewhere (Coulson and Coulson 2008). This natal dispersal (sensu Greenwood 1980) involves prospecting at several colonies in the years before breeding for the first time (Coulson 2002).
The degree of philopatry and natal dispersal may vary depending on the species, year, climate, availability of food and quality of habitat (Dugger et al. 2010) and understanding these life-history traits is important as they affect patterns of gene flow, and genetic structure and dynamics of populations (Osorio-Beristain and Drummond 1993; Steiner and Gaston 2005; Bouzat et al. 2009).
The Humboldt Penguin (Spheniscus humboldti) breeds along the Pacific coast of Chile and Peru, foraging in the nutrient-rich waters of the Humboldt Current (Williams 1995). In central Chile, Humboldt Penguins breed twice annually: a spring event occurs mainly between August and January and an autumn event occurs between April and June. The species has a declining world population (probably <10 000 mature individuals; BirdLife International 2013; but see Mattern et al. 2004). This species is also is considered Vulnerable globally. Research recommendations for improving the conservation status of the Humboldt Penguin have included studies on breeding biology and life history (Araya et al. 2000). Despite some local efforts in providing information on these subjects (e.g. Simeone et al. 2002), little is still known of the age at first breeding, nest-site fidelity, philopatry and dispersal, among other relevant life-history traits. In this study we report for the first time direct evidence of both natal philopatry and dispersal of wild-ranging Humboldt Penguins tagged as chicks at their natal colony in central Chile. We also examined and report on the age at first breeding and the role of nest-site characteristics in nest-site selection of first-time breeders.
Methods
From December 1994 to December 2006 we conducted a study on the breeding biology and ecology of the Humboldt Penguin at Pájaro Niño Island (33°21′S, 71°41′W), a 3-ha island in Algarrobo, central Chile (Fig. 1). Pájaro Niño (250 pairs) and Cachagua Island (600 pairs) are the two largest colonies of Humboldt Penguins in central Chile (32–38°S) and combined comprise ~95% of the total Penguin population in this region (Simeone et al. 2003). During the period of study, the breeding population at Pájaro Niño fluctuated between 131 and 326 pairs (Simeone and Bernal 2000).

|
At the Pájaro Niño colony we established monitoring plots on three sections of the island, the area for each of the plots was 0.41, 0.16 and 0.18 ha and partitioned into a 10 × 10-m grid; the three plots included ~45% of the breeding population of the island. Within the gridded plots, nests were assigned a unique number (based on grid and nest) and classified into one of the following types of nest: dirt burrow, rock crevice, rock-covered and vegetation-protected. The colony was monitored twice a week during the main periods of nesting activity (April–May and October–November) and twice per month during the rest of the year. All nests within the plots were individually checked for contents and, if birds were present, bird identity was determined (see below). Additionally, once per year (normally between October and November) we did an exhaustive search for tagged Penguins around the island to check for birds that had moved from the study plots.
From December 1994 to December 2001, we tagged Penguin chicks 60–70 days old or 3.0 to 3.8 kg (i.e. before fledging) with subcutaneous transponder chips (Trovan Ltd, Douglas, Isle of Man, UK). These chips provided a unique identification code based on a combination of 10 numbers and letters. During subsequent nest inspections, we checked for tagged birds using a Trovan chip-reader LID 500, which allows detection of chips within 10 cm. Chips were placed under the skin on top of the head, approximately at the midline between the eyes, using a Trovan needle and injector. The chips were so placed for ease of reading the chip as Penguins tend to peck the chip-reader, allowing it to pick up the signal with little disturbance to the Penguin. Also, the chip tends to move less when placed on top of the head than when placed under the skin of the back where they tend to migrate caudally (R. S. Wallace, pers. obs.).
In 2004, we searched for tagged birds at four other colonies: Concón Islet (32°53′S, 71°31′W), 55 km north of Pájaro Niño, on 15 May and 25 November; Cachagua Island (32°35′S, 71°27′W), 90 km north of Pájaro Niño, on 10–12 May and 21–23 November; Pájaros 1 (29°35′S, 71°33′W), 420 km north of Pájaro Niño, on 6–8 November; and Pupuya Island (33°58′S, 71°53′W), 70 km south of Pájaro Niño, on 29 May. At these colonies we did an exhaustive search of nests and checked all birds on nests using the same procedure as at Pájaro Niño. Coverage at these colonies included 80–90% of the land area and excluded nests at inaccessible sites (e.g. cliffs, sea caves).
We considered a bird to be philopatric if it returned to the natal colony and was seen on a nest attending eggs or chicks. Birds in nests, either alone or as a pair, and without confirmed eggs or chicks were considered to be prospecting (Ainley et al. 1983).
Because many Penguin nests in the colony disappear between years (owing to collapse, filling in with soil, drying of vegetation), it was not possible to determine directly whether Penguins selected the same type of nest as their natal one or chose a nest based on availability of sites at the time our observations were made. To overcome this problem, we used an exact binomial calculation to estimate the two-tail probability that total philopatric Penguins would use the same type of nest as its natal one (P = 0.5) or a nest of another type (P = 0.5) (Zar 1999).
Results
From 1994 to 2001, we tagged a total of 241 Humboldt Penguin chicks from Pájaro Niño Island (hereafter, the natal colony) with transponder chips (Table 1). These birds were ~90% of all chicks produced within the study plots during the period of study. The remaining chicks departed or died before we tagged them.

|
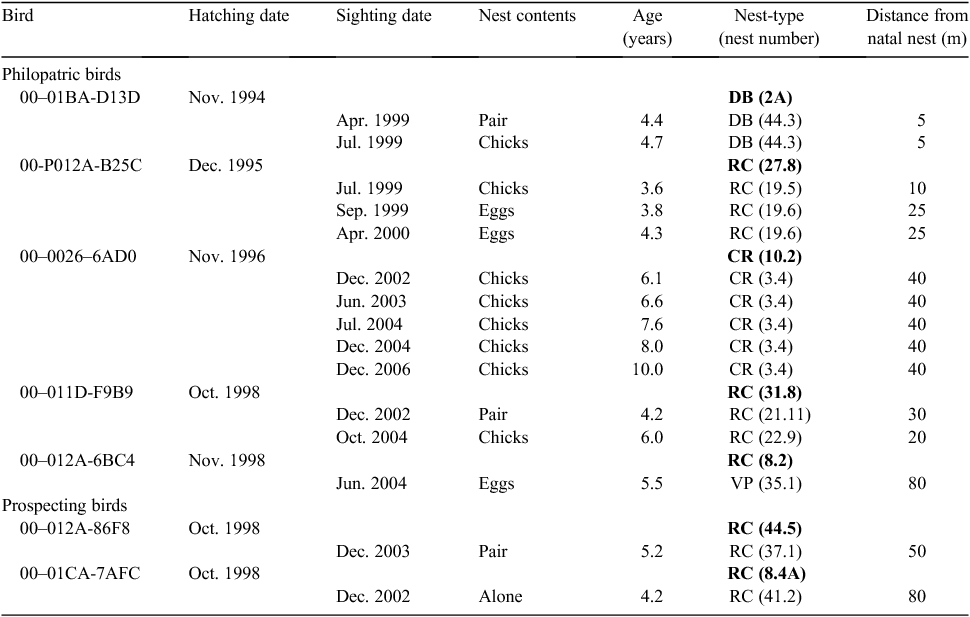
Of the 241 tagged birds, only 7 (3%) were found at least once as adults at the natal colony: 5 individuals were breeding (philopatric birds) and 2 were prospecting (Table 2). Two birds (00-01BA-D13D and 00-011D-F9B9; Table 2) were prospecting for nesting sites at 4.4 and 4.2 years old and bred for the first time 3 months and 2 years later. Two other birds (00-012A-86F8 and 00-01CA-7AFC, Table 2) were prospecting for nesting sites at 4.2 and 5.2 years old, but were not seen again at the colony. The nests of philopatric birds ranged from 5 to 80 m from their natal nests (mean ± s.d. = 30 ± 25 m, n = 7, note that the two birds each used two different nests, cf. Table 2); the two prospecting birds occupied nests 50 and 80 m from their natal nests (Table 2).
Of the seven philopatric and prospecting birds, six chose the same type of nest as their natal nests. Including nesting of two philopatric birds that used different nests during the study period but of the same types (see birds 00-P012A-B25C and 00–011D-F9B9, Table 2) the Penguins used the same type of nests as their natal ones in eight of nine cases (P = 0.039).
Four birds were recorded breeding at two other colonies: Concón and Cachagua Islands (Table 3). The age of these birds ranged from 5.4 to 6.1 years (mean ± s.d. = 5.7 ± 0.3 years). Only one of these four used a nest similar to the natal nest-type.

|
Discussion
The proportion of tagged birds re-sighted at the natal colony here (3%) is low but such numbers appear to be common of Spheniscus penguins. Whittington et al. (2005) estimated that, depending on year and location, 5–9% of African Penguins (S. demersus) banded as chicks returned to breed at their natal colony. This is likely to reflect the high mortality of most marine birds in the first year of life. During this critical period, most juveniles appear to die because they are not able to learn successfully how to feed themselves (Hamer et al. 2002; Gaston 2004). Rates of first-year mortality of up to 58% have been reported in first-year juvenile Magellanic Penguins (Spheniscus magellanicus) (Scolaro 1987); between 31% (La Cock and Hänel 1987) and 88% (La Cock et al. 1987) among African Penguins; and 83% in Little Penguins (Eudyptula minor) (Sidhu et al. 2007). In our study area, incidental mortality in gill-nets is an important cause of death in Humboldt Penguins (Simeone et al. 1999) and this is likely to increase the already high natural rates of first-year mortality. Note, however, that most of the studies cited above used flipper-bands to mark birds, which may increase mortality in first-year birds and thus bias the results (Jackson and Wilson 2002). Effects may, however, depend largely on the types of bands (Boersma and Rebstock 2010).
To our knowledge, this is the first comprehensive attempt to estimate levels of philopatry and dispersal in free-ranging Humboldt Penguins. Previously, Schlosser et al. (2009) made indirect estimates of philopatry based on genetic analysis, and estimated philopatry of 75% for Penguins on Pájaro Niño and rates of dispersal of 14–22%.
We are aware of our small sample size and low numbers of resightings, but they seem representative considering the small population in the region and its current overall conservation status (BirdLife International 2013). We are reasonably confident of our estimates of philopatry, as sampling effort at the natal colony was intense and constant over time. However, it is feasible that we missed prospecting birds that came ashore for short periods or failed to find returning birds that moved outside our study plots for breeding, this despite our annual checks of nests outside the plots. Furthermore, dispersal may have been underestimated because we spent little time on the other islands visited.
Most philopatric and prospecting Penguins were seen close to their natal nests and used the same types of nests as their natal ones. It is thus conceivable that Penguins, when choosing a nest for first time, use structural aspects of their natal nest as cues for selection of their nesting site. Other seabirds (e.g. albatrosses, murres) breed for the first time close (within 50–100 m) to the nest where they were raised and explanations for this behaviour include social attraction and adaptation to microhabitat (Halley et al. 1995; Sagar et al. 1998). We suggest that first-time breeding Humboldt Penguins seek a nesting site that looks familiar to them, which is ultimately based on the single experience they have of a nest, the one in which they hatched.
We are confident that our observations of philopatric Humboldt Penguins on Pájaro Niño accurately reflects their age of first breeding because birds were tagged as chicks and our monitoring effort on the island was intense. Our results, ranging from 3.6 to 6.1 years compare well with ages of first breeding recorded for African Penguins, of 3.8–6.2 years (Whittington et al. 2005) and the age of sexual maturity of Magellanic Penguins, at 4 years old (Garcia-Borboroglu and Boersma 2013). Age at first breeding in seabirds can vary widely between regions and between cohorts as a result of geographical and temporal variation in the cost of reproduction and availability of food (Croxall and Rothery 1995; Crawford et al. 2001; Whittington et al. 2005). Our data are likely to be representative of the study colony. However, we suggest similar studies be carried out at other colonies along the extensive breeding range of the species because rates of philopatry, dispersal and other relevant life-history traits may vary depending on local environmental conditions.
Acknowledgements
Research grants were provided by the Milwaukee County Zoo and the Zoological Society of the Milwaukee County, the Windway Foundation and the Penguin Taxon Advisory Group. Subsecretaría de Pesca allowed penguin tagging (permits 1288, 1599, 2187, 3087 and 3309). Help in the field was provided by M. Bernal, L. Cabezas, M. Flores and R. Villablanca. C. Anguita prepared Fig. 1, and C. Duarte provided statistical advice. Research permits were provided by the Consejo de Monumentos Nacionales and access to Pájaro Niño Island was facilitated by the Cofradía Náutica del Pacífico. We are very grateful to all of them.
References
Ainley, D., Le Resche, R. E., and Sladen, W. J. L. (1983). ‘Breeding Biology of the Adélie Penguin.’ (University of California Press: Berkeley, CA.)Araya, B., Garland, D., Espinoza, G., Sanhuesa, A., Simeone, A., Teare, A., Zavalaga, C., Lacy, R., and Ellis, S. (2000). Population and habitat viability assessment for the Humboldt Penguin (Spheniscus humboldti). Final report. IUCN/SSC Conservation Breeding Specialist Group, Apple Valley, MN.
BirdLife International (2013). Species factsheet: Humboldt Penguin Spheniscus humboldti. (BirdLife International, Cambridge, UK.) Available at http://www.birdlife.org/datazone/speciesfactsheet.php?id=3862 [Verified 14 August 2013].
Boersma, P. D., and Rebstock, G. A. (2010). Effects of double bands on Magellanic Penguins. Journal of Field Ornithology 81, 195–205.
| Effects of double bands on Magellanic Penguins.Crossref | GoogleScholarGoogle Scholar |
Bouzat, J. L., Walker, B. G., and Boersma, P. D. (2009). Regional genetic structure in the Magellanic Penguin (Spheniscus magellanicus) suggests metapopulation dynamics. Auk 126, 326–334.
| Regional genetic structure in the Magellanic Penguin (Spheniscus magellanicus) suggests metapopulation dynamics.Crossref | GoogleScholarGoogle Scholar |
Bradley, J. S., Gunn, B. M., Skira, I. J., Meathrel, C. E., and Wooller, R. D. (1999). Age-dependent prospecting and recruitment to a breeding colony of Short-tailed Shearwaters Puffinus tenuirostris. Ibis 141, 277–285.
| Age-dependent prospecting and recruitment to a breeding colony of Short-tailed Shearwaters Puffinus tenuirostris.Crossref | GoogleScholarGoogle Scholar |
Coulson, J. C. (2002). Colonial breeding in seabirds. In ‘Biology of Marine Birds’. (Eds E. A. Schreiber and J. Burger.) pp 87–113. (CRC Press: Boca Raton, FL.)
Coulson, J. C., and Coulson, B. A. (2008). Measuring immigration and philopatry in seabirds; recruitment to Black-legged Kittiwake colonies. Ibis 150, 288–299.
| Measuring immigration and philopatry in seabirds; recruitment to Black-legged Kittiwake colonies.Crossref | GoogleScholarGoogle Scholar |
Crawford, R. J. M., Dyer, B. M., and Upfold, L. (2001). Age at first breeding of Bank Cormorants, Phalacrocorax neglectus, and Cape Cormorants, Phalacrocorax capensis. Ostrich 72, 145–148.
| Age at first breeding of Bank Cormorants, Phalacrocorax neglectus, and Cape Cormorants, Phalacrocorax capensis.Crossref | GoogleScholarGoogle Scholar |
Croxall, J. P., and Rothery, P. (1995). Population change in Gentoo Penguins Pygoscelis papua at Bird Island, South Georgia: potential roles of adult survival, recruitment and deferred breeding. In ‘The Penguins: Ecology and Management’. (Eds P. Dann, I. Norman and P. Reilly.) pp 26–38. (Surrey Beatty: Sydney.)
Danchin, E., Boulinier, T., and Massot, M. (1998). Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality. Ecology 79, 2415–2428.
| Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality.Crossref | GoogleScholarGoogle Scholar |
Dugger, K. M., Ainley, D. G., Lyver, P. O’B., Bartond, K., and Ballard, G. (2010). Survival differences and the effect of environmental instability on breeding dispersal in an Adélie Penguin meta-population. Proceedings of the National Academy of Science of the United States of America 107, 12375–12380.
| Survival differences and the effect of environmental instability on breeding dispersal in an Adélie Penguin meta-population.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovFGqu7s%3D&md5=a64114402af0f863520592bbcf8de1b8CAS |
Garcia-Borboroglu, P., and Boersma, P. D. (2013). ‘Penguins: Natural History and Conservation.’ (University of Washington Press: Seattle, WA.)
Gaston, A. J. (2004). ‘Seabirds: A Natural History.’ (T. and A. D. Poyser: London.)
Gauthier, G., Milot, E., and Weimerskirch, H. (2010). Small-scale dispersal and survival in a long-lived seabird, the Wandering Albatross. Journal of Animal Ecology 79, 879–887.
| 20337756PubMed |
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Animal Behaviour 28, 1140–1162.
| Mating systems, philopatry and dispersal in birds and mammals.Crossref | GoogleScholarGoogle Scholar |
Halley, D. J., Harris, M. P., and Wanless, S. (1995). Colony attendance patterns and recruitment in immature Common Murres (Uria aalge). Auk 112, 947–957.
| Colony attendance patterns and recruitment in immature Common Murres (Uria aalge).Crossref | GoogleScholarGoogle Scholar |
Hamer, K. C., Schreiber, E. A., and Burger, J. (2002). Breeding biology, life histories, and life history-environment interactions in seabirds. In ‘Biology of Marine Birds’. (Eds E. A. Schreiber and J. Burger.) pp 217–261. (CRC Press: Boca Raton, FL.)
Ibarguchi, G., Gaston, A. J., and Friesen, V. L. (2011). Philopatry, morphological divergence, and kin groups: structuring in Thick-billed Murres Uria lomvia within a colony in Arctic Canada. Journal of Avian Biology 42, 134–150.
| Philopatry, morphological divergence, and kin groups: structuring in Thick-billed Murres Uria lomvia within a colony in Arctic Canada.Crossref | GoogleScholarGoogle Scholar |
Jackson, S., and Wilson, R. P. (2002). The potential costs of flipper-bands to penguins. Functional Ecology 16, 141–148.
| The potential costs of flipper-bands to penguins.Crossref | GoogleScholarGoogle Scholar |
La Cock, G. D., and Hänel, C. (1987). Survival of African Penguins Spheniscus demersus at Dyer Island, Southern Cape, South Africa. Journal of Field Ornithology 58, 284–287.
La Cock, G. D., Duffy, D. C., and Cooper, J. (1987). Population dynamics of the African Penguin Spheniscus demersus at Marcus Island in the Benguela Upwelling ecosystem: 1979–85. Biological Conservation 40, 117–126.
| Population dynamics of the African Penguin Spheniscus demersus at Marcus Island in the Benguela Upwelling ecosystem: 1979–85.Crossref | GoogleScholarGoogle Scholar |
Mattern, T., Ellenberg, U., Luna-Jorquera, G., and Davis, L. S. (2004). Humboldt Penguin census on Isla Chañaral, Chile: recent increase or past underestimate of penguin numbers? Waterbirds 27, 368–376.
| Humboldt Penguin census on Isla Chañaral, Chile: recent increase or past underestimate of penguin numbers?Crossref | GoogleScholarGoogle Scholar |
Osorio-Beristain, M., and Drummond, H. (1993). Natal dispersal and deferred breeding in the Blue-footed Booby. Auk 110, 234–239.
Ovenden, J. R., Wust-Saucy, A., Bywater, R., Brothers, N., and White, R. W. G. (1991). Genetic evidence for philopatry in a colonially nesting seabird, the Fairy Prion (Pachyptila turtur). Auk 108, 688–694.
| Genetic evidence for philopatry in a colonially nesting seabird, the Fairy Prion (Pachyptila turtur).Crossref | GoogleScholarGoogle Scholar |
Sagar, P. M., Stahl, J. C., and Molloy, J. (1998). Sex determination and natal philopatry of Southern Buller’s Mollymawks (Diomedea bulleri bulleri). Notornis 45, 271–278.
Schjørring, S. (2001). Ecologically determined natal philopatry within a colony of Great Cormorants. Behavioral Ecology 12, 287–294.
| Ecologically determined natal philopatry within a colony of Great Cormorants.Crossref | GoogleScholarGoogle Scholar |
Schlosser, J. A., Dubach, J. M., Garner, T. W. J., Araya, B., Bernal, M., Simeone, A., Smith, K. A., and Wallace, R. S. (2009). Evidence for gene flow differs from observed dispersal patterns in the Humboldt Penguin, Spheniscus humboldti. Conservation Genetics 10, 839–849.
| Evidence for gene flow differs from observed dispersal patterns in the Humboldt Penguin, Spheniscus humboldti.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntlSgsrk%3D&md5=ac4b8edd3773759b85c51d0bacc5f986CAS |
Scolaro, J. A. (1987). A model life table for Magellanic Penguins (Spheniscus magellanicus) at Punta Tombo, Argentina. Journal of Field Ornithology 58, 432–441.
Sidhu, L. A., Catchpole, E. A., and Dann, P. (2007). Mark-recapture-recovery modeling and age-related survival in Little Penguins (Eudyptula minor). Auk 124, 815–827.
| Mark-recapture-recovery modeling and age-related survival in Little Penguins (Eudyptula minor).Crossref | GoogleScholarGoogle Scholar |
Simeone, A., and Bernal, M. (2000). Effects of habitat modification on breeding seabirds: a case study in central Chile. Waterbirds 23, 449–456.
| Effects of habitat modification on breeding seabirds: a case study in central Chile.Crossref | GoogleScholarGoogle Scholar |
Simeone, A., Bernal, M., and Meza, J. (1999). Incidental mortality of Humboldt Penguins Spheniscus humboldti in gill nets, central Chile. Marine Ornithology 27, 157–161.
Simeone, A., Araya, B., Bernal, M., Diebold, E. N., Grzybowski, K., Michaels, M., Teare, J. A., Wallace, R. S., and Willis, M. J. (2002). Oceanographic and climatic factors influencing breeding and colony attendance patterns of Humboldt Penguins Spheniscus humboldti in central Chile. Marine Ecology Progress Series 227, 43–50.
| Oceanographic and climatic factors influencing breeding and colony attendance patterns of Humboldt Penguins Spheniscus humboldti in central Chile.Crossref | GoogleScholarGoogle Scholar |
Simeone, A., Luna-Jorquera, G., Bernal, M., Garthe, S., Sepúlveda, F., Villablanca, R., Ellenberg, U., Contreras, M., Muñoz, J., and Ponce, T. (2003). Breeding distribution and abundance of seabirds on islands off north-central Chile. Revista Chilena de Historia Natural 76, 323–333.
| Breeding distribution and abundance of seabirds on islands off north-central Chile.Crossref | GoogleScholarGoogle Scholar |
Steiner, U. K., and Gaston, A. J. (2005). Reproductive consequences of natal dispersal in a highly philopatric seabird. Behavioral Ecology 16, 634–639.
| Reproductive consequences of natal dispersal in a highly philopatric seabird.Crossref | GoogleScholarGoogle Scholar |
Whittington, P., Klages, N., Crawford, R., Wolfaardt, A., and Kemper, J. (2005). Age at first breeding of the African Penguin. Ostrich 76, 14–20.
| Age at first breeding of the African Penguin.Crossref | GoogleScholarGoogle Scholar |
Williams, T. D. (1995). ‘The Penguins.’ (Oxford University Press: Oxford, UK.)
Zar, J. H. (1999). ‘Biostatistical Analysis’, 4th edn. (Prentice Hall: Upper Saddle River, NJ.)