Exploring the interplay of biotic interactions and salinity stress in freshwater invertebrate assemblages: a response to Kefford et al. (2022)
Bruce C. Chessman A *
A *
A Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, UNSW Sydney, NSW 2052, Australia.
Marine and Freshwater Research 73(5) 578-584 https://doi.org/10.1071/MF21314
Submitted: 26 October 2021 Accepted: 4 January 2022 Published: 22 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Controlled mesocosm experiments can add substantially to our knowledge of the influence of environmental factors on freshwater assemblages by partitioning the possible effects of different drivers. Reporting results of such an experiment, Bray et al. (2019) concluded that effects of salinity on salt-sensitive stream invertebrates were substantially modified by interspecific biotic interactions with salt-tolerant invertebrates from a high-salinity stream. Chessman (2021) questioned this conclusion on three grounds: (1) confounding of the experimental design, (2) lack of evidence that purported diverse effects of biotic interactions were beyond mere stochastic variation, and (3) absence of mechanistic explanations for supposed effects grounded in organism biology and ecology. Chessman (2021) also conducted an independent statistical analysis of publicly available data from the experiment, which did not support the study’s conclusions. Kefford et al. (2022) dispute Chessman’s (2021) findings by analysing previously unpublished data from the experiment, which they claim demonstrates that the experimental design was not confounded, and criticise Chessman’s (2021) statistical analysis. Here, I respond to their new analysis and criticisms, explaining why they do not dispel any of the concerns expressed by Chessman (2021).
Keywords: biotic interaction, experimental design, freshwater, invertebrate, mesocosm, salinity, statistical confounding, stream.
Introduction
A perennial quest in freshwater ecology is to understand how multiple physical, chemical, and biological factors interact to influence aquatic communities at multiple spatial and temporal scales (Downes 2010; Friberg 2010; Thorp 2014). Controlled microcosm and mesocosm experiments can add substantially to our knowledge in this sphere by partitioning the possible effects of different environmental drivers in a way that is seldom possible in observational field studies (Ledger et al. 2009; Barmentlo et al. 2019), although their ability to replicate processes occurring in nature may be questioned (Crossland and La Point 1992; Schindler 1998).
In a recent example, Bray et al. (2019) conducted a mesocosm experiment to test the response of salt-sensitive stream invertebrates to two manipulated independent variables: salinity and the abundance of salt-tolerant stream invertebrates. Their experiment involved five salinity treatments (including a salinity control) applied to two invertebrate assemblages. One assemblage was derived from invertebrate samples from a single low-salinity stream, the Cotter River, which Bray et al. (2019) assumed to contain both salt-sensitive and salt-tolerant invertebrates. For simplicity, this assemblage is referred to hereafter as the ‘pure assemblage’. Each mesocosm containing this assemblage was stocked with three substratum colonisation trays plus leaf packs and two kick samples from the Cotter River. The second assemblage was a mixture of invertebrates from the low-salinity stream and a high-salinity stream, Cunningham Creek, which Bray et al. (2019) assumed to contain only salt-tolerant invertebrates. This assemblage is referred to for simplicity as the ‘mixed assemblage’. Each mesocosm containing this assemblage was stocked with three substratum colonisation trays plus leaf packs and one kick sample from the Cotter River, as well as one kick sample from Cunningham Creek.
The procedure for formulating the assemblages meant that both would initially have contained both salt-sensitive and salt-tolerant invertebrates, but in different quantities. The pure assemblage would initially have contained more salt-sensitive invertebrates, because it was stocked with more invertebrate samples from the low-salinity stream. The mixed assemblage would initially have contained more salt-tolerant invertebrates, because it included invertebrates from the high-salinity stream, whereas the pure assemblage did not. After comparing abundances of putatively salt-sensitive taxa among treatments at the end of the experiment, Bray et al. (2019) claimed support for the hypothesis that ‘salinity effects were modified by interspecific biotic interactions between salt-tolerant organisms, collected from a high salinity site, and a community expected to be more salt-sensitive, collected from a low salinity site’.
Chessman (2021) questioned this conclusion on three grounds. First, he noted that the study design was subject to procedural confounding because differences in start-of-experiment abundances of salt-tolerant invertebrates were tied to differences in start-of-experiment abundances of salt-sensitive invertebrates. Second, Chessman (2021) queried the practice of Bray et al. (2019) of attributing apparent differences between the two assemblages in end-of-experiment density–EC (electrical conductivity) relationships of individual taxa to unspecified biotic interactions without demonstrating that the spectrum of apparent differences could not be merely a manifestation of stochastic variation. Third, Chessman (2021) observed that Bray et al. (2019) did not provide any mechanistic basis in the biology or ecology of the various salt-sensitive taxa to explain why they would respond to salt-tolerant invertebrates in the particular ways suggested. Chessman (2021) also conducted a statistical analysis of Bray et al.’s (2019) publicly available data, which found no support for their hypothesis.
Kefford et al. (2022) dispute Chessman’s (2021) findings by analysing previously unpublished data from the experiment, which they claim demonstrates that the experimental design was not confounded, as well as by criticising Chessman’s (2021) statistical analysis. Here, I respond to their new analysis and criticisms, explaining why they do not dispel any of the concerns expressed by Chessman (2021).
The new results do not eliminate confounding
The additional data and analyses presented by Kefford et al. (2022) compare the putatively salt-sensitive faunal component in the salinity-control mesocosms between the pure and mixed assemblages ~1 week into the experiment. They report results of univariate analyses (method not stated) of taxon richness, total abundance, EPT (Ephemeroptera, Plecoptera, and Trichoptera) abundance and OCH (Odonata, Coleoptera and Hemiptera) abundance, as well as multivariate analyses (permutational multivariate analysis of variance and analysis of similarities) of assemblage proportional composition. None of these analyses found a statistically significant difference between the two assemblages.
These analyses likely had low statistical power, because they incorporated only the salinity-control mesocosms, there were only four such mesocosms for each assemblage, and variability in the response variables was high. Moreover, Kefford et al. (2022) offer no explanation of why the salt-sensitive faunal component would be the same in the pure and mixed assemblages a week into the experiment when it must have differed between the two assemblages at the start of the experiment because the pure assemblage received an extra kick-net sample from the Cotter River. Conceivably, something caused greater mortality of salt-sensitive invertebrates in the pure assemblage than in the mixed assemblage during the first week, evening out the numbers. But what could do that? It could not be the salt-tolerant invertebrates, because they were less abundant in the pure assemblage and so would have caused less mortality there. However, competitive or predatory interactions among salt-sensitive invertebrates might have caused greater mortality in the pure assemblage, because salt-sensitive invertebrates were initially more abundant there. If so, the two assemblages differed in sensitive–sensitive biotic interactions as well as potentially in sensitive–tolerant biotic interactions, and the experimental design was indeed confounded.
Interpretation of density–EC relationships
Kefford et al. (2022) state that Bray et al. (2019) did not statistically test end-of-experiment differences in density–EC relationships of individual taxa between the pure and mixed assemblages because the focus of their study was on community-level responses. However, the proposition that individual salt-sensitive taxa demonstrated a variety of effects of sensitive–tolerant biotic interactions on salinity responses is central to their conclusions. Bray et al. (2019) devote two of their five Results paragraphs, four of their six Discussion paragraphs, and three of their five figures either entirely or primarily to responses of single taxa.
Kefford et al. (2022) also state that Bray et al. (2019) acknowledged uncertainty in their findings for individual taxa by using phrases such as ‘appeared to show’ and ‘appeared to depend’. While such phrases are often used, Bray et al. (2019) also make unqualified claims such as that ‘at the population level, however, salinity and tolerant–sensitive taxa interactions caused a range of species-specific and context-dependent responses’ and that their ‘results reinforced [that] interspecific biological interactions both mediated salinity effects and were important on their own, irrespective of salinity toxicity, influencing taxa and community responses.’ Moreover, the abstract of Bray et al. (2019) includes no qualifications at all.
Response to statistical criticisms
Kefford et al. (2022) criticise Chessman’s (2021) statistical comparison of the two assemblages in terms of end-of-experiment density–EC relationships of individual taxa on multiple grounds. First, they argue that Chessman’s (2021) analysis had low statistical power, because it included a non-parametric method (rank correlation). This method was used because large numbers of zero densities for some taxa meant that assumptions of the parametric equivalent could not be met. Second, they object to multiple testing of a null hypothesis that they regard as implausible (that density–EC relationships did not differ between the two assemblages), an issue that they elaborate on at great length in their Discussion. Third, Kefford et al. (2022) point to a risk of an inflated rate of type I error due to separate testing of multiple taxa. However, Chessman (2021) allowed for multiple testing by comparing the number of statistically significant differences in EC–density relationships between the two assemblages with the number expected by chance, rather than using a Bonferroni correction, which would have inflated the type II error rate (Moran 2003; Nakagawa 2004).
Kefford et al. (2022) also assert that multiple testing is inappropriate because it assumes that the individual salt-sensitive taxa responded to salinity and biotic interactions independently of one another. However, such an assumption is implicit in the approach of Bray et al. (2019), whereby they subjectively assess model fits for the density–EC relationship taxon by taxon. If apparent differences in the density–EC relationship of a salt-sensitive taxon between the pure and mixed assemblages may be influenced by other salt-sensitive taxa, then attributing those differences to interactions with salt-tolerant invertebrates is obviously problematic.
Kefford et al. (2022) also criticise Chessman (2021) for testing ‘the null hypothesis of no difference in the central tendency of correlation coefficients between treatments’, because of ‘different responses potentially cancelling one another’. The apparent responses of individual salt-sensitive taxa did indeed ‘cancel one another’, because Chessman (2021) showed that the number of those taxa whose density–EC correlations differed between the two assemblages in one direction was similar to, and statistically indistinguishable from, the number whose density–EC correlations differed between the two assemblages in the opposite direction. Kefford et al. (2022) do not propose any mechanism that could explain such equivalence in terms of biotic interactions. They state that they ‘expected that invertebrates obtained from the higher-salinity stream would be better able to tolerate salinity treatments and to outcompete or prey upon taxa from the low-salinity stream’, but do not articulate how such competition and predation would alter responses to salinity. Logically, though, the competitive and predatory impact of salt-tolerant invertebrates on salt-sensitive taxa would be greater at higher salinities where the salt-tolerant invertebrates would enjoy a physiological advantage. Thus, greater exposure to salt-tolerant invertebrates would be expected to amplify toxic effects of higher salinity on salt-sensitive taxa. Consequently, if supposed effects of sensitive–tolerant interactions were real, differences in density–EC relationships between the two assemblages should have been predominantly in one direction – the one indicating a greater salinity impact on salt-sensitive taxa in the mixed assemblage.
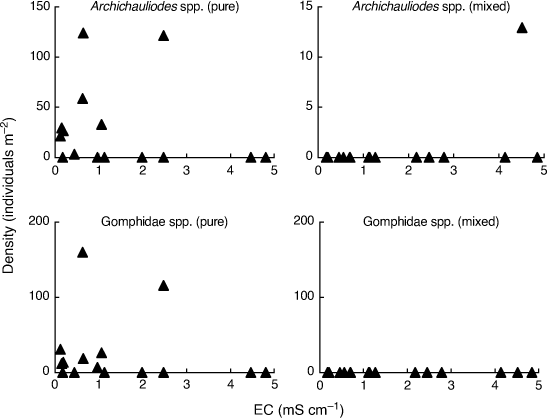
Finally, Kefford et al. (2022) address Chessman’s (2021) observations on the two taxa (Archichauliodes spp. and Corynoneura spp.) that had the strongest apparent difference in density–EC relationships between the two assemblage types. Chessman (2021) first noted that both taxa had a negative correlation with EC in the pure assemblage and a positive correlation with EC in the mixed assemblage. Interpreting this contrast in terms of sensitive–tolerant biotic interactions would require that greater exposure to salt-tolerant invertebrates somehow changed the salinity responses of both taxa from negative to positive, which Chessman (2021) considered implausible. Second, Chessman (2021) pointed out that Archichauliodes spp. and Corynoneura spp. are phylogenetically and ecologically distinct, and so ‘it was unlikely that they would interact with salt-tolerant invertebrates in the same way’. Kefford et al. (2022) do not respond to the first point and misrepresent the second point as Chessman (2021) supposedly asserting that it was ‘unlikely that the different responses could be the result of interactions with organisms from the high-salinity stream’.
A reality check
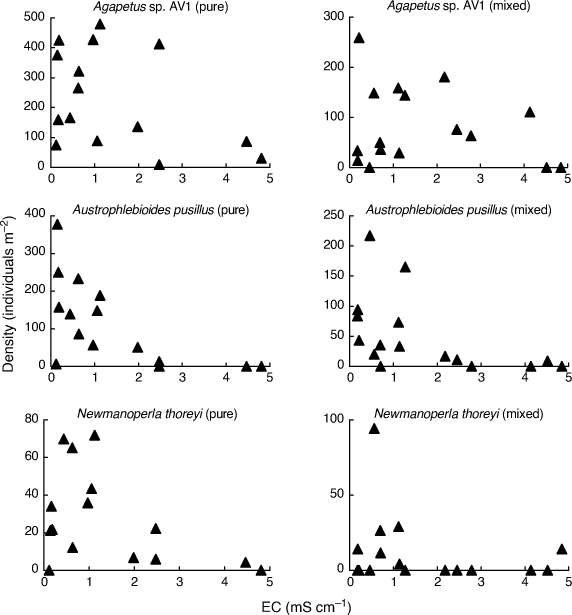
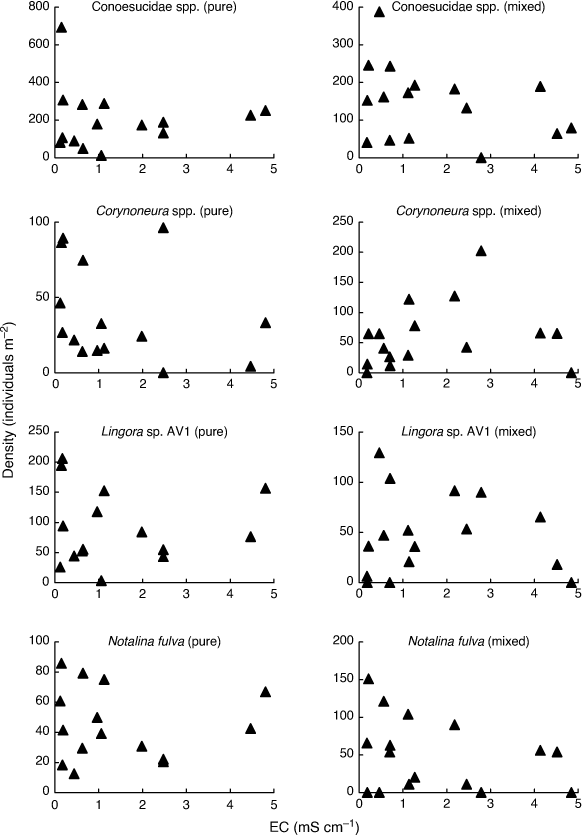
In order to fully appreciate the high level of stochasticity in the data analysed by Bray et al. (2019), it is necessary to examine densities of individual taxa in individual mesocosms. Fig. 1–3 use their publicly available data, downloaded from the Dryad Digital Repository at the link provided in Bray et al. (2019, see https://doi.org/10.5061/dryad.n541d0t), to illustrate such data for nine (out of 88) putatively salt-sensitive taxa that they highlight as examples of apparent sensitive–tolerant biotic interactions. Densities are plotted against EC separately for each assemblage type, and y-axes are scaled according to the maximum density for each type, because Kefford et al. (2022) suggest that density–EC relationships should be considered independently of overall differences in density.
|
|
|
These nine taxa demonstrate three basic patterns. Three taxa (Agapetus sp. AV1, Austrophlebioides pusillus and Newmanoperla thoreyi) show a pattern of highly variable density at low EC and lower density at high EC for both assemblage types (Fig. 1). Their sensitivity to salinity is obvious but any difference in salinity response between assemblage types is obscure.
Four taxa (Conoesucidae spp., Corynoneura spp., Lingora sp. AV1 and Notalina fulva) show at best a weak relationship to EC for both assemblage types, suggesting that they are perhaps not very salt-sensitive (Fig. 2). Bray et al. (2019) provided no direct evidence that their putatively salt-sensitive invertebrate taxa actually are salt-sensitive, but simply treated a taxon as salt-sensitive because it was not known to occur in Cunningham Creek. However, their site on the Cotter River lies in native forest whereas their site on Cunningham Creek lies in cleared farmland, and so the absence of certain taxa from the latter could be due to many factors other than sensitivity to salinity. For example, Zalizniak et al. (2006) reported upper 96-h LC50 values of EC for Notalina fulva of 16–20 mS cm–1, at least 10 times the EC Bray et al. (2019) report for Cunningham Creek (1.6 mS cm–1) and 3–4 times the maximum EC in their experiment (5 mS cm–1). Thus, it is questionable whether salinity was the factor causing the apparent absence of Notalina fulva from Cunningham Creek.
Finally, two large-bodied predators (Archichauliodes spp. and Gomphidae spp.) have zero densities in so many mesocosms that a confident comparison of density–EC relationships between the two assemblage types is impossible (Fig. 3).
Discussion
Incongruously, the data analysis presented by Kefford et al. (2022) has many of the features that they criticise in Chessman’s (2021) analysis. Kefford et al.’s (2022) analysis likely has low statistical power and appears to test an implausible null hypothesis, namely that the salt-sensitive faunal component did not differ between the two assemblages 1 week into the experiment, even though it differed at both the start and the end of the experiment. Moreover, their analysis also involves multiple testing with variables that are not all independent of one another, for example total abundance and EPT abundance.
Kefford et al. (2022) also invoke a straw man argument, implying that Chessman (2021) proposed that ‘if the effect of salinity on taxa were dependent on biotic interactions, such dependencies would be restricted to closely related species or those from the same functional group’. As explained above, Chessman (2021) did not suggest that effects of biotic interactions of salinity responses need be confined to particular types of salt-sensitive taxa, but simply stated that two phylogenetically and ecologically distinct salt-sensitive taxa would be unlikely to interact with salt-tolerant invertebrates in the same way. The point is that salt-sensitive taxa with similar characteristics would logically be expected to interact with salt-tolerant invertebrates more similarly than salt-sensitive taxa with disparate characteristics. Therefore, if supposed effects of salt-tolerant invertebrates on the salinity responses of salt-sensitive taxa are real, the magnitude and direction of those effects should relate to the biology and ecology of the salt-sensitive taxa concerned. However, neither Bray et al. (2019) nor Kefford et al. (2022) provides any evidence of such relationships.
In addition, Kefford et al. (2022) still provide no evidence that the apparent salinity responses of salt-sensitive taxa varied appreciably more with greater or lesser exposure to salt-tolerant invertebrates than expected from normal stochastic variation in invertebrate assemblages. Their proposition about the effect of the salt-tolerant invertebrates rests on significant differences between assemblage types in the end-of-experiment composition of the salt-sensitive faunal component (Bray et al. 2019). However, as Chessman (2021) pointed out, this result is ambiguous, because it could be due to the start-of-experiment difference between the two assemblages in either the salt-tolerant or the salt-sensitive faunal component (or both). As explained above, the new analysis does not remove this ambiguity.
Kefford et al. (2022) describe the purpose of the experiment reported by Bray et al. (2019) as ‘to mimic a situation where a freshwater stream was salinised.’ The design of Bray et al.’s (2019) experiment is indeed amenable to exploring some questions about how different invertebrate assemblages respond to salinisation. However, its ability to mimic possible proliferation of salt-tolerant invertebrates was probably limited by the brevity of the experiment (75 days) and the isolation, small size and artificial character of the mesocosms. These factors likely constrained colonisation by salt-tolerant species, especially non-volant ones, and reproduction within the mesocosms, especially by insects with a terrestrial life-history phase.
Although suited to other purposes, Bray et al.’s (2019) experiment does not enable partitioning of the effect of interactions between salt-tolerant invertebrates from the high-salinity stream and salt-sensitive invertebrates from the low-salinity stream, as is required to test the study hypothesis. The hypothesis could have been tested validly if Bray et al. (2019) had implemented standard experimental control, keeping the quantity of invertebrates from the low-salinity stream constant while varying the quantity of invertebrates from the high-salinity stream. Kefford et al. (2022) suggest that Bray et al. (2019) did not do so because the two assemblages would then have differed in density as well as in composition. However, effects of density and composition could have been partitioned with a 2 × 2 factorial design incorporating the following four assemblages: (1) pure – low density, (2) pure – high density, (3) mixed – low density, and (4) mixed – high density. With 32 mesocosms available, eight could have been allocated to each assemblage, comprising two replicates for each of four salinity levels. If resulting statistical power was not considered adequate, the experiment could have been repeated.
Data availability
The data presented are available from the link provided by Bray et al. (2019), reproduced above.
Conflicts of interest
The work reported by Bray et al. (2019) and Kefford et al. (2022) was partly funded by Australian Research Council Linkage Project LP160100093. The present author is listed as one of six chief investigators in the grant application for that project, but was not involved in the work described by Bray et al. (2019) and Kefford et al. (2022).
Declaration of funding
The preparation of this article did not receive any specific funding.
Acknowledgements
The present author thanks the authors of the original study and the Dryad Digital Repository for making the study data available, and an anonymous reviewer for instructive comments on the original manuscript.
References
Barmentlo, SH, Schrama, M, van Bodegom, PM, de Snoo, GR, Musters, CJM, and Vijver, MG (2019). Neonicotinoids and fertilizers jointly structure naturally assembled freshwater macroinvertebrate communities. The Science of the Total Environment 691, 36–44.| Neonicotinoids and fertilizers jointly structure naturally assembled freshwater macroinvertebrate communities.Crossref | GoogleScholarGoogle Scholar | 31306875PubMed |
Bray, JP, Reich, J, Nichols, SJ, Kon Kam King, G, Mac Nally, R, Thompson, R, O’Reilly-Nugent, A, and Kefford, BJ (2019). Biological interactions mediate context and species-specific sensitivities to salinity. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 374, 20180020.
| Biological interactions mediate context and species-specific sensitivities to salinity.Crossref | GoogleScholarGoogle Scholar |
Chessman, BC (2021). No support for purported effects of salt-tolerant stream invertebrates on the salinity responses of salt-sensitive stream invertebrates. Marine and Freshwater Research 72, 439–442.
| No support for purported effects of salt-tolerant stream invertebrates on the salinity responses of salt-sensitive stream invertebrates.Crossref | GoogleScholarGoogle Scholar |
Crossland, NO, and La Point, TW (1992). The design of mesocosm experiments. Environmental Toxicology and Chemistry 11, 1–4.
| The design of mesocosm experiments.Crossref | GoogleScholarGoogle Scholar |
Downes, BJ (2010). Back to the future: little-used tools and principles of scientific inference can help disentangle effects of multiple stressors on freshwater ecosystems. Freshwater Biology 55, 60–79.
| Back to the future: little-used tools and principles of scientific inference can help disentangle effects of multiple stressors on freshwater ecosystems.Crossref | GoogleScholarGoogle Scholar |
Friberg, N (2010). Pressure-response relationships in stream ecology: introduction and synthesis. Freshwater Biology 55, 1367–1381.
| Pressure-response relationships in stream ecology: introduction and synthesis.Crossref | GoogleScholarGoogle Scholar |
Kefford, BJ, Bray, JP, Nichols, SJ, Reich, J, Mac Nally, R, O’Reilly-Nugent, A, Kon Kam King, G, and Thompson, R (2022). Understanding salt-tolerance and biota–stressor interactions in freshwater invertebrate communities. Marine and Freshwater Research 73, 140–146.
| Understanding salt-tolerance and biota–stressor interactions in freshwater invertebrate communities.Crossref | GoogleScholarGoogle Scholar |
Ledger, ME, Harris, RML, Armitage, PD, and Milner, AM (2009). Realism of model ecosystems: an evaluation of physicochemistry and macroinvertebrate assemblages in artificial streams. Hydrobiologia 617, 91–99.
| Realism of model ecosystems: an evaluation of physicochemistry and macroinvertebrate assemblages in artificial streams.Crossref | GoogleScholarGoogle Scholar |
Moran, MD (2003). Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403–405.
| Arguments for rejecting the sequential Bonferroni in ecological studies.Crossref | GoogleScholarGoogle Scholar |
Nakagawa, S (2004). A farewell to Bonferroni: the problems of low statistical power and publication bias. Behavioural Ecology 15, 1044–1045.
| A farewell to Bonferroni: the problems of low statistical power and publication bias.Crossref | GoogleScholarGoogle Scholar |
Schindler, DW (1998). Replication versus realism: the need for ecosystem-scale experiments. Ecosystems 1, 323–334.
| Replication versus realism: the need for ecosystem-scale experiments.Crossref | GoogleScholarGoogle Scholar |
Thorp, JH (2014). Metamorphosis in river ecology: from reaches to macrosystems. Freshwater Biology 59, 200–210.
| Metamorphosis in river ecology: from reaches to macrosystems.Crossref | GoogleScholarGoogle Scholar |
Zalizniak, L, Kefford, BJ, and Nugegoda, D (2006). Is all salinity the same? The effect of ionic compositions on the salinity tolerance of five species of freshwater invertebrates. Marine and Freshwater Research 57, 75–82.
| Is all salinity the same? The effect of ionic compositions on the salinity tolerance of five species of freshwater invertebrates.Crossref | GoogleScholarGoogle Scholar |


