ROWLEY REVIEW. Sex determination in birds: a review
Craig A. SmithComparative Development Group, Murdoch Children’s Research Institute and University of Melbourne, Department of Paediatrics, Royal Children’s Hospital, Parkville, Vic. 3052, Australia. Email: craig.smith@mcri.edu.au
Emu 110(4) 364-377 https://doi.org/10.1071/MU10030
Submitted: 28 April 2010 Accepted: 5 August 2010 Published: 30 November 2010
Abstract
In birds, sex determination occurs at fertilisation by the inheritance of sex chromosomes. This review summarises our current understanding of sex determination in birds, with emphasis on the molecular genetics of male versus female development during embryonic life. Recent studies in the Chicken (Gallus gallus domesticus) have revealed some remarkable features of avian sex determination, such as the finding that sex appears to be determined autonomously within cells throughout the body, and the demonstration that the key, sex-linked gene, DMRT1, is required for testis formation and hence male development. However, despite these recent advances, the mechanism of avian sex determination is still not entirely clear. Understanding sex determination in birds has important implications for the conservation of threatened species, and for the global poultry industry.
Introduction
Birds are well known for their striking sexual dimorphisms, often characterised by gaudy plumage or ornamental feathering in males versus the more drab or cryptic colouration of females. The array of sex-specific features seen in birds and other animals all stem from a key developmental process: sex determination. In its broadest sense, sex determination can be defined as the earliest developmental event whereby sex is established. In birds, and most other animals, sex is determined early in life, at or shortly after fertilisation. In therian mammals (marsupials and ‘placentals’), sex is set at fertilisation by the inheritance of the sex chromosomes. Embryos inheriting two X chromosomes develop as females, whereas those inheriting an X and a Y chromosome develop as males. The SRY gene, located on the Y chromosome, represents the master sex determinant in higher mammals. In XY embryos, SRY initiates formation of testes during embryonic life, whereas formation of ovaries occurs in the absence of SRY (reviewed in Capel 1998). However, birds lack SRY and have a non-homologous set of sex chromosomes, designated ZZ male and ZW female (Takagi and Sasaki 1974; Griffiths 1991; Coriat et al. 1993; McBride et al. 1997; Mizuno et al. 2002; Stiglec et al. 2007). Despite some important advances in the past few years, our understanding of avian sex determination is still incomplete. This review will summarise the current knowledge of avian sex determination at the genetic and developmental levels. Avian sex chromosomes will be described, and the possible mechanism of sex determination will be considered. Sexual differentiation of the embryonic gonads will be outlined and genes implicated in the genetic regulation of this process will be described. Lastly, the implications of understanding sex determination in birds will be discussed.
Avian sex chromosomes
All birds have sex chromosomes and sex is determined at fertilisation by the inheritance of these chromosomes. Sex determination (male v. female development) must be regulated at the genetic level by one or more genes located on the sex chromosomes. In birds, male is the homogametic sex, carrying two Z sex chromosomes. Females are heterogametic, inheriting two different sex chromosomes, Z and W. This nomenclature reflects the fact that the sex chromosome system of birds is unrelated to that of mammals. Avian ZZ–ZW and mammalian XX–XY sex chromosomes have evolved from different pairs of autosomes that were present in reptilian ancestors (Fridolfsson et al. 1998; Nanda et al. 1999; Stiglec et al. 2007; Marshall Graves 2008). Birds generally have a high number of chromosomes (2n > 70 in most species), comprising several pairs of macrochromosomes, and many small almost indistinguishable microchromosomes (Griffin et al. 2007). The sex chromosomes are among the macrochromosome group, whereas the relative size of the Z and W varies among species (Takagi and Sasaki 1974) (shown in Fig. 1). Early karyotyping studies showed that the Z and W are generally very different in size among neognathous (flying) birds. For example, in the Chicken (Gallus gallus domesticus), the large Z chromosome comprises 7% of the haploid genome, whereas the smaller W comprises 1.5% (reviewed in Marshall Graves and Shetty 2001). Among the more primitive palaeognathous birds (flightless ratites, such as the Emu (Dromaius novaehollandiae), Ostrich (Struthio camelus), Southern Cassowary (Casuarius casuarius), and the tinamous (Tinamidae)) the Z and W sex chromosomes are usually homomorphic, that is, they appear morphologically very similar or identical (Ogawa et al. 1998) (Fig. 1b). This is thought to represent a primitive or basal evolutionary state of sex chromosome among birds, with differentiation of the sex chromosomes accompanying the evolution of the neognathous birds, which split from the ratites c. 120 million years ago (van Tuinen and Hedges 2001). (Note, however, that the palaeognathous tinamou has differentiated Z and W chromosomes (Tsuda et al. 2007).) It is unclear why the ratite Z and W have differentiated so little over time, unless their sex chromosomes are newly acquired. This seems unlikely given the homology with other birds. Alternatively, other sex-advantage genes may not have accumulated on the ratite Z or W, so that no suppression of recombination, and hence sex chromosome differentiation, has occurred (J. A. Marshall Graves, pers. comm.). Cross-species chromosome painting and comparative gene mapping have shown that the large avian Z sex chromosome is highly conserved among groups, including the ratites (Shetty et al. 1999; Nishida-Umehara et al. 2007). (Chromosome painting involves fluorescently labelling an entire chromosome of one species and using that to probe to chromosomes of another species. If homologous, the probe will ‘paint’ the corresponding chromosome.) For example, Nanda and colleagues recently reported that the Z is highly conserved across at least 14 species representing 11 families (Nanda et al. 2008). The Z sex chromosome carries ~1000 genes (Bellott et al. 2010), most of which are ostensibly unrelated to sex. However, most recently, it has been show that the Chicken Z chromosome, like the mammalian X chromosome, is enriched for testis-expressed genes (Bellott et al. 2010). One of these is DMRT1 (Doublesex and Mab-3 Related Transcription factor, #1), which has a key role in testis development and may represent the master avian testis determinant (Smith et al. 2009a) (described below).
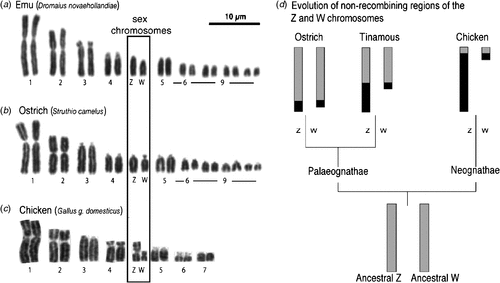
|
The Z and W chromosomes of birds have evolved from an ancestral autosomal pair and they are therefore homologous. However, in most birds, the W has lost most of its genes and it now represents a degraded version of the Z. The W is largely composed of repetitive heterochromatic DNA and, unlike the Z, it is not well conserved among different avian groups (Tone et al. 1982; Saitoh and Mizuno 1992; Itoh et al. 2008). Most ratites exhibit an early stage of sex-chromosome evolution. Little degradation of the W has occurred, its gene content closely resembles that of the Z, and the two chromosomes are of similar size (Fig. 1). When the Chicken Z sex chromosome is fluorescently labelled and hybridised to metaphase spreads of Emu, Ostrich, Southern Cassowary and Rhea spp. chromosomes, it paints both the Z and W of all four ratites (Nishida-Umehara et al. 2007). This indicates that extensive homology exists between the ratite Z and W, with little evidence of W degradation. In contrast, the Z chromosome of the Chicken and other neognathous birds only paints the Z and not the W. In ratites, the Z and W pair over most of their length during female meiosis, exchanging genetic material, as with autosomes. However, this region of pairing, the so-called PseudoAutosomal Region (PAR), is small in the Chicken (Fig. 1d) (Schmid et al. 2005, reviewed in Mank and Ellegren 2007). Taken together, the data indicate that sex chromosomes are poorly differentiated in ratites, but highly differentiated among neognathous birds. Among the latter group, the Z and W sex chromosomes have evidently diverged from each other independently several times during avian evolution (Mank and Ellegren 2007; Itoh et al. 2008). Despite the differences among ratites and carinates, all birds have so-called ‘genotypic sex determination’, whereby the sex chromosomes control development as male or female. The avian sex-determining gene(s) must be present in all birds, including ratites. Given the strong homology of the Z sex chromosome across all groups, and a gene deficit on the degraded W of most birds, it seems unlikely that different sex-determining genes occur in different avian groups, as appears to be the case in bony fishes (Koopman and Loffler 2003; Volff et al. 2003). Rather, it is thought that a single sex-determining mechanism applies to all birds, as also in therian mammals.
The molecular mechanism of avian sex determination
The basic mechanism of avian sex determination has remained a mystery for decades. Sex may be determined by Z chromosome dosage (two for a male, one for a female), or it could depend upon a female gene carried on the W sex chromosome. These two possibilities are known as the Z dosage and dominant W hypotheses respectively and they are not necessarily mutually exclusive (Ellegren 2000; Smith and Sinclair 2004; Ellegren et al. 2007). Which of these applies? This question could readily be answered by observing the sexual phenotype of sex chromosome aneuploids (birds carrying too few or too many sex chromosomes) (Fig. 2). Hence, a bird with a ZZW genotype that developed as a male despite the presence of the W would support the Z dosage hypothesis, as would a Z0 bird (with only one Z) that was female. Alternatively, a ZZW female bird or a Z0 male bird would support the dominant W hypothesis (Fig. 2). However, such sex chromosome aneuploids have not been definitively documented in birds (Clinton 1998) and it has been suggested that such genotypes may be lethal to embryos (Marshall Graves 2003). Marney Thorne and Bruce Sheldon, working at the CSIRO Animal Production Division, described a line of triploid Chickens, with the genome present in triplicate (3A rather than 2A), including the sex chromosomes (Lin et al. 1995; Thorne 1995; Thorne et al. 1997). Whereas 3A:ZZZ birds developed as fairly normal males, 3A:ZZW birds developed as intersexes. At hatching, the 3A:ZZW birds had a right testis and a transient left ‘ovotestis’ with both ovarian and testicular structures (Lin et al. 1995). Phenotypically female at hatching, the birds eventually lost the ovarian component of the left gonad and they became male at sexual maturity. This suggests that Z dosage is more important for sex determination in the Chicken, although the W may have some effect. These studies were complicated, however, owing to the fact that the entire genome was present in triplicate, not just the sex chromosomes. More recently, a breeding female Great Reed-Warbler (Acrocephalus arundinaceus) was described that was inferred to be a 2A:ZZW, based on Z microsatellite analysis (Arlt et al. 2004). Although no karyotyping was carried out to show definitively the presence of a W sex chromosome, these data support the dominant W hypothesis over Z dosage. Hence, there is no consistent evidence from these previous studies that clearly supports one hypothesis over the other.
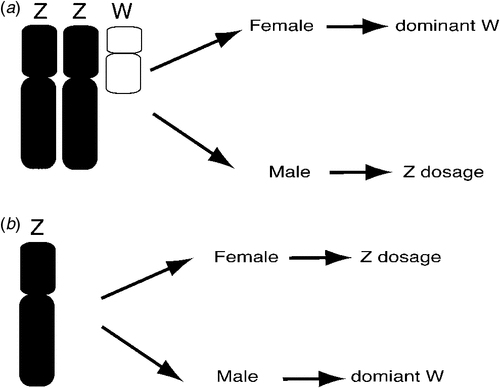
|
Most recently, Zhao and colleagues, working at the Roslin Institute (Edinburgh, UK), presented a detailed study of three rare but naturally occurring gynandromorphic Chickens (Zhao et al. 2010). Gynandromorphs are lateral sex chimeras, male on one side of the body and female on the other (Fig. 3). On the male side, these bizarre birds exhibited a male wattle, large masculine breast muscle, and a large spur-bearing leg. The female side had no male-type wattle, smaller breast muscle and a smaller spur-less leg. Zhao and colleagues examined the sex-chromosome composition of the two sides, hoping to find the sex-chromosome aneuploidy that would settle the Z dosage versus dominant W debate. However, cells on the male side contained mainly ZZ sex chromosomes, and those on the female side were predominantly ZW. Internally, the gonads reflected the relative contributions of ZZ and ZW cells: testes were present if most cells were ZZ, ovaries if most were ZW and ‘ovotestes’ had a mixture of both ZZ and ZW cells. Hence, the gynandromorphs did not shed light on which sex chromosome carries the key sex determinant or determinants. This study, however, does reveal an important new facet of avian sex determination. It suggests that sex determination is cell autonomous in tissues throughout the body, and is not driven exclusively by hormonal signalling. A long-held dogma in vertebrate development is that sex-determining genes control differentiation of the embryonic gonads into testes or ovaries, which then release hormones (testosterone or oestrogens) to masculinse or feminise the rest of the body. There are some notable exceptions in mammals (the formation of pouch and scrotum in wallabies, for example, which precedes gonadal differentiation (reviewed in (Renfree and Short 1988)). The dogma that hormones released from the developing testes or ovaries masculinse or feminise the body could not completely apply to the gynandromorphic Chickens described by Zhao et al. (2010); hormones in the circulation would not be restricted to one side of the body. The logical conclusion is that sexual identity is largely controlled by direct genetic affects in each cell, that is that it is cell autonomous (Zhao et al. 2010).
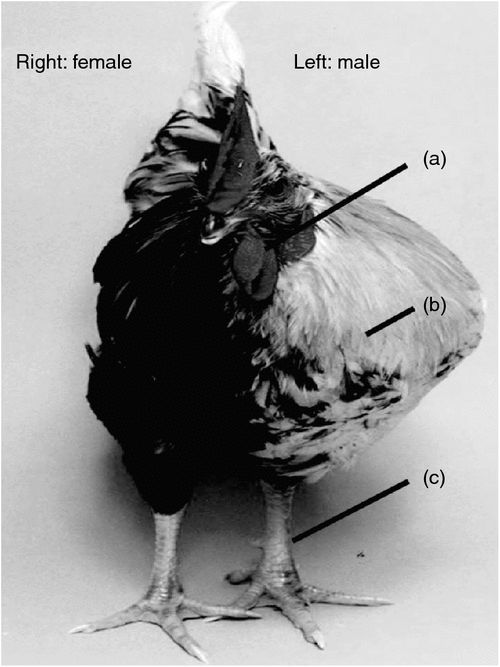
|
Zhao and colleagues also carried out studies on embryonic Chicken gonads experimentally manipulated to carry a mixture of male (ZZ) and female (ZW) cells. These experiments showed that female (ZW) cells would not integrate into male (ZZ) gonads, and vice versa. This again suggested that cells ‘know’ their sex autonomously and steadfastly retain it (Zhao et al. 2010). The authors further postulated that the higher dose of Z-linked genes (the ‘Z transcriptome’) in ZZ cells would confer ‘maleness’ in those cells. This idea stems from the finding that there is no chromosome-wide Z inactivation in birds, as occurs for the X chromosome in female mammals to equalise the dosage of X-linked genes between the sexes (Kuroda et al. 2001; Melamed and Arnold 2007). Although some genes are dosage compensated, male birds (with two Z chromosomes) have on average twice the level of Z-linked gene expression compared with females (with only one Z) (Ellegren et al. 2007; Itoh et al. 2007; McQueen and Clinton 2009; Zhang et al. 2010). In each cell, the higher level of many Z-linked genes could trigger maleness, or a specific gene could be involved. Other studies also support the idea that cells could have a sexual identity that is at least partly independent of gonadal development. A gynandormorphic Zebra Finch (Taeniopygia guttata), for example, had a male-type brain on the left and a female-type brain on the right (Agate et al. 2003), whereas sexually dimorphic gene expression has been observed in Chicken embryos well before the onset of gonadal differentiation into ovaries or testes (Scholz et al. 2006; Smith et al. 2007). Similarly, it has been shown that male Japanese Quails (Coturnix japonica) manipulated to carry female brains show female rather than male behaviours, supporting a cell- or tissue-autonomous sexual function, independent of hormonal signalling (Gahr 2003). However, although sex may be established early in avian embryos, this information must be relayed to a genetic switch in the gonad itself that triggers formation of testes or ovaries. Several lines of evidence suggest that this switch is the Z-linked DMRT1 gene (described below).
Gonadal sex differentiation in avian embryos
A genetic trigger must operate within the gonads of avian embryos to signal formation of testes or ovaries. This trigger must respond to the constitution of the sex chromosome. Much of our understanding of sex determination and gonadal development in birds comes from developmental studies in the Chicken embryo, which has long been a favoured experimental model. Owing to its value as a model in developmental biology, immunology and genetics, and its importance as a major food resource, the genome of the Chicken was one of the first non-mammalian vertebrate genomes to be sequenced (Hillier et al. 2004). This has provided a wealth of genomic data that has enhanced our understanding of avian biology, including sex determination and gonadal development.
In birds, the gonads differentiate into testes or ovaries during embryonic life. Gonadal development has been most extensively studied in the Chicken embryo (Clinton 1998; Mizuno et al. 2002; Smith and Sinclair 2004). Those few other birds that have been examined at the embryonic stages show a broadly similar pattern of gonadal morphogenesis (Gasc 1978). There is no solid evidence of temperature or other environmental factors having a natural effect upon avian sex determination or gonadal development in birds, as is well documented in reptiles. In the closest living relative to birds, the crocodilians, the temperature of incubation of the egg determines sex (Ferguson and Joanen 1982; Johnston et al. 1995; Merchant-Larios et al. 2010). However, sex-biased mortality of embryos has been reported in some species, such as the Australian Brush-turkey (Alectura lathami), resulting in skewed sex-ratios at hatching (Göth and Booth 2005).
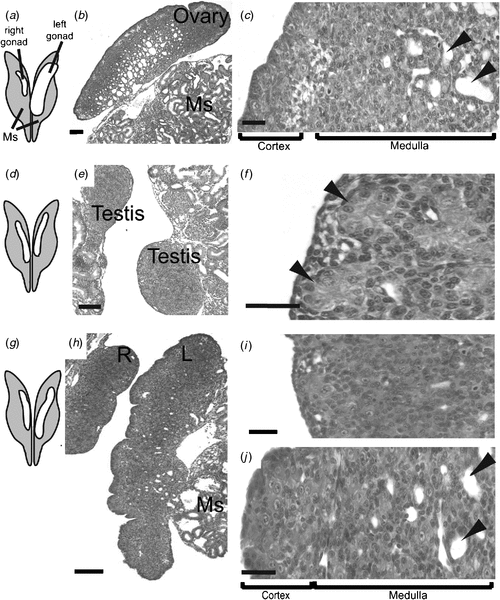
In the Chicken embryo, the embryonic gonads become apparent as thickenings on the ventral medial surface of the embryonic (mesonephric) kidneys by Day 3.5 of incubation (Hamburger-Hamilton stage 19) (Hamburger and Hamilton 1951) At this stage, the paired organs are morphologically indistinguishable between the sexes and are considered ‘indifferent’ or ‘bipotential’ (although, as noted above, there are already likely to be sex differences in gene expression). The undifferentiated gonads are composed of an outer epithelial layer, and underlying cords of cells interspersed with loose mesenchyme (the so-called medulla) (C. A. Smith, pers. obs.). Germ cells (future sperm and ova) originate outside the gonads, in an extra-embryonic region anterior to the head, called the germinal crescent (Fujimoto et al. 1976). They subsequently migrate into the embryo and through the developing vascular system to the gonads, where they predominantly populate the outer cortical region. It has been reported that germ cells become asymmetrically distributed in the gonads before sexual differentiation, being more numerous in the left gonad, in both sexes (Vallisneri et al. 1990; Zaccanti et al. 1990). During sexual differentiation, the gonads develop into either bilateral testes (ZZ) or unilateral ovaries (ZW) (Fig. 4a). In genetic males (ZZ) the cords of cells in the medulla condense into seminiferous cords, and the outer epithelium becomes reduced to a monolayer of squamous epithelium (Fig. 4a). Germ cells become enclosed in the cords, where they enter transient mitotic arrest. In female Chicken embryos (ZW), gonadal development is asymmetric. Somatic cells of the left gonadal cortex proliferate, germ cells accumulate in the cortex and enter the early stages of meiosis. The inner medulla becomes fragmented. The right gonad grows initially but never develops a thickened cortex. The medulla becomes fragmented, as occurs in the left gonad, but the right gonad eventually becomes reduced to a small rudiment. This asymmetry has been well characterised at the molecular level, where it has been show to depend upon the homeobox gene, PITX2. This gene is preferentially expressed in the left gonad, where it stimulates cell proliferation (Guioli and Lovell-Badge 2007; Ishimaru et al. 2008; Rodriguez-Leon et al. 2008). It is thought that the regression of the right gonad (and its accompanying oviduct) is necessary because the body cavity of a gravid female can hold only one ovary and duct with developing eggs, and two ovaries and ducts would be energetically expensive for flying. However, this may not be the complete answer, as some bird species have both left and right functional reproductive organs.
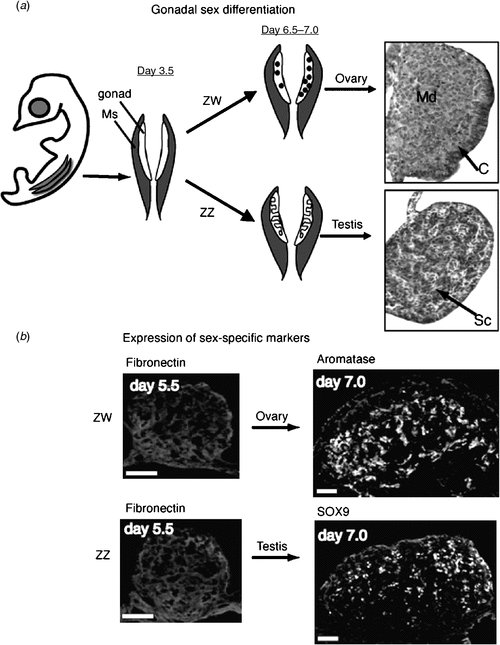
|
At the molecular level, the SOX9 gene is activated male-specifically at the time of gonadal sex differentiation (from Day 6.5, developmental stage 29–30; Fig. 4b) (Kent et al. 1996; Morais da Silva et al. 1996). SOX9 encodes a conserved transcription factor, part of the SOX family of developmental regulatory genes, of which the mammalian SRY gene is the founding member. Expression of the SOX9 gene is upregulated in the developing testes of amniotic vertebrates (reptiles, birds, mammals) (Kent et al. 1996; Smith et al. 2005; Shoemaker et al. 2007). Evidence from mouse embryos indicates that SOX9 regulates the differentiation of the Sertoli cell lineage in the developing testis (Kobayashi et al. 2005). Sertoli cells are the first type of cell to differentiate in male gonads; they become organised into the characteristic testis cords, they enclose germ cells and they signal the differentiation of hormone-producing Leydig cells. In mammals, current evidence indicates that the Y-linked Sry gene activates SOX9 as one of the first steps in the testis-determining pathway (Sekido and Lovell-Badge 2008). SRY is absent in birds, so SOX9, which is autosomal, must be directly or indirectly activated by a signal from the sex chromosomes. In female embryonic Chicken gonads (ZW), SOX9 is never activated. Instead, at the same time (from Day 6, stage 29–30), the oestrogen-synthesising enzyme, aromatase, is activated female-specifically (Fig. 4b). This and other lines of evidence indicate that oestrogen plays a key role in ovarian differentiation in avian embryos.
The importance of oestrogen
The steroid hormone, oestrogen, plays a central role in gonadal sex differentiation in the Chicken (Scheib 1983; Vaillant et al. 2001; Hudson et al. 2005). In embryonic Chicken gonads, all of the steroidogenic enzymes required to produce the oestrogen precursor steroids, testosterone and androstenedione, are present in both sexes (Nakabayashi et al. 1998; Nishikimi et al. 2000). There is no evidence that androgens influence the early gonadal sex differentiation in birds. The two final-step enzymes required to make 17-β-oestradiol from androgens, P450 aromatase and 17-β-hydroxysteroid dehydrogenase (17-βHSD), are only expressed in female gonads from Day 6 of embryogenesis (the onset of ovarian differentiation; Fig. 4a) (Andrews et al. 1997; Smith et al. 1997; Nakabayashi et al. 1998). The importance of oestrogen for female development can be demonstrated by administering a single dose of an aromatase enzyme inhibitor in ovo, which results in testis development and female-to-male sex reversal (Elbrecht and Smith 1992; Abinawanto et al. 1996; Vaillant et al. 2001). As shown in Fig. 5, treatment with fadrozole at Day 3.5 results in greatly reduced aromatase enzyme activity and induces masculinisation of the gonads in ZW (female) embryos. Gonads are stunted, the left fails to form a proper ovary and the right becomes a testis (Fig. 5). Oestrogen receptor α (ER-α), which mediates the action of oestrogen, is expressed in the outer epithelial layer of the left gonads of both male and female embryos (Nakabayashi et al. 1998). Hence, the role of oestrogen in females is to promote proliferation of the outer cortical layer of the left gonad. This is the site of germ-cell meiosis in females. Little if any ER-α is expressed in the right gonad, which explains its failure to elaborate a thickened ovarian cortex. The significance of ER-α expression in the left male gonad is unknown, but it explains the ability of exogenously administered oestrogen to feminise the left gonad of ZZ embryos, by promoting cortical proliferation. The trigger for aromatase and 17-βHSD activation in the female gonad is not currently known, but the transcription factor, FOXL2, is likely to be involved (Govoroun et al. 2004; Hudson et al. 2005). In mammals, loss-of-function mutations of FOXL2 compromise ovarian development (Ottolenghi et al. 2005; Pailhoux et al. 2005; Garcia-Ortiz et al. 2009; Uhlenhaut et al. 2009) and FOXL2 can activate the Aromatase promoter region (Pannetier et al. 2006).
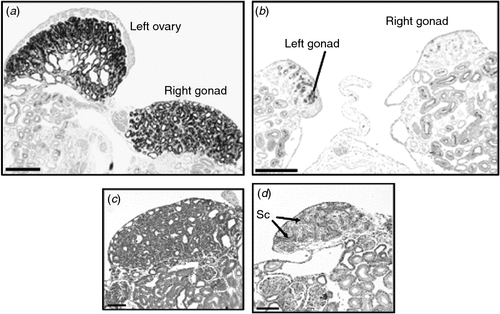
|
In contrast to the key role of oestrogen in female development, no equivalent role has been shown for testosterone in early male development. In males, however, Anti-Müllerian Hormone (AMH) may be important for testicular morphogenesis. AMH is synthesised and released by Sertoli cells and is responsible for regression of the Müllerian duct. (This duct otherwise forms the oviduct of females.) AMH is expressed in both sexes (since the right Müllerian duct also regresses in females) but is more highly expressed in males (Oreal et al. 1998). Interestingly, if testes at embryonic Day 13 are grafted into early (Day 3) female Chicken embryos, the gonads are masculinised, forming partial or complete testes, with Müllerian duct regression (Maraud et al. 1990). It is likely that the masculinising factor involved is AMH. This hormone may therefore have an important role in early testicular development. In contrast, in mammals, where AMH is also male enriched, the hormone has a more downstream role.
Does the W sex chromosome participate in avian sex determination?
The avian W sex chromosome is largely heterochromatic with only a few characterised genes, none of which have an obvious role in sex determination. Yamada and colleagues carried out an extensive expression-based screen of Day 3 and Day 4 Chicken embryos but did not uncover any clear W-linked genes that might regulate gonadal (ovarian) differentiation ((Yamada et al. 2004; Koyama et al. 2007). One W-linked gene that was initially a strong candidate ovary-determining factor is HINTW (Histidine triad NucleoTide binding protein) also known as WPKCI or ASW) (Hori et al. 2000; O’Neill et al. 2000). HINTW is conserved in all carinate birds. In the Chicken embryo, HINTW is expressed throughout the body of female (ZW) embryos, including the gonads. HINTW encodes an unusual isoform of a nucleotide binding protein of the HIT family. It is unusual because it specifically lacks the AMP hydrolase enzyme activity normally found in these proteins. However, a paralagous gene, called HINTZ, is located on the Z sex chromosome. HINTZ does encode a bona fide HIT protein, a dimeric enzyme with AMP-lysine hydrolase activity intact. It has been suggested that HINTW may function in avian sex determination by inhibiting the (male-promoting) activity of HINTZ (Pace and Brenner 2003). In vitro enzyme data support a dominant negative influence of HINTW on HINTZ enzymatic activity (Parks et al. 2004). However, the HINTW gene is apparently absent in ratites, and overexpression in early Chicken embryos does not induce ovarian development, undermining this gene as a candidate female determinant (Smith et al. 2009b). At present, therefore, there is little evidence in support of the dominant W hypothesis for avian sex determination.
The DMRT1 gene and testis development
The alternative to the dominant W hypothesis for avian sex determination is the Z dosage model, or the Z : Autosome ratio. A large number of genes are carried by the Z chromosome and, in the absence of global dosage compensation mechanism, many Z-linked genes are expressed at a higher level in males compared with females (Itoh et al. 2007; Arnold et al. 2008). Among these Z-linked genes is a candidate avian sex determinant that is implicated in male development in many animals. The gene is DMRT1 (Doublesex and Mab-3 Related Transcription factor, # 1). DMRT1 encodes a zinc finger-like transcription factor, and is structurally related to genes that have a male role in animals as distant as flies (Drosophila) and worms (Caenorhabditis elegans) (Raymond et al. 1998). We and others have found that DMRT1 has a conserved male upregulated expression pattern in reptile, bird and mouse embryos (Raymond et al. 1999; Smith et al. 1999; Shan et al. 2000). In the Chicken embryo, DMRT1 is expressed specifically in the urogenital system of both males (ZZ) and females (ZW), but is more highly expressed in males. Mammals with DMRT1 loss-of-function mutations have impaired testicular development (Raymond et al. 2000; Õunap et al. 2004). In lower vertebrates, a duplicated and diverged copy of DMRT1, called DMY/DMRT1bY, is the male determinant in the Medaka Fish (Oryzias latipes) (Matsuda et al. 2002; Nanda et al. 2002), whereas a truncated copy, called DMW, antagonises DMRT1 function and participates in ovarian determinant in the African Clawed Frog (Xenopus laevis) (Yoshimoto et al. 2008, 2010). Hence, this gene and its homologues clearly have a central role in vertebrate gonadal development. DMRT1 is Z-linked in all birds, including the ratites (Shetty et al. 2002). This fact, together with its gonadal expression pattern in developing Chicken embryos, makes DMRT1 a very strong candidate for an avian testis determinant.
To test the role of DMRT1 in avian gonadal development, we used an avian retroviral vector to deliver DMRT1 RNA interference molecules into early (Day 0) Chicken embryos. The replication competent viral vector, called RCASBP, spreads from cell to cell following initial infection (Logan and Tabin 1998), delivering short hairpin RNAs (shRNAs) directed against DMRT1. RNAi relies on the ability of short antisense RNA molecules to bind to target messenger RNA (mRNA) sequences, marking them for degradation or blocking their translation into proteins, or both. In this way, gene expression is ‘knocked down’ or silenced. Knockdown of DMRT1 expression using virally delivered RNAi caused feminisation of genetic males (ZZ) by Day 10 of development (Smith et al. 2009a). In control females infected with virus carrying non-silencing scrambled control shRNA, the gonads developed normally. The right gonad regressed, whereas the left formed a typical ovary, with thickened outer cortex and the inner medulla that was fragmented (Fig. 6a–c). In control males, bilateral testes developed, characterised by compact seminiferous cords and a thin outer gonadal (germinal) epithelium (Fig. 6d–f). Female embryos subjected to DMRT1 knockdown appeared normal. However, males subjected to DMRT1 knockdown exhibited a left ovarian-type gonad and a right testicular or poorly differentiated gonad (Fig. 6g–i). The left gonad had a female-type thickened outer cortex and fragmented medulla (Fig. 6j). Gene-expression analysis, using quantitative reverse transcription polymerase chain reaction (RT–PCR), confirmed knockdown of DMRT1 transcripts (Fig. 7a). In control males (ZZ) the DMRT1 protein is expressed in Sertoli cells through the seminiferous cords of the developing testis (Fig. 7a). In DMRT1 knockdown males, little if any DMRT1 protein is detectable (Fig. 7b). At the mRNA level, endogenous DMRT1 expression is greatly reduced in the knockdown embryos (Fig. 7c). In the ZZ DMRT1 knockdown gonads, expression of the testicular marker, SOX9, was significantly reduced, whereas the female marker, Aromatase, was ectopically activated (Fig. 7d, e). Taken together, these data show that the Z-linked gene, DMRT1, is required for proper male development in the Chicken embryo.
|
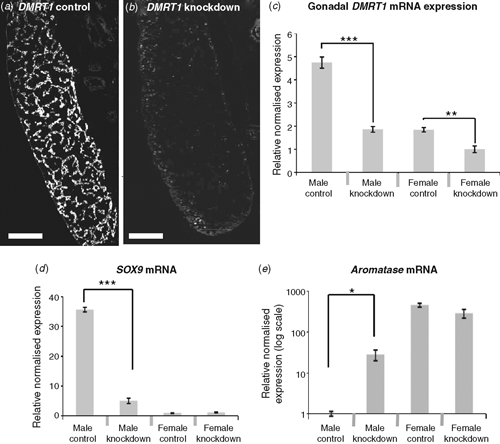
|
The DMRT1 knockdown experiments support the Z dosage model of avian sex determination, whereby DMRT1 dosage directs testicular versus ovarian development (higher in males). Is DMRT1 the master avian sex-determining switch gene? It is expressed at the gonads before and during sexual differentiation, it is conserved on the Z in all birds, and knockdown induces male-to-female sex reversal in the Chicken embryo. It is theoretically possible that another Z-linked gene lies upstream of DMRT1 in the testis pathway, although this seems unlikely. In addition, in tissues outside the gonads, the gynandromorphic Chicken study (Zhao et al. 2010) suggests that other Z-linked genes may operate to regulate sex outside the gonads, because DMRT1 is not expressed outside the urogenital system. DMRT1 is required for testis development, but is unlikely to be the master sex-determining switch in other tissues or the embryo as a whole, since cells appear to know their sex before formation of the gonads (Zhao et al. 2010). Hence, there are still several questions surrounding avian sex determination, and the picture is not yet complete. The nature of the cell autonomous sex-determining switch outside the gonads remains to be defined. Is it controlled by several Z-linked genes, or by different genes in different tissues, or by an as yet undefined W-linked gene?
Avian sex chromosomes and sex-determining genes: applications to wild and domesticated birds
The analysis of avian sex chromosomes and sex-determining genes has important implications for both wild and domesticated birds. First, DNA sequences derived from the sex chromosomes provide valuable tools for the molecular sexing of birds. This allows the sexing of fledgling or immature birds, and mature birds that are not sexually dimorphic, and provides critical information for the breeding of rare or endangered species. Second, by genetically manipulating key sex-determining genes, the sex of commercially produced birds can potentially be altered. This is of value in the poultry industry, for example, where females are required for laying, whereas males are required for meat production.
Birds can be sexed at the molecular level by utilising sex-linked DNA sequences. For example, repetitive sequences of the XhoI family occupy ~70% of the W sex chromosome of the Chicken (Kodama et al. 1987; Klein and Ellendorff 2000). Amplification of these elements by polymerase chain reaction (PCR) is now routinely used to sex Chicken embryos (Clinton et al. 2001). Females show a DNA band on an agarose gel whereas males do not. However, such repetitive elements are rapidly evolving and the W-linked XhoI repeat is absent outside the Galliformes (which includes the Chicken, turkeys, pheasants and other gamefowl). Alternative W-linked sequences that are more widely conserved have been utilised to sex a variety of avian species, from many different families. For example, the conserved W-linked gene, CHD-W (ChromoHelicase DNA-binding protein-W linked) has a homologue on the Z (CHD-Z) but the two sequences can be distinguished by different sized introns, or by the presence of unique restriction enzyme sites (Griffiths et al. 1996; Chang et al. 2008). This has allowed the use of the sex-linked CHD gene for the molecular sexing of several different birds, including rare species (Ellegren 1996; Griffiths et al. 1998; Wang et al. 2007). The last remaining Spix’s Macaw (Cyanopsitta spixii) was sexed in this way (Griffiths and Tlwarl 1995). The CHD-W and CHD-Z genes can be readily distinguished in all birds, with the exception of the ratites (Emu, Ostrich, Southern Cassowary, rhea, kiwi (Apteryx spp.) and tinamous). This is consistent with the near-complete homology of the Z and W sex chromosomes in most members of this group (Ogawa et al. 1998; Nishida-Umehara et al. 2007). However, even among this group, molecular sexing has been successfully carried out, using an unrelated W-linked (female-specific) sequence (Huynen et al. 2002). Hence, all birds can now be sexed at the molecular level, enhancing studies on avian reproductive biology, ecology, breeding and conservation.
References
Abinawanto, , Shimada, K., Yoshida, K., and Saito, N. (1996). Effects of aromatase inhibitor on sex differentiation and levels of P450 (17 alpha) and P450 arom messenger ribonucleic acid of gonads in chicken embryos. General and Comparative Endocrinology 102, 241–246.| Effects of aromatase inhibitor on sex differentiation and levels of P450 (17 alpha) and P450 arom messenger ribonucleic acid of gonads in chicken embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XivV2qtbY%3D&md5=7223417c85823a34ad7d25f269000b8fCAS |
Agate, R. J., Grisham, W., Wade, J., Mann, S., Wingfield, J., Schanen, C., Palotie, A., and Arnold, A. P. (2003). Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proceedings of the National Academy of Sciences of the United States of America 100, 4873–4878.
| Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt12ntrY%3D&md5=65c3cf037e0344fac201b0974ed9750aCAS | 12672961PubMed |
Andrews, J. E., Smith, C. A., and Sinclair, A. H. (1997). Sites of estrogen receptor and aromatase expression in the chicken embryo. General and Comparative Endocrinology 108, 182–190.
| Sites of estrogen receptor and aromatase expression in the chicken embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntlOhtL8%3D&md5=e72611377a2493a01e761f30d7c9f059CAS | 9356214PubMed |
Arlt, D., Bensch, S., Hansson, B., Hasselquist, D., and Westerdahl, H. (2004). Observation of a ZZW female in a natural population: implications for avian sex determination. Proceedings of the Royal Society of London. Series B. Biological Sciences 271, S249–S251.
| Observation of a ZZW female in a natural population: implications for avian sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsVWjs7w%3D&md5=616b390a0be43028ce8e9055201b653cCAS |
Arnold, A. P., Itoh, Y., and Melamed, E. (2008). A bird’s-eye view of sex chromosome dosage compensation. Annual Review of Genomics and Human Genetics 9, 109–127.
| A bird’s-eye view of sex chromosome dosage compensation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1SnsbnN&md5=53cf48f6806bdcd1ebf9e48d01d6e470CAS | 18489256PubMed |
Bellott, D. W., Skaletsky, H., Pyntikova, T., Mardis, E. R., Graves, T., Kremitzki, C., Brown, L. G., Rozen, S., et al (2010). Convergent evolution of chicken Z and human X chromosomes by expansion and gene acquisition. Nature 466, 612–616.
| Convergent evolution of chicken Z and human X chromosomes by expansion and gene acquisition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXos1aitb8%3D&md5=f064cbffb10441874b0234fed41dfd90CAS | 20622855PubMed |
Capel, B. (1998). Sex in the 90s: SRY and the switch to the male pathway. Annual Review of Physiology 60, 497–523.
| Sex in the 90s: SRY and the switch to the male pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitVOhsb4%3D&md5=dd413ff4228b218e6c3479752e1bb7bdCAS | 9558474PubMed |
Chang, H.-W., Cheng, C.-A., Gu, D.-L., Chang, C.-C., Su, S.-H., Wen, C.-H., Chou, Y.-C., Chou, T.-C., et al (2008). High-throughput avian molecular sexing by SYBR green-based real-time PCR combined with melting curve analysis. BMC Biotechnology 8, 12.
| High-throughput avian molecular sexing by SYBR green-based real-time PCR combined with melting curve analysis.Crossref | GoogleScholarGoogle Scholar | 18269737PubMed |
Clinton, M. (1998). Sex determination and gonadal development: a bird’s eye view. Journal of Experimental Zoology 281, 457–465.
| Sex determination and gonadal development: a bird’s eye view.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1czislegtg%3D%3D&md5=cfd92086c7991ff60a4546bd1e3b8d9eCAS | 9662832PubMed |
Clinton, M., Haines, L., Belloir, B., and McBride, D. (2001). Sexing chick embryos: a rapid and simple protocol. British Poultry Science 42, 134–138.
| Sexing chick embryos: a rapid and simple protocol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjsFSgu7g%3D&md5=37024d12c997a9b97d55421ca30656b3CAS | 11337963PubMed |
Coriat, A. M., Muller, U., Harry, J. L., Uwanogho, D., and Sharpe, P. T. (1993). PCR amplification of SRY-related gene sequences reveals evolutionary conservation of the SRY-box motif. PCR Methods and Applications 2, 218–222.
| 1:CAS:528:DyaK3sXksVagu74%3D&md5=2c2e107b8f552feb896de1acd4536e4bCAS | 8443573PubMed |
Elbrecht, A., and Smith, R. G. (1992). Aromatase enzyme activity and sex determination in chickens. Science 255, 467–470.
| Aromatase enzyme activity and sex determination in chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XhslSlsrk%3D&md5=08e9f40eed76c846fce54685f8ec903bCAS | 1734525PubMed |
Ellegren, H. (1996). First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proceedings of the Royal Society of London. Series B. Biological Sciences 263, 1635–1641.
| First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhtVCiu7Y%3D&md5=35567f04e25ed699f043ac7ddb9d38bfCAS |
Ellegren, H. (2000). Evolution of the avian sex chromosomes and their role in sex determination. Trends in Ecology & Evolution 15, 188–192.
| Evolution of the avian sex chromosomes and their role in sex determination.Crossref | GoogleScholarGoogle Scholar |
Ellegren, H., Hultin-Rosenberg, L., Brunstrom, B., Dencker, L., Kultima, K., and Scholz, B. (2007). Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biology 5, 40.
| Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes.Crossref | GoogleScholarGoogle Scholar | 17883843PubMed |
Ferguson, M. W., and Joanen, T. (1982). Temperature of egg incubation determines sex in Alligator mississippiensis. Nature 296, 850–853.
| Temperature of egg incubation determines sex in Alligator mississippiensis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL387ntlaiuw%3D%3D&md5=1ffadf2aadd9f11781e1a2c41300c495CAS | 7070524PubMed |
Fridolfsson, A. K., Cheng, H., Copeland, N. G., Jenkins, N. A., Liu, H. C., Raudsepp, T., Woodage, T., Chowdhary, B., Halverson, J., and Ellegren, H. (1998). Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proceedings of the National Academy of Sciences of the United States of America 95, 8147–8152.
| Evolution of the avian sex chromosomes from an ancestral pair of autosomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXks1Wltbs%3D&md5=47e1955e232b5780546c18ab88e9125eCAS | 9653155PubMed |
Fujimoto, T., Ukeshima, A., and Kiyofuji, R. (1976). The origin, migration and morphology of the primordial germ cells in the chick embryo. Anatomical Record 185, 139–153.
| The origin, migration and morphology of the primordial germ cells in the chick embryo.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE283gtl2lsg%3D%3D&md5=a1d092a8345fdb2c106bddb446017c48CAS | 945007PubMed |
Gahr, M. (2003). Male Japanese Quails with female brains do not show male sexual behaviors. Proceedings of the National Academy of Sciences of the United States of America 100, 7959–7964.
| Male Japanese Quails with female brains do not show male sexual behaviors.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlt1Wqt7o%3D&md5=ef2c8456a00e9178df470725fce28e4bCAS | 12802009PubMed |
Garcia-Ortiz, J. E., Pelosi, E., Omari, S., Nedorezov, T., Piao, Y., Karmazin, J., Uda, M., Cao, A., et al (2009). Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Developmental Biology 9, 36.
| Foxl2 functions in sex determination and histogenesis throughout mouse ovary development.Crossref | GoogleScholarGoogle Scholar | 19538736PubMed |
Gasc, J. M. (1978). Growth and sexual differentiation in the gonads of chick and duck embryos. Journal of Embryology and Experimental Morphology 44, 1–13.
| 1:STN:280:DyaE1c7nt1yqtg%3D%3D&md5=a539a61af2a4f43b3a44e04b27429d51CAS | 650128PubMed |
Göth, A., and Booth, D. T. (2005). Temperature-dependent sex ratio in a bird. Biology Letters 1, 31–33.
| Temperature-dependent sex ratio in a bird.Crossref | GoogleScholarGoogle Scholar | 17148121PubMed |
Govoroun, M. S., Pannetier, M., Pailhoux, E., Cocquet, J., Brillard, J. P., Couty, I., Batellier, F., and Cotinot, C. (2004). Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Developmental Dynamics 231, 859–870.
| Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFakur3K&md5=1a9e5a04f183a00850daa25fc3df9d0cCAS | 15517586PubMed |
Griffin, D. K., Robertson, L. B., Tempest, H. G., and Skinner, B. M. (2007). The evolution of the avian genome as revealed by comparative molecular cytogenetics. Cytogenetic and Genome Research 117, 64–77.
| The evolution of the avian genome as revealed by comparative molecular cytogenetics.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2svlvVenug%3D%3D&md5=3d9d3e66ced06cb7acbe0cc02d854fb4CAS | 17675846PubMed |
Griffiths, R. (1991). The isolation of conserved DNA sequences related to the human sex-determining region Y gene from the Lesser Black-backed Gull (Larus fuscus). Proceedings of the Royal Society of London. Series B. Biological Sciences 244, 123–128.
| The isolation of conserved DNA sequences related to the human sex-determining region Y gene from the Lesser Black-backed Gull (Larus fuscus).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXlsFahurk%3D&md5=e9b2e73c79932ea1412a2e0adfc35d77CAS |
Griffiths, R., and Tlwarl, B. (1995). Sex of the last wild Spix’s Macaw. Nature 375, 454.
| Sex of the last wild Spix’s Macaw.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmtFCgs70%3D&md5=25159e11caac05cc27b92da003fdbd3aCAS | 7777052PubMed |
Griffiths, R., Daan, S., and Dijkstra, C. (1996). Sex identification in birds using two CHD genes. Proceedings of the Royal Society of London. Series B. Biological Sciences 263, 1251–1256.
| Sex identification in birds using two CHD genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XmsFCitLw%3D&md5=326eafc238bdb3b8062c42634889b444CAS |
Griffiths, R., Double, M. C., Orr, K., and Dawson, R. J. (1998). A DNA test to sex most birds. Molecular Ecology 7, 1071–1075.
| A DNA test to sex most birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlslGmt7c%3D&md5=865575e8ccef2b7f96e7833bbc857648CAS | 9711866PubMed |
Guioli, S., and Lovell-Badge, R. (2007). PITX2 controls asymmetric gonadal development in both sexes of the chick and can rescue the degeneration of the right ovary. Development 134, 4199–4208.
| PITX2 controls asymmetric gonadal development in both sexes of the chick and can rescue the degeneration of the right ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtFegsQ%3D%3D&md5=6e8dcb2a3f5d3203556be25b72de6833CAS | 17959721PubMed |
Hamburger, V., and Hamilton, H. L. (1951). A series of normal stages in the development of the chick embryo. Journal of Morphology 88, 49–92.
| A series of normal stages in the development of the chick embryo.Crossref | GoogleScholarGoogle Scholar |
Hillier, L. W., Miller, W., Birney, E., Warren, W., Hardison, R. C., Ponting, C. P., Bork, P., Burt, D. W., et al (2004). Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432, 695–716.
| Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVGmtb7M&md5=a93e35816a470a70c5058a182bccd6eeCAS | 15592404PubMed |
Hori, T., Asakawa, S., Itoh, Y., Shimizu, N., and Mizuno, S. (2000). Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implication of its role in female sex determination. Molecular Biology of the Cell 11, 3645–3660.
| 1:CAS:528:DC%2BD3cXnslWntb8%3D&md5=125c44ff66847c83fc2496465308ffe3CAS | 11029061PubMed |
Hudson, Q. J., Smith, C. A., and Sinclair, A. H. (2005). Aromatase inhibition reduces expression of FOXL2 in the embryonic chicken ovary. Developmental Dynamics 233, 1052–1055.
| Aromatase inhibition reduces expression of FOXL2 in the embryonic chicken ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmtFKgsbc%3D&md5=f4c874f116d5986c83b75f1c1c166febCAS | 15830351PubMed |
Huynen, L., Millar, C. D., and Lambert, D. M. (2002). A DNA test to sex ratite birds. Molecular Ecology 11, 851–856.
| A DNA test to sex ratite birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktFahs7o%3D&md5=5456757fb925d0634f1df2fd8838ce70CAS | 11972770PubMed |
Ishimaru, Y., Komatsu, T., Kasahara, M., Katoh-Fukui, Y., Ogawa, H., Toyama, Y., Maekawa, M., Toshimori, K., et al (2008). Mechanism of asymmetric ovarian development in chick embryos. Development 135, 677–685.
| Mechanism of asymmetric ovarian development in chick embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsFyhsbg%3D&md5=384d22ecd8cf53b97791a8aa52cd0c1aCAS | 18199582PubMed |
Itoh, Y., Melamed, E., Yang, X., Kampf, K., Wang, S., Yehya, N., Van Nas, A., Replogle, K., et al (2007). Dosage compensation is less effective in birds than in mammals. Journal of Biology 6, 2.
| Dosage compensation is less effective in birds than in mammals.Crossref | GoogleScholarGoogle Scholar | 17352797PubMed |
Itoh, Y., Kampf, K., and Arnold, A. P. (2008). Molecular cloning of Zebra Finch W chromosome repetitive sequences: evolution of the avian W chromosome. Chromosoma 117, 111–121.
| Molecular cloning of Zebra Finch W chromosome repetitive sequences: evolution of the avian W chromosome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjsFGitbo%3D&md5=449de7022fb3ad67fb4cb5a25a292853CAS | 17972090PubMed |
Johnston, C. M., Barnett, M., Sharpe, P. T., Graves, J. A. M., Renfree, M. B., Capel, B., and Mireille, D. (1995). The molecular biology of temperature-dependent sex determination. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences 350, 297–303.
| The molecular biology of temperature-dependent sex determination.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK287isVajtg%3D%3D&md5=5f1e4c15ac0ea4788472fc9689c845acCAS |
Kent, J., Wheatley, S. C., Andrews, J. E., Sinclair, A. H., and Koopman, P. (1996). A male-specific role for Sox9 in vertebrate sex determination. Development 122, 2813–2822.
| 1:CAS:528:DyaK28XlvFCkt7g%3D&md5=b99b080228f8edf17742baeeb0efc69cCAS | 8787755PubMed |
Klein, S., and Ellendorff, F. (2000). Localisation of Xho1 repetitive sequences on autosomes in addition to the W chromosome in chickens and its relevance for sex diagnosis. Animal Genetics 31, 104–109.
| Localisation of Xho1 repetitive sequences on autosomes in addition to the W chromosome in chickens and its relevance for sex diagnosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtVyjurk%3D&md5=1f18ad0bb34e53e5bcb34070ebfe0bebCAS | 10782208PubMed |
Kobayashi, A., Chang, H., Chaboissier, M. C., Schedl, A., and Behringer, R. R. (2005). Sox9 in testis determination. Annals of the New York Academy of Sciences 1061, 9–17.
| Sox9 in testis determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhvVamurg%3D&md5=01d96ece48b06ffb4b2bc8cca29c8c35CAS | 16467253PubMed |
Kodama, H., Saitoh, H., Tone, M., Kuhara, S., Sakaki, Y., and Mizuno, S. (1987). Nucleotide sequences and unusual electrophoretic behavior of the W chromosome-specific repeating DNA units of the domestic fowl, Gallus gallus domesticus. Chromosoma 96, 18–25.
| Nucleotide sequences and unusual electrophoretic behavior of the W chromosome-specific repeating DNA units of the domestic fowl, Gallus gallus domesticus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXovVyntA%3D%3D&md5=90c67a0e2fd3d6187e5309a160238adcCAS | 2893693PubMed |
Koopman, P., and Loffler, K. A. (2003). Sex determination: the fishy tale of Dmrt1. Current Biology 13, R177–R179.
| Sex determination: the fishy tale of Dmrt1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhvFaitLg%3D&md5=70d4965bc2720c77214f36491118ac72CAS | 12620206PubMed |
Koyama, Y., Yamada, D., Saito, Y., Sato, T., Miyai, S., Tasaki, M., Kato, J., Kasumi, T., et al (2007). Sequence analysis of full-length cDNA of sex chromosome-linked novel gene 2d-2F9 in Gallus gallus. Bioscience, Biotechnology, and Biochemistry 71, 561–570.
| Sequence analysis of full-length cDNA of sex chromosome-linked novel gene 2d-2F9 in Gallus gallus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXivV2itb0%3D&md5=caee668de020f5b9a21f1eb86c596314CAS | 17284846PubMed |
Kuroda, Y., Arai, N., Arita, M., Teranishi, M., Hori, T., Harata, M., and Mizuno, S. (2001). Absence of Z-chromosome inactivation for five genes in male chickens. Chromosome Research 9, 457–468.
| Absence of Z-chromosome inactivation for five genes in male chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnt1ektb8%3D&md5=329135b00004a5e02ba8fa0a63f63c1fCAS | 11592480PubMed |
Lin, M., Thorne, M. H., Martin, I. C. A., Sheldon, B. L., and Jones, R. C. (1995). Development of the gonads in the triploid (ZZW and ZZZ) fowl, Gallus domesticus, and comparison with normal diploid males (ZZ) and females (ZW). Reproduction, Fertility and Development 7, 1185–1197.
| Development of the gonads in the triploid (ZZW and ZZZ) fowl, Gallus domesticus, and comparison with normal diploid males (ZZ) and females (ZW).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK283kvFeiuw%3D%3D&md5=261a394eda262b11ae0047afde918373CAS |
Logan, M., and Tabin, C. (1998). Targeted gene misexpression in chick limb buds using avian replication-competent retroviruses. Methods (San Diego, Calif.) 14, 407–420.
| Targeted gene misexpression in chick limb buds using avian replication-competent retroviruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtFant7s%3D&md5=90298cc2d0f3e35ec547607ad3578338CAS | 9608511PubMed |
Mank, J. E., and Ellegren, H. (2007). Parallel divergence and degradation of the avian W sex chromosome. Trends in Ecology & Evolution 22, 389–391.
| Parallel divergence and degradation of the avian W sex chromosome.Crossref | GoogleScholarGoogle Scholar |
Maraud, R., Vergnaud, O., and Rashedi, M. (1990). New insights on the mechanism of testis differentiation from the morphogenesis of experimentally induced testes in genetically female chick embryos. American Journal of Anatomy 188, 429–437.
| New insights on the mechanism of testis differentiation from the morphogenesis of experimentally induced testes in genetically female chick embryos.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3czmsV2qtg%3D%3D&md5=01616cdec1a3984d77010dacc9c329afCAS | 2392999PubMed |
Marshall Graves, J. A. (2003). Sex and death in birds: a model of dosage compensation that predicts lethality of sex chromosome aneuploids. Cytogenetic and Genome Research 101, 278–282.
| Sex and death in birds: a model of dosage compensation that predicts lethality of sex chromosome aneuploids.Crossref | GoogleScholarGoogle Scholar | 14684995PubMed |
Marshall Graves, J. A. (2008). Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annual Review of Genetics 42, 565–586.
| Weird animal genomes and the evolution of vertebrate sex and sex chromosomes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFemtbvO&md5=48ed450bb6750ba10131784652ea4337CAS | 18983263PubMed |
Marshall Graves, J. A., and Shetty, S. (2001). Sex from W to Z: evolution of vertebrate sex chromosomes and sex determining genes. Journal of Experimental Zoology 290, 449–462.
| Sex from W to Z: evolution of vertebrate sex chromosomes and sex determining genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntFamsLg%3D&md5=a6ece9ce2c8b41b6fca9ee47a1d2f996CAS | 11555852PubMed |
Matsuda, M., Nagahama, Y., Shinomiya, A., Sato, T., Matsuda, C., Kobayashi, T., Morrey, C. E., Shibata, N., et al (2002). DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417, 559–563.
| DMY is a Y-specific DM-domain gene required for male development in the medaka fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XktVehtb8%3D&md5=45094fa2a87291a1ff48f9eba6c3b11aCAS | 12037570PubMed |
McBride, D., Sang, H., and Clinton, M. (1997). Expression of Sry-related genes in the developing genital ridge/mesonephros of the chick embryo. Journal of Reproduction and Fertility 109, 59–63.
| Expression of Sry-related genes in the developing genital ridge/mesonephros of the chick embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhs1Gntb8%3D&md5=677a0257a4ea23ae19bcf68698d2cf76CAS | 9068414PubMed |
McQueen, H. A., and Clinton, M. (2009). Avian sex chromosomes: dosage compensation matters. Chromosome Research 17, 687–697.
| Avian sex chromosomes: dosage compensation matters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1WhsLrK&md5=6118b1c4936bd9612ed8edcdc2aa200bCAS | 19802708PubMed |
Melamed, E., and Arnold, A. P. (2007). Regional differences in dosage compensation on the chicken Z chromosome. Genome Biology 8, R202.
| Regional differences in dosage compensation on the chicken Z chromosome.Crossref | GoogleScholarGoogle Scholar | 17900367PubMed |
Merchant-Larios, H., Diaz-Hernandez, V., and Marmolejo-Valencia, A. (2010). Gonadal morphogenesis and gene expression in reptiles with temperature-dependent sex determination. Sexual Development 4, 50–61.
| Gonadal morphogenesis and gene expression in reptiles with temperature-dependent sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtlahu74%3D&md5=3b50a54c145801033d31858ec88196dbCAS | 20090307PubMed |
Mizuno, S., Kunita, R., Nakabayashi, O., Kuroda, Y., Arai, N., Harata, M., Ogawa, A., Itoh, Y., Teranishi, M., and Hori, T. (2002). Z and W chromosomes of chickens: studies on their gene functions in sex determination and sex differentiation. Cytogenetic and Genome Research 99, 236–244.
| Z and W chromosomes of chickens: studies on their gene functions in sex determination and sex differentiation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3szms1Srtw%3D%3D&md5=e4975ee7b1e0ad5901fe51a42a19814bCAS | 12900570PubMed |
Morais da Silva, S., Hacker, A., Harley, V., Goodfellow, P., Swain, A., and Lovell-Badge, R. (1996). Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nature Genetics 14, 62–68.
| Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xls1Sqtbc%3D&md5=7e147b626058a2b9c5a8708c6c3e5a5fCAS | 8782821PubMed |
Nakabayashi, O., Kikuchi, H., Kikuchi, T., and Mizuno, S. (1998). Differential expression of genes for aromatase and estrogen receptor during the gonadal development in chicken embryos. Journal of Molecular Endocrinology 20, 193–202.
| Differential expression of genes for aromatase and estrogen receptor during the gonadal development in chicken embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXislartrw%3D&md5=b3db51ff54a67119d72055ebce75c689CAS | 9584834PubMed |
Nanda, I., Sick, C., Münster, U., Kaspers, B., Schartl, M., Staeheli, P., and Schmid, M. (1998). Sex chromosome linkage of chicken and duck type 1 interferon genes: further evidence of evolutionary conservation of the Z chromosome in birds. Chromosoma 107, 204–210.
| Sex chromosome linkage of chicken and duck type 1 interferon genes: further evidence of evolutionary conservation of the Z chromosome in birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjvVyitr8%3D&md5=78d7dc6dabb80b6e1ba79fabd7f48a31CAS | 9639659PubMed |
Nanda, I., Shan, Z., Schartl, M., Burt, D. W., Koehler, M., Nothwang, H., Grutzner, F., Paton, I. R., et al (1999). 300 million years of conserved synteny between chicken Z and human chromosome 9. Nature Genetics 21, 258–259.
| 300 million years of conserved synteny between chicken Z and human chromosome 9.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXitVCit7k%3D&md5=b9652b8023222ff1fed6551faef691c6CAS | 10080173PubMed |
Nanda, I., Kondo, M., Hornung, U., Asakawa, S., Winkler, C., Shimizu, A., Shan, Z., Haaf, T., et al (2002). A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proceedings of the National Academy of Sciences of the United States of America 99, 11 778–11 783.
| A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntFWqt78%3D&md5=edc943a7fa6787462397a19a54b58703CAS |
Nanda, I., Schlegelmilch, K., Haaf, T., Schartl, M., and Schmid, M. (2008). Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination. Cytogenetic and Genome Research 122, 150–156.
| Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M%2FktFSgtg%3D%3D&md5=ab7af522ffceffb7ab1ea0387bffc0a3CAS | 19096210PubMed |
Nishida-Umehara, C., Tsuda, Y., Ishijima, J., Ando, J., Fujiwara, A., Matsuda, Y., and Griffin, D. K. (2007). The molecular basis of chromosome orthologies and sex chromosomal differentiation in palaeognathous birds. Chromosome Research 15, 721–734.
| The molecular basis of chromosome orthologies and sex chromosomal differentiation in palaeognathous birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVChu7%2FF&md5=05bb50d090b5e35c9acb62c90a74a8a8CAS | 17605112PubMed |
Nishikimi, H., Kansaku, N., Saito, N., Usami, M., Ohno, Y., and Shimada, K. (2000). Sex differentiation and mRNA expression of P450c17, P450arom and AMH in gonads of the chicken. Molecular Reproduction and Development 55, 20–30.
| Sex differentiation and mRNA expression of P450c17, P450arom and AMH in gonads of the chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXnvFyntr8%3D&md5=9789a8ca7e1644c247abaa13ec11b6a9CAS | 10602270PubMed |
O’Neill, M., Binder, M., Smith, C., Andrews, J., Reed, K., Smith, M., Millar, C., Lambert, D., and Sinclair, A. (2000). ASW: a gene with conserved avian W-linkage and female specific expression in chick embryonic gonad. Development Genes and Evolution 210, 243–249.
| ASW: a gene with conserved avian W-linkage and female specific expression in chick embryonic gonad.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXisVGlt7o%3D&md5=daa98381c56a58de9b47ae3f8ec2a286CAS | 11180828PubMed |
Ogawa, A., Murata, K., and Mizuno, S. (1998). The location of Z- and W-linked marker genes and sequence on the homomorphic sex chromosomes of the ostrich and the emu. Proceedings of the National Academy of Sciences of the United States of America 95, 4415–4418.
| The location of Z- and W-linked marker genes and sequence on the homomorphic sex chromosomes of the ostrich and the emu.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXis1Ohu7s%3D&md5=00b272e47d4a859e6bc2fb192902bb92CAS | 9539751PubMed |
Oreal, E., Pieau, C., Mattei, M. G., Josso, N., Picard, J. Y., Carre-Eusebe, D., and Magre, S. (1998). Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Developmental Dynamics 212, 522–532.
| Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXlt1ahs7s%3D&md5=39fa9429b5a137275a62ad04e509a6f2CAS | 9707325PubMed |
Ottolenghi, C., Omari, S., Garcia-Ortiz, J. E., Uda, M., Crisponi, L., Forabosco, A., Pilia, G., and Schlessinger, D. (2005). Foxl2 is required for commitment to ovary differentiation. Human Molecular Genetics 14, 2053–2062.
| Foxl2 is required for commitment to ovary differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlslyjs7Y%3D&md5=45e2057320f1e2a43082178bc6673021CAS | 15944199PubMed |
Õunap, K., Uibo, O., Zordania, R., Kiho, L., Ilus, T., Õiglane-Shlik, E., and Bartsch, O. (2004). Three patients with 9p deletions including DMRT1 and DMRT2: a girl with XY complement, bilateral ovotestes, and extreme growth retardation, and two XX females with normal pubertal development. American Journal of Medical Genetics. Part A 130A, 415–423.
| Three patients with 9p deletions including DMRT1 and DMRT2: a girl with XY complement, bilateral ovotestes, and extreme growth retardation, and two XX females with normal pubertal development.Crossref | GoogleScholarGoogle Scholar | 15481033PubMed |
Pace, H. C., and Brenner, C. (2003). Feminizing chicks: a model for avian sex determination based on titration of Hint enzyme activity and the predicted structure of an Asw-Hint heterodimer. Genome Biology 4, R18.
| Feminizing chicks: a model for avian sex determination based on titration of Hint enzyme activity and the predicted structure of an Asw-Hint heterodimer.Crossref | GoogleScholarGoogle Scholar | 12620103PubMed |
Pailhoux, E., Vigier, B., Schibler, L., Cribiu, E. P., Cotinot, C., and Vaiman, D. (2005). Positional cloning of the PIS mutation in goats and its impact on understanding mammalian sex-differentiation. Genetics, Selection, Evolution 37, S55–S64.
| Positional cloning of the PIS mutation in goats and its impact on understanding mammalian sex-differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1ens7w%3D&md5=6532f1604813ce8a3aaa6270e8ed3868CAS |
Pannetier, M., Fabre, S., Batista, F., Kocer, A., Renault, L., Jolivet, G., Mandon-Pepin, B., Cotinot, C., Veitia, R., and Pailhoux, E. (2006). FOXL2 activates P450 aromatase gene transcription: towards a better characterization of the early steps of mammalian ovarian development. Journal of Molecular Endocrinology 36, 399–413.
| FOXL2 activates P450 aromatase gene transcription: towards a better characterization of the early steps of mammalian ovarian development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtFSrtL4%3D&md5=f7a27d53662573611c4e8799cc64359cCAS | 16720712PubMed |
Parks, K. P., Seidle, H., Wright, N., Sperry, J. B., Bieganowski, P., Howitz, K., Wright, D. L., and Brenner, C. (2004). Altered specificity of Hint-W123Q supports a role for Hint inhibition by ASW in avian sex determination. Physiological Genomics 20, 12–14.
| Altered specificity of Hint-W123Q supports a role for Hint inhibition by ASW in avian sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKktb8%3D&md5=fd5c12f4b756b327dc83fdc8212e8416CAS | 15507519PubMed |
Raymond, C. S., Shamu, C. E., Shen, M. M., Seifert, K. J., Hirsch, B., Hodgkin, J., and Zarkower, D. (1998). Evidence for evolutionary conservation of sex-determining genes. Nature 391, 691–695.
| Evidence for evolutionary conservation of sex-determining genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXht1GmtLw%3D&md5=af5241acbaf08e8aeeea2ef8189f918eCAS | 9490411PubMed |
Raymond, C. S., Kettlewell, J. R., Hirsch, B., Bardwell, V. J., and Zarkower, D. (1999). Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Developmental Biology 215, 208–220.
| Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmvFyrtb4%3D&md5=6dac2b1013d1ac3a02de51076659c19dCAS | 10545231PubMed |
Raymond, C. S., Murphy, M. W., O’Sullivan, M. G., Bardwell, V. J., and Zarkower, D. (2000). Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes & Development 14, 2587–2595.
| Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnslGru70%3D&md5=ab6aca20ada583d31a1364cc9a412017CAS | 11040213PubMed |
Renfree, M. B., and Short, R. V. (1988). Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences 322, 41–53.
| Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1M3ktV2mtg%3D%3D&md5=321ee207f428c8fae579751b14446fd8CAS |
Rodriguez-Leon, J., Rodriguez Esteban, C., Marti, M., Santiago-Josefat, B., Dubova, I., Rubiralta, X., and Izpisua Belmonte, J. C. (2008). Pitx2 regulates gonad morphogenesis. Proceedings of the National Academy of Sciences of the United States of America 105, 11 242–11 247.
| Pitx2 regulates gonad morphogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVShur3N&md5=38e39c206e965114e24cffe8482ca23eCAS |
Saitoh, Y., and Mizuno, S. (1992). Distribution of XhoI and EcoRI family repetitive DNA sequences into separate domains in the chicken W chromosome. Chromosoma 101, 474–477.
| Distribution of XhoI and EcoRI family repetitive DNA sequences into separate domains in the chicken W chromosome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XlvVGqsr4%3D&md5=4fa61cc13aad3802065c1b3dbdffce3bCAS | 1424991PubMed |
Scheib, D. (1983). Effects and role of estrogens in avian gonadal differentiation. Differentiation 23, S87–S92.
| 6444180PubMed |
Schmid, M., Nanda, I., Hoehn, H., Schartl, M., Haaf, T., Buerstedde, J. M., Arakawa, H., Caldwell, R. B., Weigend, S., Burt, D. W., et al (2005). Second report on chicken genes and chromosomes 2005. Cytogenetic and Genome Research 109, 415–479.
| Second report on chicken genes and chromosomes 2005.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M3mtlSmsw%3D%3D&md5=d649c7f13576204b1ffcf1a3e463901eCAS | 15905640PubMed |
Scholz, B., Kultima, K., Mattsson, A., Axelsson, J., Brunstrom, B., Halldin, K., Stigson, M., and Dencker, L. (2006). Sex-dependent gene expression in early brain development of chicken embryos. BMC Neuroscience 7, 12.
| Sex-dependent gene expression in early brain development of chicken embryos.Crossref | GoogleScholarGoogle Scholar | 16480516PubMed |
Sekido, R., and Lovell-Badge, R. (2008). Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453, 930–934.
| Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXntVCqtbc%3D&md5=0761a98fbe89676c108ff37670d6b26aCAS | 18454134PubMed |
Shan, Z., Nanda, I., Wang, Y., Schmid, M., Vortkamp, A., and Haaf, T. (2000). Sex-specific expression of an evolutionarily conserved male regulatory gene, DMRT1, in birds. Cytogenetics and Cell Genetics 89, 252–257.
| Sex-specific expression of an evolutionarily conserved male regulatory gene, DMRT1, in birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXmsF2msbk%3D&md5=20c7b5e43d13506757aba8022b6b8aabCAS | 10965136PubMed |
Shetty, S., Griffin, D. K., and Marshall Graves, J. A. (1999). Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Research 7, 289–295.
| Comparative painting reveals strong chromosome homology over 80 million years of bird evolution.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXltV2jtrk%3D&md5=8d0c8f8a7c130dc8ed09822a3bd776aeCAS | 10461874PubMed |
Shetty, S., Kirby, P., Zarkower, D., and Marshall Graves, J. A. (2002). DMRT1 in a ratite bird: evidence for a role in sex determination and discovery of a putative regulatory element. Cytogenetic and Genome Research 99, 245–251.
| DMRT1 in a ratite bird: evidence for a role in sex determination and discovery of a putative regulatory element.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXmtVKlu78%3D&md5=a965ec704ca568620b3fa527511c92cdCAS | 12900571PubMed |
Shoemaker, C., Ramsey, M., Queen, J., and Crews, D. (2007). Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination. Developmental Dynamics 236, 1055–1063.
| Expression of Sox9, Mis, and Dmrt1 in the gonad of a species with temperature-dependent sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltlWnsbo%3D&md5=23f9bf907f82015668932a8cd19a2665CAS | 17326219PubMed |
Smith, C. A., and Sinclair, A. H. (2004). Sex determination: insights from the chicken. BioEssays 26, 120–132.
| Sex determination: insights from the chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXitVCks7o%3D&md5=0ec65f405aeba88551b7522cebd86ec9CAS | 14745830PubMed |
Smith, C. A., Andrews, J. E., and Sinclair, A. H. (1997). Gonadal sex differentiation in chicken embryos: expression of estrogen receptor and aromatase genes. Journal of Steroid Biochemistry and Molecular Biology 60, 295–302.
| Gonadal sex differentiation in chicken embryos: expression of estrogen receptor and aromatase genes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXksF2it7g%3D&md5=06b90f29b5d3986fcf25ecebad1a90acCAS | 9219920PubMed |
Smith, C. A., McClive, P. J., Western, P. S., Reed, K. J., and Sinclair, A. H. (1999). Conservation of a sex-determining gene. Nature 402, 601–602.
| Conservation of a sex-determining gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjsVCm&md5=229b0c59c59c9c8cd015695ee7f29204CAS | 10604464PubMed |
Smith, C. A., McClive, P. J., Hudson, Q., and Sinclair, A. H. (2005). Male-specific cell migration into the developing gonad is a conserved process involving PDGF signalling. Developmental Biology 284, 337–350.
| Male-specific cell migration into the developing gonad is a conserved process involving PDGF signalling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpt1eksr8%3D&md5=c62d547173c77495451a21ba517cb553CAS | 16005453PubMed |
Smith, C. A., Roeszler, K. N., Hudson, Q. J., and Sinclair, A. H. (2007). Avian sex determination: what, when and where? Cytogenetic and Genome Research 117, 165–173.
| Avian sex determination: what, when and where?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2svlvVemuw%3D%3D&md5=441fd6f6dd54f163a86fe3ca57663826CAS | 17675857PubMed |
Smith, C. A., Roeszler, K. N., Ohnesorg, T., Cummins, D. M., Farlie, P. G., Doran, T. J., and Sinclair, A. H. (2009a). The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461, 267–271.
| The avian Z-linked gene DMRT1 is required for male sex determination in the chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVGiurvN&md5=02254a204d3b59cab23cfa187136a076CAS | 19710650PubMed |
Smith, C. A., Roeszler, K. N., and Sinclair, A. H. (2009b). Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination. International Journal of Developmental Biology 53, 59–67.
| Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktFGmtrg%3D&md5=b8fe870b688645a554f062c7fff1b7c7CAS | 19123127PubMed |
Stiglec, R., Ezaz, T., and Marshall Graves, J. A. (2007). A new look at the evolution of avian sex chromosomes. Cytogenetic and Genome Research 117, 103–109.
| A new look at the evolution of avian sex chromosomes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2svlvVemsA%3D%3D&md5=0e8ff9fff846abd85f1f8e5688ff193bCAS | 17675850PubMed |
Takagi, N., and Sasaki, M. (1974). A phylogenetic study of bird karyotypes. Chromosoma 46, 91–120.
| A phylogenetic study of bird karyotypes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2c3mtl2hug%3D%3D&md5=77fabac68ce67ef183ef077277a5aed9CAS | 4134896PubMed |
Thorne, M. H. (1995). Genetics of poultry reproduction. In ‘Poultry Production’. (Ed. P. Hunton.) pp. 411–434. (Elsevier: Amsterdam.)
Thorne, M. H., Nicholas, F. W., Moran, C., and Sheldon, B. L. (1997). Genetic analysis of triploidy in a selected line of chickens. Journal of Heredity 88, 495–498.
| 1:STN:280:DyaK1c%2FotFahuw%3D%3D&md5=01fbcb8e9010057ba0fe7d715ac30f7cCAS | 9419888PubMed |
Tone, M., Nakano, N., Takao, E., Narisawa, S., and Mizuno, S. (1982). Demonstration of W chromosome-specific repetitive DNA sequences in the domestic fowl, Gallus g. domesticus. Chromosoma 86, 551–569.
| Demonstration of W chromosome-specific repetitive DNA sequences in the domestic fowl, Gallus g. domesticus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXivVyksw%3D%3D&md5=82d734361c6797ec41e0d6649be94231CAS | 7172866PubMed |
Tsuda, Y., Nishida-Umehara, C., Ishijima, J., Yamada, K., and Matsuda, Y. (2007). Comparison of the Z and W sex chromosomal architectures in Elegant Crested Tinamou (Eudromia elegans) and Ostrich (Struthio camelus) and the process of sex chromosome differentiation in palaeognathous birds. Chromosoma 116, 159–173.
| Comparison of the Z and W sex chromosomal architectures in Elegant Crested Tinamou (Eudromia elegans) and Ostrich (Struthio camelus) and the process of sex chromosome differentiation in palaeognathous birds.Crossref | GoogleScholarGoogle Scholar | 17219176PubMed |
Uhlenhaut, N. H., Jakob, S., Anlag, K., Eisenberger, T., Sekido, R., Kress, J., Treier, A. C., Klugmann, C., et al (2009). Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130–1142.
| Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslartrs%3D&md5=4806e44815629e05a82b9bb28fc65229CAS | 20005806PubMed |
Vaillant, S., Dorizzi, M., Pieau, C., and Richard-Mercier, N. (2001). Sex reversal and aromatase in chicken. Journal of Experimental Zoology 290, 727–740.
| Sex reversal and aromatase in chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXos12qsL4%3D&md5=192afe95a89bd7be9d668e4767cdaf2eCAS | 11748621PubMed |
Vallisneri, M., Quaglia, A., Stagni, A. M., and Zaccanti, F. (1990). Differences between male and female protogonia in chick embryos before sex differentiation of the gonads. Bollettino della Societa Italiana di Biologia Sperimentale 66, 91–98.
| 1:STN:280:DyaK3c3htFOhtA%3D%3D&md5=56e0cf37ed8fddebee0f218ac8c85643CAS | 2322448PubMed |
van Tuinen, M., and Hedges, S. B. (2001). Calibration of avian molecular clocks. Molecular Biology and Evolution 18, 206–213.
| 1:CAS:528:DC%2BD3MXotVOmuw%3D%3D&md5=9715608d8d63a445cadf9d30cc25daceCAS | 11158379PubMed |
Volff, J. N., Kondo, M., and Schartl, M. (2003). Medaka dmY/dmrt1Y is not the universal primary sex-determining gene in fish. Trends in Genetics 19, 196–199.
| Medaka dmY/dmrt1Y is not the universal primary sex-determining gene in fish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXis12ns7s%3D&md5=889c7686b56a3b6578b4ef96e75c1064CAS | 12683972PubMed |
Wang, L. C., Chen, C. T., Lee, H. Y., Li, S. H., Lir, J. T., Chin, S. C., Pu, C. E., and Wang, C. H. (2007). Sexing a wider range of avian species based on two CHD1 introns with a unified reaction condition. Zoo Biology 26, 425–431.
| Sexing a wider range of avian species based on two CHD1 introns with a unified reaction condition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlelsrfM&md5=38f6cc3919e86bca0b5afa4d32b380fbCAS | 19360591PubMed |
Yamada, D., Koyama, Y., Komatsubara, M., Urabe, M., Mori, M., Hashimoto, Y., Nii, R., Kobayashi, M., Nakamoto, A., et al (2004). Comprehensive search for chicken W chromosome-linked genes expressed in early female embryos from the female-minus-male subtracted cDNA macroarray. Chromosome Research 12, 741–754.
| Comprehensive search for chicken W chromosome-linked genes expressed in early female embryos from the female-minus-male subtracted cDNA macroarray.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVOjsr8%3D&md5=89ddf3741e784fbc1cb61302cb3bb3e0CAS | 15505409PubMed |
Yoshimoto, S., Okada, E., Umemoto, H., Tamura, K., Uno, Y., Nishida-Umehara, C., Matsuda, Y., Takamatsu, N., Shiba, T., and Ito, M. (2008). A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America 105, 2469–2474.
| A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXis1Khurk%3D&md5=4e89751a2a7dfc6a05ab5bf8eaeb365cCAS | 18268317PubMed |
Yoshimoto, S., Ikeda, N., Izutsu, Y., Shiba, T., Takamatsu, N., and Ito, M. (2010). Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: implications of a ZZ/ZW-type sex-determining system. Development 137, 2519–2526.
| Opposite roles of DMRT1 and its W-linked paralogue, DM-W, in sexual dimorphism of Xenopus laevis: implications of a ZZ/ZW-type sex-determining system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtF2mt7bJ&md5=b6fe80d229cae589e40a4175711a34ceCAS | 20573695PubMed |
Zaccanti, F., Vallisneri, M., and Quaglia, A. (1990). Early aspects of sex differentiation in the gonads of chick embryos. Differentiation 43, 71–80.
| Early aspects of sex differentiation in the gonads of chick embryos.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3czitlCnsw%3D%3D&md5=71fc50d99a23a0f1c7d0e9a3cfc5e413CAS | 2373289PubMed |
Zhang, S. O., Mathur, S., Hattem, G., Tassy, O., and Pourquie, O. (2010). Sex-dimorphic gene expression and ineffective dosage compensation of Z-linked genes in gastrulating chicken embryos. BMC Genomics 11, 13.
| Sex-dimorphic gene expression and ineffective dosage compensation of Z-linked genes in gastrulating chicken embryos.Crossref | GoogleScholarGoogle Scholar | 20055996PubMed |
Zhao, D., McBride, D., Nandi, S., McQueen, H. A., McGrew, M. J., Hocking, P. M., Lewis, P. D., Sang, H. M., and Clinton, M. (2010). Somatic sex identity is cell autonomous in the chicken. Nature 464, 237–242.
| Somatic sex identity is cell autonomous in the chicken.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFSmtb8%3D&md5=3101dfaec23c7bb26c4c35ca55fac88dCAS | 20220842PubMed |

