Temporal change of the song of a local population of the Grey Warbler (Gerygone igata): has its song changed over time?
Joseph F. Azar A C , Ben D. Bell A and Marta Borowiec BA Centre for Biodiversity & Restoration Ecology, School of Biological Sciences, Victoria University of Wellington, PO Box 600, Wellington 6041, New Zealand.
B Museum of Natural History, University of Wroclaw, Sienkiewicza 21, PL-50-335 Wroclaw, Poland.
C Corresponding author. Email: azar.joseph@gmail.com
Emu 114(1) 80-85 https://doi.org/10.1071/MU13039
Submitted: 20 March 2013 Accepted: 17 July 2013 Published: 10 October 2013
Abstract
The songs of bird species can vary from place to place and such variation may reflect ecological heterogeneity within the habitat. However, there is little understanding of how this process occurs over time within the same population. Here, change in song over time in a local population of the New Zealand Grey Warbler (Gerygone igata) was investigated. Spectral and temporal aspects of the song were compared in the same population at an interval of 7 years (2002 and 2009). There was a significant shift in the song syllables to a higher frequency but no difference in the temporal structure of the song. The frequency difference in song suggests that interspecific interactions may have led to song-frequency displacement, which in turn may be caused by the reintroduction of other native species to the study area.
Additional keyword: bird song.
Introduction
Song dialects are a form of vocal variation that is characteristic of birds that learn their songs. Habitat characteristics used by bird species may explain some geographical variation and interspecific differences in song structure (Warren 2002). Further, variation in song may arise through cultural evolution, which can be described as alteration or change in a learned behavioural trait from one generation to the next (Byers et al. 2010). The temporal stability of song varies within taxa, even within the same geographical boundaries, with some dialects persisting unchanged for many generations whereas others can quickly arise and disappear (Podos and Warren 2007). For example, song elements of the Yellowhammer (Emberiza citrinella) are transmitted culturally through a considerable number of generations (Hansen 1999). Other examples of species that maintain songs unchanged for decades or longer include the Rufous-collared Sparrow (Zonotrichia capensis) (Handford 1988), Chaffinch (Fringilla coelebs) (Ince et al. 1980) and Wood Thrush (Hylocichla mustelina) (Whitney 1992). In contrast, some species can modify their songs in periods of 1 year or less, such as the emergence of new song types in colonies of Yellow-rumped Caciques (Cacicus cela) (Trainer 1989), and removal of syllables, differentiation of song into parts and and increase in duration of song in the Lazuli Bunting (Passerina amoena) (Greene et al. 1997).
Despite many observations and studies regarding local song dialects, factors that influence the formation of new dialects or the resistance to change in songs are still not known (Catchpole and Slater 2008). However, the same factors argued to influence formation of geographical song dialects could also influence evolution of avian song over time (Luther and Baptista 2010), such as change in the physical transmission properties of the environment (Slabbekoorn et al. 2007), sexual selection (MacDougall-Shackleton 1997) and song learning (Slabbekoorn and Smith 2002a). The accuracy of song learning varies considerably, both within and between species, and copying errors can result in the emergence of new variations of the song (Catchpole and Slater 2008).
Change in song structure can also be influenced by competition for signal space between species using the same acoustic environment (Slabbekoorn and Smith 2002b; Kirschel et al. 2009). For example, songs diverge in Darwin’s Finches (Geospizinae) when a new species enters the community (Grant and Grant 2010) and songs of Eurasian Blue Tits (Cyanistes caeruleus) lack a species-specific trill in areas where Great Tits (Parus major) are absent (Doutrelant and Lambrechts 2001).
Here we explore whether songs in a local population of the Grey Warbler (Greygone igata) have changed over time and the possibility of cultural evolution of song, using recordings of songs made 7 years apart (2002 and 2009) within the same population of Warblers.
Methods
Study species
The Grey Warbler is an endemic New Zealand passerine in the family Acanthizidae (Heather and Robertson 2000). Males are territorial, with some territories maintained year round, and pairs form annually before the start of the breeding season. Only males sing, and songs are important in maintaining territories (Gill 1982). The song is described as a soft, sweet, trilling warble, sometimes subdued, regular and cricket-like (Buller 1888). It is a long plaintive, rambling indeterminate trill, of approximately eight notes, usually lasting 5 s but sometimes as long as 12 s (Fig. 1). The birds do not weave several patterns into one song, but rather appear to have several themes, and to sing by repetition (Andersen 1926). Songs are sometimes broken off suddenly, in which case the song seems not to be complete (Paul and McKenzie 1975).
Analysis of songs
Songs of Grey Warblers were recorded in the Zealandia Sanctuary (41°18′S, 174°44′E; see http://www.visitzealandia.com/, accessed July 2013), a 2.5-km2 valley of secondary forest 2 km west of Wellington, on the North Island of New Zealand. The forest is protected from a suite of mammalian predators introduced to New Zealand by a barrier fence 9 km long and 2.2 m high (Campbell and Atkinson 2002). The upperstorey of the forest is predominantly a mixture of evergreen native and exotic tree species, and there is a well-developed native understorey. In 2002, songs were recorded by M. Borowiec and B. D. Bell. From this sample, the songs of 10 individuals recorded at different locations within the Zealandia Sanctuary were sampled (hereafter referred to as the 2002 songs). To investigate possible temporal changes within the song of this species, the songs of 10 birds were recorded in 2009 by J. F. Azar (hereafter referred to as the 2009 songs). In both years, songs were recorded in spring and always in the morning. Songs sampled in 2002 were recorded on a Sony TCD-D10 Pro II DAT recorder (Sony, Tokyo), equipped with a Telinga parabola microphone (Telinga, Tobo, Sweden). The 2009 songs were recorded on a Marantz PMD670 solid-state recorder (Marantz Europe, Eindhoven, the Netherlands), equipped with a Telinga Pro 7 parabola microphone (Telinga). Both 2002 and 2009 songs were digitised at a sampling frequency of 44.1 kHz and 16-bit sample size.
The main parts of the Grey Warbler’s song were classified into two phrases: the ‘start phrase’ (S), consisting of four syllables; and a ‘repeated phrase’ (R1), which consists of three syllables, and is given 1–6 times (Fig. 1). The song also includes faint whistled notes of low frequency between syllables, which were difficult to detect in some recordings owing to the quality of recordings or interference from ambient noise. As a result, these whistled notes were not included in the analysis (Fig. 1). For each individual song, spectrograms were produced, viewed and measured using Raven Pro 1.4 software (Cornell Laboratory of Ornithology, Ithaca, NY), with a Hann-filter and a Fast Fourier Transform (FFT) value of 678 points. Overlap was set to 50%, giving a frequency resolution of 86.1 Hz.
Four songs from each individual Warbler were analysed. Syllables in each phrase formed the basic unit of analysis and four parameters were measured: (1) lowest frequency (kHz), (2) maximum frequency (frequency with maximum energy, in kHz), (3) highest frequency (kHz) and (4) duration of syllable (s). For each individual the mean values of each syllable were calculated, then the means of each phrase determined. One-sample t-tests and Mann–Whitney U tests were used to compare means of the measured parameters of phrases between the 2 years. All statistical tests were performed using SPSS 18 (SPSS Inc., Chicago, IL, USA).
To investigate the possible effect on Grey Warbler song of the frequency of songs of species reintroduced to Zealandia Sanctuary, we calculated the mean maximum frequency of the songs of 10 individuals of species reintroduced to the Sanctuary after 2000: North Island Saddleback (Philesturnus rufusater), reintroduced in 2002; Bellbird (Anthornis melanura), reintroduced from 2002–03; North Island Robin (Petroica longipes), reintroduced in 2001–02; Whitehead (Mohoua albicilla), reintroduced in 2001–02; and North Island Kaka (Nestor meridionalis septentrionalis), reintroduced in 2000 (Bell 2008).
To determine if the equipment used to record the songs in the 2 years compared (a DAT recorder in 2002 and a solid-state digital recorder in 2009) affected the analysis of songs, we used both types of equipment to record a series of generated tones simultaneously. The recorded tones were then analysed using Raven Pro 1.4 software (Cornell Laboratory of Ornithology). There was no difference in the measured frequency or duration of the recorded tones, ruling out an equipment effect on the mean frequencies determined (Azar 2012).
Results
The phrases in the Grey Warbler’s song had a narrow frequency bandwidth, and the lowest and highest frequencies were highly positively correlated with maximum frequency of both the start phrase and repeated phrases (Table 1). We therefore reduced the dataset and used the maximum frequency as a representative measure for frequency characteristics of song elements.

|
The values of maximum frequency were normally distributed (Kolmogorov–Smirnov test; P > 0.5) and the variances between groups (2002 and 2009 songs) were not unequal (Levene’s Test for Equality of Variances for all three song parts; all P > 0.05), so a one-sample test was appropriate to test differences between groups. An independent samples t-test was conducted to compare maximum frequency song parameters between the 2 years compared. The duration of syllables was not normally distributed (Kolmogorov–Smirnov; P < 0.05) so the non-parametric Mann–Whitney U test was used to compare duration of phrases between the 2 years compared.
The structure of songs of Grey Warblers in 2002 and 2009 differed in maximum frequency (spectrally) but not in duration (temporally) (Table 2).

|
Maximum frequencies in all parts of the song (start phrase (S), 1–3 repeats of the repeated phrase (R1, R2, R3)) were significantly higher in 2009 than in 2002 (Table 3). Repeated phrase (R1) can be repeated up to six times; however, the analysis was restricted to the first three repeats (R1, R2, R3) because some birds will stop after the 3 or 4th repeat. The average increase in frequency between 2002 and 2009 songs was 0.34–0.45 kHz. There was no significant change in the mean duration of phrases for the start phrase (S), or one and two repeats of the repeated phrase (R1, R2) between 2002 and 2009 (n = 10 for each song part), but R3 was 0.11 s longer in 2002 than in 2009 (P > 0.01) (Table 4).

|

|
Mean frequencies of songs (±s.e.) of birds reintroduced to the Sanctuary were: Bellbird 2.3 kHz ± 1.2, Stitchbird 4.8 kHz ± 0.8, Kaka 2.3 kHz ± 0.7, North Island Robin 4.6 kHz ± 1.5, Saddleback 4.3 kHz ± 0.7 and Whitehead 3.2 kHz ± 0.2 (n = 10 for all species).
Discussion
There was a marked difference in the frequency characteristics of Grey Warbler songs between 2002 and 2009. The increase in the frequencies of all parts of the songs of Grey Warblers between 2002 and 2009 could be a response to (1) increased levels of ambient low-frequency noise, (2) change in the acoustic competition in the environment, (3) change in acoustic properties of the habitat, (4) influence of seasonal change in breeding density. At first glance, the use of higher frequencies in all phrases in 2009 might suggest a shift to a higher song frequency as an adaptation to increased levels of ambient low-frequency noise, as suggested for the Great Tit (Slabbekoorn and Peet 2003; Slabbekoorn and den Boer-Visser 2006). Similar results have been reported for the Common Blackbird (Turdus merula) in Austria (Nemeth and Brumm 2009), Song Sparrow in the Netherlands (Melospiza melodia) (Wood et al. 2006) and House Finch in western USA (Haemorhous mexicanus) (Fernandez-Juricic et al. 2005). In these species, there was a positive correlation between song frequency and level of ambient noise in their habitat. The ambient noise between the two periods in Zealandia was not measured, but there was no anecdotal evidence of an increase of ambient noise in the study area. Moreover, anthropogenic noise usually occurs within the range of 1–4 kHz, with most energy at 1–2 kHz (Skiba 2000). The frequency of Grey Warbler song syllables was in the range of 2.5–5.0 kHz, a range less likely to be masked by ambient noise.
Grey Warblers did not show a significant change in the duration of their song syllables between 2002 and 2009. Increased duration of song can also be an adaptation to high levels of ambient noise, for example, King Penguins (Aptenodytes patagonicus) produce longer calls with more syllables under windy and noisy conditions (Lengagne et al. 1999). However, production of longer notes can be constrained by the size of birds and the volume of air that can be exhaled to produce the notes. Other studies have shown that city birds may have a faster delivery rate of song and a shorter interval between songs (Slabbekoorn and den Boer-Visser 2006), possibly as adaptation to increased anthropogenic noise.
Interspecific interactions may lead to displacement of song characters of different species, which in turn may be caused by the introduction or reintroduction of avian species or by increase in the relative densities of avian species that use a similar frequency bandwidth. In Zealandia Sanctuary, the Whitehead was reintroduced to the sanctuary in 2001 and 2002. Its song had a mean maximum frequency of 3.1 kHz ± 0.2 (s.e.) (n = 10) (Fig. 2c), which more or less equals the mean maximum frequency of 3.0 kHz ± 0.5 (s.e.) (n = 10) for Grey Warblers in 2002 (Fig. 2a). Both species are now fairly common in Zealandia Sanctuary, so between 2002 and 2009 acoustic competition may have influenced the shift in frequency of Grey Warbler songs. The advantages of avoiding acoustic competition could be considerable, with any masking or interference minimised and the efficiency of the communication system in the environment maximised. This would be particularly important where two species show similarities in song structure, such as sharing the same frequency range (Catchpole and Slater 2008). There is now some evidence to suggest that the singing behaviour of males is not only affected by the songs of conspecific neighbours, but also the songs of other species with which they share their habitat (Brumm 2006). For example, Red-eyed Vireos (Vireo olivaceus) and Least Flycatchers (Empidonax minimus) can modify their singing patterns to avoid competition with each other (Ficken et al. 1974). Popp et al. (1985) analysed the singing patterns of four forest species in Wisconsin, NJ, USA, and found that each attempted to avoid temporal overlap of the songs of the others, often by singing as soon as there was a sound gap. This result was also confirmed by playback experiments on the Ovenbird (Seiurus aurocapilla) (Popp and Ficken 1987).
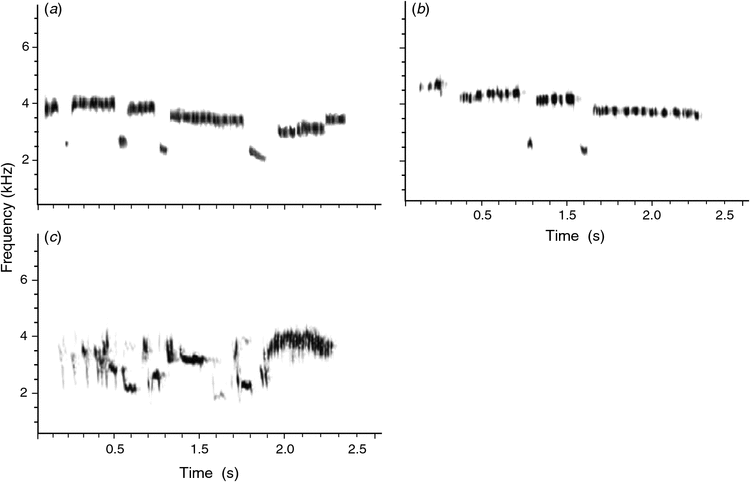
|
An alternative explanation for differences in song frequency over time relates to signal transmission in the habitat that might possibly be associated with different seasons and weather conditions. Atmospheric conditions can affect sound transmission properties of the habitat, as sound attenuation increases with the increase in temperature, and is reduced with increasing humidity (Wiley and Richards 1982). However, both sets of samples were taken over periods of weeks, recordings in both years being made in the spring, with most recording in October. The mean October average temperature was 1.0°C below normal in both years (see the National Institute of Water and Atmospheric Research (NIWA), http://www.niwa.co.nz/climate/summaries/seasonal, accessed 10 July 2013). Atmospheric differences between 2002 and 2009 therefore seem unlikely.
Another explanation for differences between years might be related to the possible influence of seasonal change in breeding density on vocal communication. Change in the breeding densities between years, and the reintroduction of species to Zealandia Sanctuary may have influenced the song of Grey Warblers in some way through acoustic competition. Intraspecific competition by males and motivational changes in singing activity can cause a shift in song features (Goretskaia 2004), and divergence in song features could result from variation in song within individuals related to either motivational status or acoustic conditions (Ripmeester et al. 2010). Information on individual variation in song is provided in the Supplementary material (Table S1).
In conclusion, our study suggests that competition on the acoustical signal (song frequency) of Grey Warblers is likely to have been an important factor in changes in their song. The reintroduction of the Whitehead to the sanctuary, which utilises a similar frequency band to that used by Grey Warbler, appears the most likely reason for the frequency shift in Grey Warbler song that we measured.
References
Andersen, J. C. (1926). ‘Bird-song and New Zealand Song Birds.’ (Whitcombe and Tombs: Auckland.)Azar, J. F. (2012). Vocal communication within a forest bird community. Ph.D. Thesis, Victoria University of Wellington, Wellington.
Bell, B. D. (2008). The birds of the south Wellington region. In ‘The Taputeranga Marine Reserve’. (Ed. J. P. A. Gardner.) pp. 424–458. (First Edition Publishers: Wellington.)
Brumm, H. (2006). Signalling through acoustic windows: Nightingales avoid interspecific competition by short-term adjustment of song timing. Journal of Comparative Physiology 192, 1279–1285.
| Signalling through acoustic windows: Nightingales avoid interspecific competition by short-term adjustment of song timing.Crossref | GoogleScholarGoogle Scholar | 16924503PubMed |
Buller, W. L. (1888). ‘A History of the Birds New Zealand’, 2nd edn. (W. L. Buller: London.)
Byers, B. E., Belinsky, K. L., and Bentley, R. A. (2010). Independent cultural evolution of two song traditions in the Chestnut‐sided Warbler. American Naturalist 176, 476–489.
| Independent cultural evolution of two song traditions in the Chestnut‐sided Warbler.Crossref | GoogleScholarGoogle Scholar | 20712515PubMed |
Campbell, D. J., and Atkinson, I. A. E. (2002). Depression of tree recruitment by the Pacific Rat (Rattus exulans Peale) on New Zealand’s northern offshore islands. Biological Conservation 107, 19–35.
| Depression of tree recruitment by the Pacific Rat (Rattus exulans Peale) on New Zealand’s northern offshore islands.Crossref | GoogleScholarGoogle Scholar |
Catchpole, C. K., and Slater, P. J. B. (2008). ‘Bird Song: Biological Themes and Variations’, 2nd edn. (Cambridge University Press: Cambridge, UK.)
Doutrelant, C., and Lambrechts, M. M. (2001). Macrogeographic variation in song: a test of competition and habitat effects in Blue Tits. Ethology 107, 533–544.
| Macrogeographic variation in song: a test of competition and habitat effects in Blue Tits.Crossref | GoogleScholarGoogle Scholar |
Fernandez-Juricic, E., Poston, R., De Collibus, K., Morgan, T., Bastain, B., Martin, C., Jones, K., and Treminio, R. (2005). Microhabitat selection and singing behavior patterns of male House Finches (Carpodacus mexicanus) in urban parks in a heavily urbanized landscape in the western US. Urban Habitats 3, 49–69.
Ficken, R. W., Ficken, M. S., and Hailman, J. P. (1974). Temporal pattern shifts to avoid acoustic interference in singing birds. Science 183, 762–763.
| Temporal pattern shifts to avoid acoustic interference in singing birds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cvgvFOksg%3D%3D&md5=fca37fe810d18540c14e4791c87a6d19CAS | 17790627PubMed |
Gill, B. J. (1982). Breeding of the Grey Warbler, Geryone igata, at Kaikoura, New Zealandia. Ibis 124, 123–147.
| Breeding of the Grey Warbler, Geryone igata, at Kaikoura, New Zealandia.Crossref | GoogleScholarGoogle Scholar |
Goretskaia, M. I. (2004). Song structure and singing behaviour of Willow Warbler Phylloscopus trochilus acredula in populations of low and high density. Bioacoustics 14, 183–195.
| Song structure and singing behaviour of Willow Warbler Phylloscopus trochilus acredula in populations of low and high density.Crossref | GoogleScholarGoogle Scholar |
Grant, B. R., and Grant, P. R. (2010). Songs of Darwin’s Finches diverge when a new species enters the community. Proceedings of the National Academy of Sciences of the United States of America 107, 20 156–20 163.
| Songs of Darwin’s Finches diverge when a new species enters the community.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFaju7vL&md5=b42320368e99bbc6eb90d5cc68edc95cCAS |
Greene, E., Muehter, V. R., and Davison, W. (1997). Lazuli Bunting (Passerina amoena). In ‘The Birds of North America’. (Eds A. Poole and F. Gill.) Number 232. (Academy of Natural Sciences and the American Union of Ornithologists: Washington, DC.)
Handford, P. (1988). Trill rate dialects in the Rufous-collared Sparrow, Zonotrichia capensis, in northwestern Argentina. Canadian Journal of Zoology 66, 2658–2670.
| Trill rate dialects in the Rufous-collared Sparrow, Zonotrichia capensis, in northwestern Argentina.Crossref | GoogleScholarGoogle Scholar |
Hansen, P. (1999). Long-term stability of song elements in the Yellowhammer Emberiza citrinella. Bioacoustics 9, 281–295.
| Long-term stability of song elements in the Yellowhammer Emberiza citrinella.Crossref | GoogleScholarGoogle Scholar |
Heather, B., and Robertson, H. (2000). ‘The Field Guide to the Birds of New Zealand.’ (Viking: Auckland.)
Ince, S. A., Slater, P. J. B., and Weismann, C. (1980). Changes with time in the songs of a population of Chaffinches. Condor 82, 285–290.
| Changes with time in the songs of a population of Chaffinches.Crossref | GoogleScholarGoogle Scholar |
Kirschel, A. N. G., Blumstein, D. T., and Smith, T. B. (2009). Character displacement of song and morphology in African Tinkerbirds. Proceedings of the National Academy of Sciences of the United States of America 106, 8256–8261.
| Character displacement of song and morphology in African Tinkerbirds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvVWmtrY%3D&md5=ffefdfe46d434d8ee2f844ac6ad8a093CAS |
Lengagne, T., Aubin, T., Lauga, J., and Jouventin, P. (1999). How do King Penguins Aptenodytes patagonicus apply the mathematical theory of information to communicate in windy conditions? Proceedings of the Royal Society of London – B. Biological Sciences 266, 1623–1628.
| How do King Penguins Aptenodytes patagonicus apply the mathematical theory of information to communicate in windy conditions?Crossref | GoogleScholarGoogle Scholar |
Luther, D., and Baptista, L. (2010). Urban noise and the cultural evolution of bird songs. Proceedings of the Royal Society of London – B. Biological Sciences 277, 469–473.
| Urban noise and the cultural evolution of bird songs.Crossref | GoogleScholarGoogle Scholar |
MacDougall-Shackleton, S. A. (1997). Sexual selection and the evolution of song repertoires. In ‘Current Ornithology. Vol. 1’. (Eds V. J. Nolan, E. D. Ketterson and C. F. Thompson.) pp. 81–124. (Thompson: New York.)
Nemeth, E., and Brumm, H. (2009). Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Animal Behaviour 78, 637–641.
| Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization?Crossref | GoogleScholarGoogle Scholar |
Paul, R. S., and McKenzie, H. R. (1975). A bushman’s seventeen years of noting birds. Part B – New Zealand Pipit, Grey Warbler, North Island Fantail and Silvereye. Notornis 22, 273–282.
Podos, J., and Warren, P. S. (2007). The evolution of geographic variation in birdsong. In ‘Advances in the Study of Behavior. Vol. 1’. (Eds H. J. Brockmann, T. J. Roper, M. Naguib, J. C. Mitani and L. W. Simmons.) pp. 403–458. (Academic Press: New York.)
Popp, J. W., and Ficken, R. W. (1987). Effects of nonspecific singing on the song of the Ovenbird. Bird Behaviour 7, 22–26.
| Effects of nonspecific singing on the song of the Ovenbird.Crossref | GoogleScholarGoogle Scholar |
Popp, J. W., Ficken, R. W., and Reinartz, J. A. (1985). Short-term temporal avoidance of interspecific acoustic interference among forest birds. Auk 102, 744–748.
Ripmeester, E. A., Kok, J. S., Van Rijssel, J. C., and Slabbekoorn, H. (2010). Habitat-related birdsong divergence: a multi-level study on the influence of territory density and ambient noise in European Blackbirds. Behavioral Ecology and Sociobiology 64, 409–418.
| Habitat-related birdsong divergence: a multi-level study on the influence of territory density and ambient noise in European Blackbirds.Crossref | GoogleScholarGoogle Scholar | 20119488PubMed |
Skiba, R. (2000). Possible ‘rain call’ selection in the Chaffinch (Fringilla coelebs) by noise intensity, an investigation of a hypothesis. Journal of Ornithology 141, 160–167.
| Possible ‘rain call’ selection in the Chaffinch (Fringilla coelebs) by noise intensity, an investigation of a hypothesis.Crossref | GoogleScholarGoogle Scholar |
Slabbekoorn, H., and den Boer-Visser, A. (2006). Cities change the songs of birds. Current Biology 16, 2326–2331.
| Cities change the songs of birds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1OhtL3L&md5=afe9c5de1fbf12017388ace2df19a1f0CAS | 17141614PubMed |
Slabbekoorn, H., and Peet, M. (2003). Birds sing at a higher pitch in urban noise. Nature 424, 267.
| Birds sing at a higher pitch in urban noise.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsVGjsr0%3D&md5=edc1d68eaeb5d111de7b47f1a3fecd81CAS | 12867967PubMed |
Slabbekoorn, H., and Smith, T. B. (2002a). Bird song, ecology and speciation. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 357, 493–503.
| Bird song, ecology and speciation.Crossref | GoogleScholarGoogle Scholar |
Slabbekoorn, H., and Smith, T. B. (2002b). Habitat-dependent song divergence in the Little Greenbul: an analysis of environmental selection pressures on acoustic signals. Evolution 56, 1849–1858.
| 12389730PubMed |
Slabbekoorn, H., Yeh, P., and Hunt, K. (2007). Sound transmission and song divergence: a comparison of urban and forest acoustics. Condor 109, 67–78.
| Sound transmission and song divergence: a comparison of urban and forest acoustics.Crossref | GoogleScholarGoogle Scholar |
Trainer, J. M. (1989). Cultural evolution in song dialects of Yellow-rumped Caciques in Panama. Ethology 80, 190–204.
| Cultural evolution in song dialects of Yellow-rumped Caciques in Panama.Crossref | GoogleScholarGoogle Scholar |
Warren, P. S. (2002). Geographic variation and dialects in songs of the Bronzed Cowbird (Molothrus aeneus). Auk 119, 349–361.
Whitney, C. L. (1992). Temporal stability of song in a local population of Wood Thrushes. Wilson Bulletin 104, 516–520.
Wiley, R. H., and Richards, D. G. (1982). Adaptations for acoustic communication in birds: sound transmission and signal detection. In ‘Acoustic Communication in Birds: Production, Perception, and Design Features of Sounds. Vol. 1’. (Eds D. E. Kroodsma and E. H. Miller.) pp. 132–181. (Academic Press: New York.)
Wood, W. E., Yezerinac, S. M., and Dufty, J. A. (2006). Song Sparrow (Melospiza melodia) song varies with urban noise. Auk 123, 650–659.
| Song Sparrow (Melospiza melodia) song varies with urban noise.Crossref | GoogleScholarGoogle Scholar |


