Feeling the heat: Australian landbirds and climate change
Andrew E. McKechnie A D , Philip A. R. Hockey B and Blair O. Wolf CA DST/NRF Centre of Excellence at the Percy FitzPatrick Institute, Department of Zoology and Entomology, University of Pretoria, Private Bag X20, Hatfield 0028, South Africa.
B Percy FitzPatrick Institute of African Ornithology, DST/NRF Centre of Excellence, University of Cape Town, Rondebosch 7701, South Africa.
C UNM Biology Department, MSC03-2020, 1 University of New Mexico, Albuquerque, NM 87131-0001, USA.
D Corresponding author. Email: aemckechnie@zoology.up.ac.za
Emu 112(2) i-vii https://doi.org/10.1071/MUv112n2_ED
Published: 23 May 2012
Introduction
Earth’s climate is warming at an unprecedented rate, with the current trend ascribed primarily to anthropogenic alteration of atmospheric concentrations of carbon dioxide and other greenhouse gases (IPCC 2007). Recent evidence suggests that warming is occurring even more rapidly than predicted by most models used in the 2007 assessment of the Intergovernmental Panel on Climate Change (IPCC) (Rahmstorf et al. 2007; van Oldenborgh et al. 2009). These observations, combined with the current lack of concerted political will to significantly reduce global carbon emissions (typified by the ineffectual outcome of the recent COP 17 climate talks in Durban), suggest that climate scenarios that are presently viewed as worst-case may in fact be the most likely future outcomes. Climate change is currently recognised as the single greatest threat to global biodiversity because its effects are felt in virtually every habitat on the planet. Although most scenarios are built around models of what the world’s climate might look like several decades from now, the reality is that significant biological effects of climate change are already being manifested as extinctions (Pounds et al. 1999; Thomas et al. 2006) and rapid shifts in the distributions of species inhabiting latitudes ranging from polar to equatorial (Chen et al. 2011). Extreme heatwaves also have dire consequences for humans – a recent report noted that, over the last 200 years, fatalities during heatwaves have outnumbered those caused by any other natural hazard in Australia, and the death-toll is likely to increase dramatically in coming decades (PricewaterhouseCoopers Australia 2011).
Australia, as a predominantly hot and arid continent with terrestrial avifauna largely confined to the region (Dingle 2004), is expected to see significant effects on avian diversity and abundance. Indeed, Australia is already something of a ‘poster-continent’ for the effects of climate change on landbirds because historical records provide unparalleled insights into just how devastating heatwaves and droughts can be for avian communities. Recent mortality events associated with heatwaves (discussed below) highlight the effects of more frequent periods of very hot weather for common and nomadic birds, but also for species considered threatened. In this editorial, we focus on the direct effects of extreme weather to draw attention to the likely severity of the effects of climate change on Australian landbirds. We also outline a conceptual framework for predicting the effects of climate change on birds in hot, arid terrestrial ecosystems, and some of the ways in which this information may be used to inform conservation decisions. One key advantage of the mechanistic, process-driven approach we describe here is that it can be used to identify potential mitigation measures, for instance via the artificial manipulation of thermal landscapes. Our message is that Australian ornithologists should be urgently seeking ways to predict how climate change will affect arid-zone bird communities, particularly with regard to already threatened avifauna, and identify appropriate mitigation strategies.
Avian mortality during heatwaves
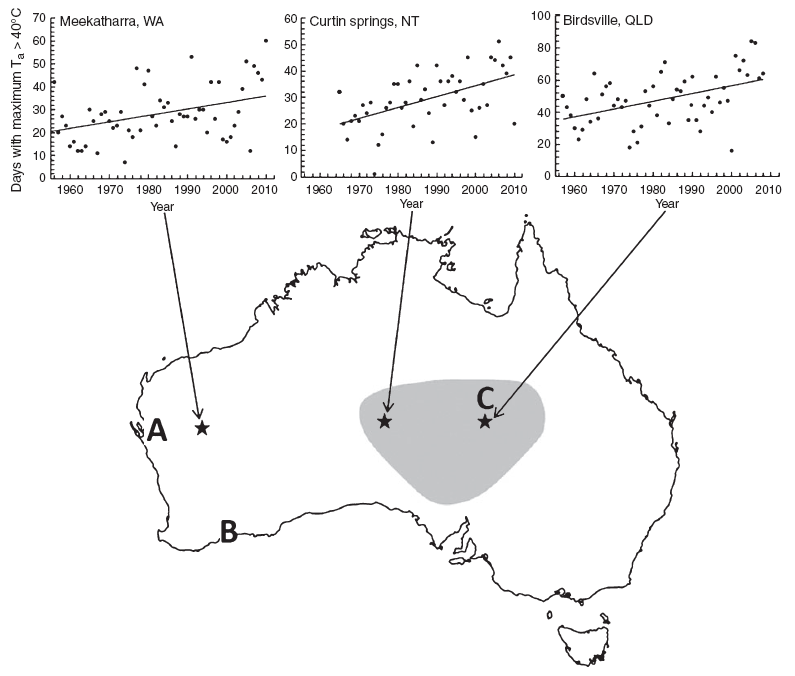
Deaths of birds during extremely hot weather are not a new occurrence in Australia; as early as 1791 the Reverend Richard Johnson, a chaplain at Port Jackson (Sydney), New South Wales (NSW), referred in a letter to temperatures so high that ‘Birds, unable to bear the heat, have great Numbers, dropped from the trees & expired’ (available at http://acms.sl.nsw.gov.au/_transcript/2010/D01866/a1769.pdf, accessed 20 December 2011). By far the most catastrophic event recorded took place in January 1932, when a severe heatwave struck a large portion of southern central Australia (Fig. 1). The April 1932 issue of the South Australian Ornithologist contained several accounts of widespread mortality, which collectively portray the deaths of many millions of birds. Finlayson (1932), for instance, provided a vivid account of thousands of dead and dying Budgerigars (Melopsittacus undulatus), Zebra Finches (Taenopygia guttata) and other birds in and around Rumbalara Siding on a day when the air temperature reached ~49°C. He noted that ‘The condition of the birds was undoubtedly a true temperature effect, and not due to thirst, as the railway people had put out several pans of water, and only a small proportion were attempting to drink’. Another observer documented the deaths of tens of thousands of birds (mainly parrots) in water troughs near Tarcoola, South Australia (SA) (McGilp 1932).
|
The accounts above provide a historical backdrop for several more recent mortality events, albeit on a much smaller scale (Fig. 1). In January 2009 a heatwave with air temperatures above 45°C for several consecutive days caused the deaths of thousands of birds, mainly Budgerigars and Zebra Finches, at the Overlander Roadhouse ~500 km north of Perth, Western Australia (WA) (Towie 2009). One of the photographs of this event shows dead Budgerigars around a pool of water, echoing Finlayson’s (1932) observation and suggesting that lethal hyperthermia rather than dehydration was the cause of most of these deaths. Another die-off occurred in January 2010, when several hundred birds died in the Hopetoun area, WA (Towie 2010). On this occasion, the fatalities included 208 endangered Carnaby’s Black-Cockatoos (Calyptorhynchus latirostris), with 145 individuals dying at the Hopetoun golf course and a further 63 individuals dying ~75 km to the east near Munglinup (Saunders et al. 2011). These deaths happened on a single extremely hot day (maximum air temperature >47°C) falling between two much milder days (maximum air temperatures <35°C). Heat-related mortality has also been reported in other parts of Australia; Low (2011) cites several such events recently reported at Ethabuka, Cravens Peak Reserve and Carlo Station in the Simpson Desert in south-western Queensland.
Birds are not the only Australian animals that have died during heatwaves. More than 30 000 flying-foxes (Pteropus spp.) have perished in heatwaves since 1994 in colonies along the eastern coast from Townsville, northern Queensland, south to Melbourne, Victoria (Welbergen et al. 2008). During a single such event in NSW on 12 January 2002, ~3500 individuals died in nine colonies when air temperatures exceeded 42°C (Welbergen et al. 2008). Heat-related deaths of birds and other animals are also not unique to Australia. Such events have been reported in the south-west of the United States of America (USA) (Miller 1963), India (Anon. 2010a, 2010b), and on Ostrich (Struthio camelus) farms in South Africa (Erasmus 2010). However, these events pale in comparison with the scale of the deaths reported in Australia, both in terms of spatial extent and the numbers of individuals involved.
Recent and predicted climate change in Australia
The mortality events discussed above reveal the vulnerability of birds to extreme heatwaves that overwhelm their physiological limits, and highlight the importance of these weather events as bottlenecks for avian survival and reproduction. A key point is that in these hot, arid ecosystems, the frequency and intensity of extreme weather events are as important, and in some cases even more important, for birds than are long-term average conditions. Historical data for weather stations across Australia convincingly show that the frequency of very hot weather events is increasing. To illustrate this point, we obtained data for three weather stations selected on the basis of their period of coverage and quality of data – Meekatharra, WA; Curtin Springs, Northern Territory; and Birdsville, Queensland, – from the Bureau of Meteorology (http://www.bom.gov.au, accessed 15 November 2011). Data from all three stations show a significant increase in the number of days per year on which maximum air temperature exceeded 40°C and thus exceeded approximate avian body temperature (Fig. 1), conditions under which birds must evaporate increasing amounts of water to dissipate heat and maintain body temperature below lethal limits. These data highlight one of the key ways in which climate change is affecting landbird communities: birds are exposed to potentially life-threatening thermal environments more frequently than in the past. Similar trends are evident in historical weather data from other hot, subtropical desert regions, such as the Sonoran Desert of the south-western USA (data from the Arizona Meteorological Network, http://ag.arizona.edu/azmet, accessed 15 November 2011).
Both the frequency of very hot weather events and extreme maximum air temperatures are predicted to increase dramatically in coming decades. Depending on the emissions scenario used, very hot days that currently represent a 1-in-20-year occurrence are likely to become 1-in-5-year or even 1-in-2-year occurrences by the end of the 21st century (IPCC 2011). Increases in the frequency of extreme daily maximum temperatures will be accompanied by increases of 2−5°C in the maximum temperatures observed over this same period (IPCC 2011).
Current approaches to predicting the effects of climate change
Recognition of the reality of climate change and its severe consequences for natural systems has left biologists scrambling to develop ways of modelling the effects of global warming on organisms. Much of the research predicting the responses of birds to climate change has involved a climate-envelope modelling approach (e.g. Peterson 2001; Erasmus et al. 2002; Peterson et al. 2002; Simmons et al. 2004), where the subset of climatic conditions currently occupied by a species is assumed to be an accurate predictor of its future range under climate change. However, the assumption implicit in this approach, namely that climate directly limits survival or reproduction, or both, is questionable. Indeed, observed recent shifts in the ranges of birds have often been inconsistent with the predictions of such models (Okes et al. 2008; Hockey and Midgley 2009; Hockey et al. 2011). There is growing realisation that the purely pattern-focused basis of the climate-envelope modelling approach is inadequate, because it overlooks a multitude of physiological and behavioural processes that mediate links between an organism’s fitness and its physical and biological environment (Huntley et al. 2010). This point has been reiterated by Williams et al. (2008), who argued that a critical step in assessing vulnerability to climate change is to distinguish between organismal sensitivity (determined by intrinsic, organismal traits) and exposure (determined by extrinsic factors related to climate and habitat). The shortcomings of the climate-envelope approach can to some extent be overcome by a second modelling approach that incorporates taxon-specific physiological data in order to map the fundamental niche, that is the hypothetical maximum extent of a taxon’s spatial distribution determined entirely by interactions between its physiology and physical conditions coupled with resource availability (Kearney and Porter 2004, 2009). This approach provides a far more biologically realistic linkage between climatic variables and the distribution of species. However, whereas the fundamental niche approach holds much promise for modelling the effects of climate change on ectotherms, its applicability to endotherms is less clear, primarily because of the greater disconnect between the body temperatures of endotherms and environmental temperatures, and their greater capacity to modify key physiological variables linked to life histories, such as egg temperatures, via behavioural mechanisms.
A third broad category of models used to predict the effects of climate change, and which is often more suited to endotherms, involves bioenergetic models that focus on specific seasonal bottlenecks in energy or water balance, or both. Examples include models that predict the effects of rising temperatures on energy balance in hibernating temperate-zone bats (Humphries et al. 2002) and those that quantify water balance in birds during extremely hot weather (McKechnie and Wolf 2010). A key advantage of these models is that they use empirical data to model directly the effects of climate change on organismal variables related to survival in a mechanistic, physiologically informed framework; a limitation is that they model only specific events during annual cycles or extreme conditions.
A new approach for predicting the effects of climate change on arid-zone birds
McKechnie and Wolf (2010) modelled the effects of increasing maximum temperatures on avian water balance during extremely hot weather and argued that catastrophic mortality events among birds inhabiting hot deserts will become much more frequent under future climatic scenarios. The effects of heatwaves and droughts on avian abundance have also recently been highlighted in North America, with negative effects most pronounced in the hot south-western deserts (Albright et al. 2010). Whereas more frequent mass die-offs will unquestionably have profound effects for arid-zone species, particularly in Australia, we might also expect a plethora of sub-lethal, cumulative effects of warmer conditions manifested during ‘typical’ rather than ‘extreme’ future conditions, and which will not necessarily be associated only with changes in the frequency of extreme weather events. For instance, data from a southern African desert bird community reveal that a suite of physiological, behavioural and reproductive variables change rapidly with increasing air temperatures in the comparatively mild range of 30−40°C (B. Smit, S. J. Cunningham, R. O. Martin, K. du Toit, A. R. Ridley, A. E. McKechnie and P. A. R. Hockey, unpubl. data), far below the temperatures typically associated with mortality events (McKechnie and Wolf 2010).
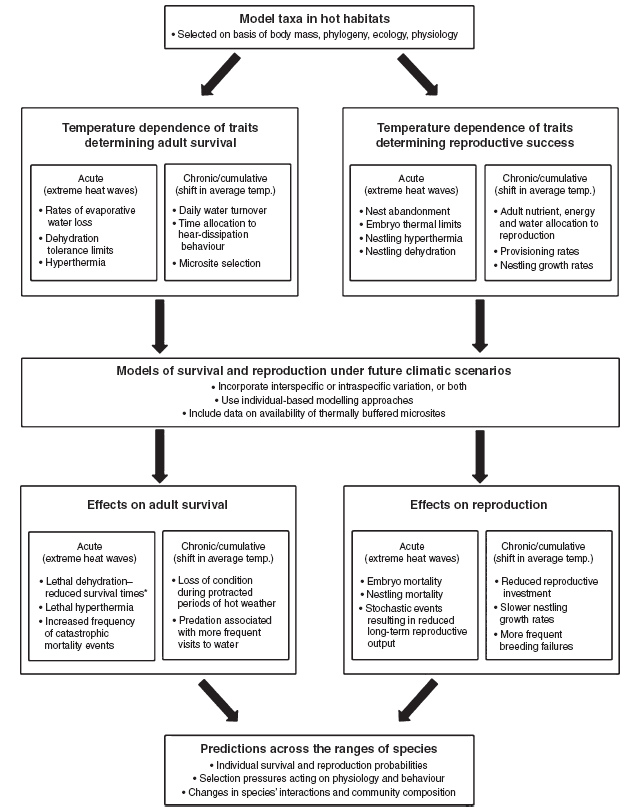
We advocate that a more rigorous approach to predicting the effects of climate change on arid-zone birds in Australia and elsewhere involves several distinct steps (Fig. 2). First, model species are selected on the basis of criteria such as body mass, phylogenetic affiliation, aspects of physiology and ecology related to heat exposure (e.g. species that forage in hotter microsites, such as open ground or exposed perches, v. species that forage in cooler microsites, like shady tree canopies), and tractability for field research. Model species should include taxa such as Columbiformes that rely predominantly on cutaneous evaporation to dissipate heat, as well as taxa such as Passeriformes that rely primarily on respiratory evaporation (e.g. panting and gular fluttering; Wolf and Walsberg 1996a), as the primary mode of evaporative cooling may have important consequences for behavioural trade-offs involving heat dissipation.
|
Second, the temperature dependence of key physiological, behavioural and reproductive variables should be empirically determined, along with physiological tolerance limits (Fig. 2). Variables examined during this step should represent a continuum from those likely to be important during acute, short-term heat exposure to those relevant to longer term, cumulative effects associated with shifts in average conditions. Key physiological variables include rates of evaporative water loss (measured via respirometry in captive birds), body temperature fluctuations (measured via field telemetry), daily water turnover (measured via the washout rate of isotopically labelled water) and field metabolic rate (measured via doubly labelled water, or by telemetric measurement of heart rate). In addition, daily changes in hydration status should be measured by comparing total body water at various times of the day by injecting birds with isotopically labelled water and measuring the dilution space once the label has equilibrated with the body water pool (Speakman 1997).
The next step is to integrate empirical data on the temperature dependence of physiological, behavioural and reproductive variables into models for survival and reproduction under future climatic scenarios (Fig. 2). Efforts should initially focus on developing convincing single-taxon models that facilitate predictions of behavioural patterns and energy and water balance on hot days over time-scales of hours. Individual-based models (Stillman 2008), in which input variables such as body mass, total body water and rates of evaporative water loss can be varied randomly within empirically determined ranges for a species, may prove the most useful modelling technique. Using individual-based models that incorporate frequency distributions of maximum air temperatures under current and future climatic scenarios, it will be possible to answer a host of species- and population-specific questions including:
-
How do the probabilities of exceeding physiological tolerance limits differ between ‘average’ mid-summer conditions and extremely hot days, and between landscapes that vary in the availability of cool microsites?
-
How will the predicted increases in air temperature affect the frequency with which birds experience life-threatening conditions?
-
What is the relative importance of direct mortality during extreme weather events versus sub-lethal but cumulative effects such as repeated breeding failure, and how will these change under future climates?
-
How will climate change alter survival probabilities and reproductive fitness across the ranges of species? Possibly the most powerful aspect of the approach proposed here is that it will allow us to model changes across different parts of the range of a species. This will provide the basis for much stronger predictions regarding shifts in range in response to climate change than are possible using existing climate-envelope modelling techniques.
Once species-specific models exist for a handful of arid-zone bird species selected on the basis of the criteria outlined above, more generalised models applicable to entire avian communities both in Australia and elsewhere can readily be developed. The overall goal of these models should be to produce ecological generalisations about the vulnerabilities of species, and provide a tool for predicting how the species that presently make up arid-zone avian communities will vary in their resilience to future climate change. In addition, such models will allow us to predict how selection pressures operating on avian physiology, behaviour and reproduction will change in the future.
Conservation implications
Climate change has profound implications for the conservation and management of arid-zone avifaunas. First, extreme heatwaves will become increasingly important as stochastic events with potentially catastrophic results for threatened species whose distributions are centred in hot, arid environments. In much the same way as exceptionally powerful cyclones can have disastrous consequences for threatened species restricted to islands (Quammen 1996), heatwaves will become increasingly important determinants of population trends in threatened arid-zone species. The recent heat-associated death of at least 208 Carnaby’s Black-Cockatoos at Hopetoun, WA, discussed above provides a sobering example. Moreover, in this instance the observed death toll was likely a gross underestimate of the actual population consequences, because many chicks would have died in nests without being detected (Saunders et al. 2011). We urge conservationists to start identifying taxa of conservation concern that are restricted to hot areas, and which are thus potentially vulnerable to significant mortality during extreme heatwaves. A prime example is the near threatened Princess Parrot (Polytelis alexandrae), a species distributed entirely within the extremely hot interior of western and central Australia, and whose population may number no more than 1000 adults during ‘poor years’ (Garnett et al. 2011). A heatwave on the scale of the 1932 event (Fig. 1) could make massive inroads into the global population of this species in a matter of hours. Another example is the critically endangered Western Ground Parrot (Pezoporus flaviventris), whose distribution is limited to coastal areas close to the sites of recent heat-associated mortality in Carnaby’s Black-Cockatoos (Garnett et al. 2011; Saunders et al. 2011).
Second, the comparatively cool microsites provided by landscape elements such as large, shady trees will also need considerable attention in the management and conservation of arid-zone birds under future climatic scenarios. In many desert landscapes, trees are the only refuges where birds can escape intense solar heat loads and experience environmental temperatures approaching air temperature, a minimum index of thermal stress. Although such microsites will not shield birds from increasing maximum air temperatures, they will be a key determinant of whether a particular species can persist in a given landscape. By providing shady microsites, otherwise undesirable exotic plants such as Prosopis spp. may in some cases increase the chances of birds persisting in very hot areas. A related issue concerns the thermal environments birds experience while drinking. Most waterholes in desert areas are unshaded, exposing drinking birds to intense solar heat loads. In very small species, environmental temperatures at midday in full sun can be 10−15°C higher than air temperature (Wolf and Walsberg 1996b). Thus, for species that rely on drinking water, a trade-off exists between avoiding dehydration by drinking regularly and avoiding hyperthermia by seeking shelter in cool microsites. No data are available on the relative roles of dehydration versus direct hyperthermia in the deaths of birds during recent mortality events, but Finlayson’s (1932) observations at Rumbalara Siding and the photographs of dead Budgerigars around a pool of water at the Overlander Roadhouse in 2009 suggest that direct hyperthermia may, at least under some conditions, be a more important source of mortality than dehydration per se. We suspect body mass may be a key issue here, with larger birds at comparatively greater risk of direct hyperthermia on account of their smaller surface area : volume ratios and lower mass-specific rates of evaporative water loss, and smaller birds conversely at greater risk of dehydration. That many individuals of smaller species have been observed apparently succumbing to direct hyperthermia during past die-offs does not necessarily conflict with this argument; small birds are more likely to be flocking species and are more likely to drink frequently, thus increasing the probability of detecting large mortality events. The trade-off between the risks of dehydration and hyperthermia could potentially be mitigated artificially by providing shade at exposed waterholes, making it possible for birds to drink while avoiding the high environmental temperatures to which they would otherwise be exposed.
Conclusion
Historical and recent accounts of bird die-offs associated with heatwaves and droughts provide striking and, in some cases, frightening insights into the ways in which climate change will affect arid-zone bird communities. Quantifying avian physiological and behavioural responses to current extreme events allows us to use the realities of today to develop predictions for tomorrow. In this editorial, we have provided an overview of but one approach to understanding how climatic warming will affect Australian landbird communities. This approach focuses on species-specific performance data and is scalable from individuals to populations and communities. Data are also needed on the direct effects of heatwaves on individual bird species and populations as well as the extent and duration of their effects. We also know little about the effects of warming on reproduction in most species and how patterns of movement vary in mobile species subjected to regional heatwaves. Australian ornithologists, both professionals and amateurs, have a leading role to play in global efforts to predict the effects of climate change on bird populations and communities, and proffer stop-gap mitigation measures.
Acknowledgements
We thank Kate Buchanan, Jon Green and an anonymous reviewer for constructive comments that greatly improved the manuscript.
References
Albright, T. P., Pidgeon, A. M., Rittenhouse, C. D., Clayton, M. K., Wardlow, B. D., Flather, C. H., Culbert, P. D., and Radeloff, V. C. (2010). Combined effects of heat waves and droughts on avian communities across the conterminous United States. Ecosphere 1, 12.| Combined effects of heat waves and droughts on avian communities across the conterminous United States.Crossref | GoogleScholarGoogle Scholar |
Anon. (2010a) Heat wave kills peacocks in Uttar Pradesh. In ‘NewzStreet’, 2 June 2010. Available at http://www.newzstreet.com/news.php?news_id=2791 [Verified 20 March 2012].
Anon. (2010b) Intense heat wave takes toll of animals, birds in Gujarat. In ‘NVONews’, 22 May 2010. Available at http://nvonews.com/2010/05/22/intense-heat-wave-takes-toll-of-animals-birds-in-gujarat/ [Verified 20 March 2012].
Chen, I.-C., Hill, J. K., Ohlemüller, R., Roy, D. B., and Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026.
| Rapid range shifts of species associated with high levels of climate warming.Crossref | GoogleScholarGoogle Scholar |
Dingle, H. (2004). The Australo-Papuan bird migration system: another consequence of Wallace’s Line. Emu 104, 95–108.
| The Australo-Papuan bird migration system: another consequence of Wallace’s Line.Crossref | GoogleScholarGoogle Scholar |
Erasmus, D. (2010). South Africa – Heatwave kills more than 2000 ostriches. In ‘Meat Trade News Daily’, 25 February 2010. Available at http://www.meattradenewsdaily.co.uk/news/250210/souith_africa___heatwave_kills_more_than__ostriches_.aspx [Verified 20 March 2012].
Erasmus, B. F. N., van Jaarsveld, A. S., Chown, S. L., Kshatriya, M., and Wessels, K. J. (2002). Vulnerability of South African taxa to climate change. Global Change Biology 8, 679–693.
| Vulnerability of South African taxa to climate change.Crossref | GoogleScholarGoogle Scholar |
Finlayson, H. H. (1932). Heat in the interior of South Australia – holocaust of bird-life. South Australian Ornithologist 11, 158–160.
Garnett, S., Szabo, J., and Dutson, G. (2011). ‘The Action Plan for Australian Birds 2010.’ (CSIRO Publishing: Melbourne.)
Hockey, P. A. R., and Midgley, G. F. (2009). Avian range changes and climate change: a cautionary tale from the Cape Peninsula. Ostrich 80, 29–34.
| Avian range changes and climate change: a cautionary tale from the Cape Peninsula.Crossref | GoogleScholarGoogle Scholar |
Hockey, P. A. R., Sirami, C., Ridley, A. R., Midgley, G. F., and Babiker, H. A. (2011). Interrogating recent range changes in South African birds: confounding signals from land use and climate change present a challenge for attribution. Diversity & Distributions 17, 254–261.
| Interrogating recent range changes in South African birds: confounding signals from land use and climate change present a challenge for attribution.Crossref | GoogleScholarGoogle Scholar |
Humphries, M. M., Thomas, D. W., and Speakman, J. R. (2002). Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 418, 313–316.
| Climate-mediated energetic constraints on the distribution of hibernating mammals.Crossref | GoogleScholarGoogle Scholar |
Huntley, B., Barnard, P., Altwegg, R., Chambers, L., Coetzee, B. W. T., Gibson, L., Hockey, P. A. R., Hole, D. G., Midgley, G. F., Underhill, L. G., and Willis, S. G. (2010). Beyond bioclimatic envelopes: dynamic species’ range and abundance modelling in the context of climate change. Ecography 33, 1–6.
IPCC (2007). ‘Climate Change 2007: Fourth Assessment Report of the Intergovernmental Panel on Climate Change.’ (Cambridge University Press: Cambridge, UK.)
IPCC (2011). ‘Intergovernmental Panel on Climate Change Special Report on Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation.’ (Cambridge University Press: Cambridge, UK.)
Kearney, M., and Porter, W. (2004). Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard. Ecology 85, 3119–3131.
| Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard.Crossref | GoogleScholarGoogle Scholar |
Kearney, M., and Porter, W. (2009). Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecology Letters 12, 334–350.
| Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges.Crossref | GoogleScholarGoogle Scholar |
Keast, A. (1960). Bird adaptations to aridity on the Australian continent. Proceedings of the International Ornithological Congress 12, 373–375.
Low, T. (2011). ‘Climate Change and Terrestrial Biodiversity in Queensland.’ (Qld Department of Environment and Resource Management: Brisbane.)
McGilp, J. N. (1932). South Australian Ornithologist 11, 160–163.
McKechnie, A. E., and Wolf, B. O. (2010). Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biology Letters 6, 253–256.
| Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves.Crossref | GoogleScholarGoogle Scholar |
Miller, A. H. (1963). Desert adaptations in birds. Proceedings of the International Ornithological Congress 13, 666–674.
Okes, N. C., Hockey, P. A. R., and Cumming, G. S. (2008). Habitat use and life history as predictors of bird responses to habitat change. Conservation Biology 22, 151–162.
| Habitat use and life history as predictors of bird responses to habitat change.Crossref | GoogleScholarGoogle Scholar |
Peterson, A. T. (2001). Predicting species’ geographical distributions based on ecological niche modeling. Condor 103, 599–605.
| Predicting species’ geographical distributions based on ecological niche modeling.Crossref | GoogleScholarGoogle Scholar |
Peterson, A. T., Ortega-Huerta, M. A., Bartley, J., Sánchez-Cordero, V., Soberón, J., Buddemeier, R. H., and Stockwell, D. R. B. (2002). Future projections for Mexican faunas under global climate change scenarios. Nature 416, 626–629.
| Future projections for Mexican faunas under global climate change scenarios.Crossref | GoogleScholarGoogle Scholar |
Pounds, J. A., Fogden, M. P. L., and Campbell, J. H. (1999). Biological response to climate change on a tropical mountain. Nature 398, 611–615.
| Biological response to climate change on a tropical mountain.Crossref | GoogleScholarGoogle Scholar |
PricewaterhouseCoopers Australia (2011). ‘Protecting Human Health and Safety During Severe and Extreme Heat Events.’ Available at http://www.pwc.com.au/industry/government/assets/extreme-heat-events-nov11.pdf [Verified 20 March 2012].
Quammen, D. (1996). ‘The Song of the Dodo: Island Biogeography and the Age of Extinctions.’ (Pimlico: London.)
Rahmstorf, S., Cazenave, A., Church, J. A., Hansen, J. E., Keeling, R. F., Parker, D. E., and Somerville, R. C. J. (2007). Recent climate observations compared to projections. Science 316, 709.
| Recent climate observations compared to projections.Crossref | GoogleScholarGoogle Scholar |
Saunders, D. A., Mawson, P., and Dawson, R. (2011). The impact of two extreme weather events and other causes of death on Carnaby’s Black Cockatoo: a promise of things to come for a threatened species? Pacific Conservation Biology 17, 141–148.
Simmons, R. E., Barnard, P., Dean, W. R. J., Midgley, G. F., Thuiller, W., and Hughes, G. (2004). Climate change and birds: perspectives and prospects from southern Africa. Ostrich 75, 295–308.
| Climate change and birds: perspectives and prospects from southern Africa.Crossref | GoogleScholarGoogle Scholar |
Speakman, J. R. (1997). ‘Doubly Labelled Water: Theory and Practise.’ (Chapman & Hall: New York.)
Stillman, R. A. (2008). MORPH – An individual-based model to predict the effect of environmental change on foraging animal populations. Ecological Modelling 216, 265–276.
| MORPH – An individual-based model to predict the effect of environmental change on foraging animal populations.Crossref | GoogleScholarGoogle Scholar |
Thomas, C. D., Franco, A. M. A., and Hill, J. K. (2006). Range retractions and extinction in the face of climate warming. Trends in Ecology & Evolution 21, 415–416.
| Range retractions and extinction in the face of climate warming.Crossref | GoogleScholarGoogle Scholar |
Towie, N. (2009). Thousands of birds die in sweltering heat. PerthNow, 13 January 2009. Available at http://www.news.com.au/perthnow/story/0,21598,24907390-2761,00.html [Verified 20 March 2012].
Towie, N. (2010). More than a hundred White-tailed Black Cockatoos dead near Hopetoun. PerthNow, 8 January 2010. Available at http://www.perthnow.com.au/news/special-features/hunderds-of-protected-carnabys-black-cockatoo-dead-near-hopetoun/story-e6frg19l-1225817390845 [Verified 20 March 2012].
van Oldenborgh, G. J., Drijfhout, S., van Ulden, A., Haarsma, R., Sterl, A., Severijns, C., Hazdgere, W., and Dijkstra, H. (2009). Western Europe is warming faster than expected. Climate of the Past 5, 1–12.
| Western Europe is warming faster than expected.Crossref | GoogleScholarGoogle Scholar |
Welbergen, J. A., Klose, S. M., Markus, N., and Eby, P. (2008). Climate change and the effects of temperature extremes on Australian flying-foxes. Proceedings of the Royal Society of London. Series B. Biological Sciences 275, 419–425.
| Climate change and the effects of temperature extremes on Australian flying-foxes.Crossref | GoogleScholarGoogle Scholar |
Williams, S. E., Shoo, L. P., Isaac, J. L., Hoffmann, A. A., and Langham, G. (2008). Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biology 6, e325.
| Towards an integrated framework for assessing the vulnerability of species to climate change.Crossref | GoogleScholarGoogle Scholar |
Wolf, B. O., and Walsberg, G. E. (1996a). Respiratory and cutaneous evaporative water loss at high environmental temperatures in a small bird. Journal of Experimental Biology 199, 451–457.
Wolf, B. O., and Walsberg, G. E. (1996b). Thermal effects of radiation and wind on a small bird and implications for microsite selection. Ecology 77, 2228–2236.
| Thermal effects of radiation and wind on a small bird and implications for microsite selection.Crossref | GoogleScholarGoogle Scholar |

