Invasive Japanese foraminifera in a south-west Australian estuary
Clément M. Tremblin A D , Maria Holzmann B , Justin H. Parker C , Aleksey Sadekov A and David W. Haig A
A D , Maria Holzmann B , Justin H. Parker C , Aleksey Sadekov A and David W. Haig A
A Oceans Graduate School, The University of Western Australia, 35 Stirling Highway, Perth, WA 6009, Australia.
B Department of Genetics and Evolution, The University of Geneva, 30 Quai Ernest Ansermet, CH-1211 Geneva, Switzerland.
C Task Fronterra Geoscience, 24 Risely Street, Ardross, WA 6153, Australia.
D Corresponding author. Email: 23126169@student.uwa.edu.au
Marine and Freshwater Research 73(3) 328-342 https://doi.org/10.1071/MF21254
Submitted: 31 August 2021 Accepted: 27 October 2021 Published: 24 November 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
An invasive foraminiferan is recorded for the first time in an Australian estuary. Trochammina hadai, originally described as endemic from Japan and subsequently found to be alien in coastal waters of California and Brazil, has been identified in estuarine sediment in the vicinity of Bunbury Port in Western Australia. Species determination is based on morphological, molecular and ecological similarities to the Japanese type. The species has not been recorded in other estuaries in Australia. Bunbury Port is a major exporter of woodchip to Japan and the introduction of T. hadai may have come from ballast water out of shallow-draught woodchip vessels. Small sediment samples of estuarine mud obtained at water depths of ~5 m contain abundant T. hadai (on average ~0.4 mm in adult diameter) that are easily recognised in microscopic view of the sediment surface by their bright reddish-brown colour. The collection of sediment samples from the estuarine floor and ballast water, and the examination of these for foraminifers, may provide a useful indicator in estuaries for the possible presence of other exotic species, particularly in the vicinity of ports.
Keywords: agglutinated foraminifera, Bunbury Port, Collie River, invasive distribution, Leschenault Inlet, Trochammina hadai, Western Australia.
Introduction
The introduction of alien species to a marine ecosystem threatens indigenous biodiversity and ecology (Bax et al. 2003). Various invasive species in shallow marine environments have been documented in ‘plague’ proportions due to rapid increases in populations in their new environments (e.g. sea anemones, gastropods, crustaceans, fish, seagrasses and algae; Ceccherelli et al. 2000; Smith et al. 2002; Hunt and Behrens Yamada 2003; Lanfranconi et al. 2009; Edelist et al. 2013; Patris et al. 2019). In Western Australian estuaries and shallow lagoons, invasive species from diverse taxonomic groups have been recorded (e.g. fish, comb jellyfish, hydroids, dinoflagellates, polychaetes, bryozoans and diatoms; Dürr and Semeniuk 2000; Bolton and Graham 2004; Wells et al. 2009; Russell et al. 2012; Smale and Childs 2012; Dias et al. 2015; Simpson et al. 2016).
Foraminifera are shelled protozoans commonly found in marine sediment. They form distinctive estuarine benthic faunas (Murray 1991; Scott et al. 2001). The tests (shells) of foraminifera are either calcareous, carbonate-cemented agglutinated, or organic-cemented agglutinated, and are usually chambered in many different arrangements that have taxonomic importance (Loeblich and Tappan 1987). Foraminifera have been recorded as prolific in estuarine sand, mud and marshes in previous studies on Western Australian estuaries (McKenzie 1962; Quilty 1977; Revets 2000; Quilty and Hosie 2006; Parker 2009; Ostrognay and Haig 2012; Haig 2020). Although some species are cosmopolitan, none has been recognised as invasive.
In our ongoing survey of estuarine foraminifera from south-west Australia, a species morphologically similar to Trochammina hadai Uchio, 1962 has been found in Leschenault Inlet and the connected Collie River adjacent to Bunbury Port (Fig. 1). T. hadai was first described from Japan in shallow water off the mouth of the Shinano River (Uchio 1962) and later reported in large populations from Lake Hamana, a brackish lagoon, south-east Japan (Matsushita and Kitazato 1990). T. hadai has subsequently been described as an invasive species in estuaries along the west coast of North America and the east coast of South America (McGann et al. 2000, 2012; Eichler et al. 2018).
|
In order to better understand whether the Western Australian Trochammina morphotype is an invasive species, this paper: (1) compares its morphology with the type material of T. hadai from Japan; (2) makes molecular comparisons between live specimens retrieved from Western Australia and the material analysed by Pawlowski et al. (1997) and Pawlowski and Holzmann (2014) from Lake Hamana, Japan and San Francisco Bay, US; (3) determines the distribution of these morphotypes elsewhere in Australia and on other continents; (4) documents the occurrence of Trochammina in Leschenault Inlet and Collie River and determines the associated foraminiferal species in the present-day assemblages; and (5) interprets the factors associated with the occurrence of the species in Leschenault Inlet and Collie River (e.g. potential vectors of introduction, such as established shipping and trade and the origination of that trade) in order to determine whether the distribution may be invasive due to anthropogenic activities.
Setting: Leschenault Inlet and Collie River estuary
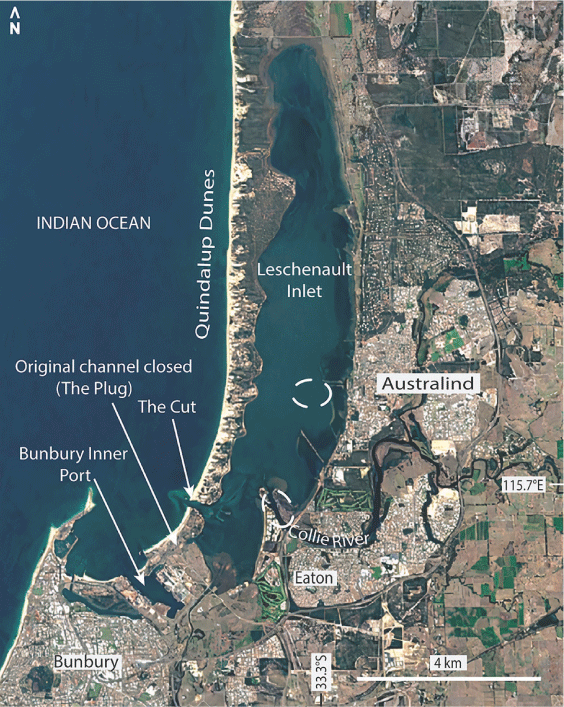
Specimens of the potential invasive foraminiferal species identified here come from Leschenault Inlet and the connected Collie River estuary adjacent to Bunbury Port. Leschenault Inlet (Fig. 1) is a long, narrow, shallow estuarine lagoon with a maximum water depth of 5 m. On the western (seaward) side, it is bordered by a ridge referred to as the Quindalup Dunes (Fig. 2) formed during the Holocene c. 7000 years ago (Brearley 2005). On its eastern side, the Mandurah–Eaton Ridge (Wurm and Semeniuk 2000) borders the estuary and includes the town of Australind. The development of the estuary is the sum of the early Holocene marine transgression that reached as far as the Pleistocene Mandurah–Eaton ridge, and the mid- to late Holocene development of the Quindalup Dunes, composed of wind-blown quartz–carbonate sand, on its seaward side (McArthur and Bettenay 1974; Semeniuk and Meagher 1981; Semeniuk et al. 1989). The Collie River flows into Leschenault Inlet towards its southern end.
Water quality in the estuarine environment varies seasonally, resulting from minor rainfall and river outflow in summer and by much increased river flow following intensive rainfall in winter (Hubertz and Cahoon 1999; Dürr and Semeniuk 2000). Water quality is also affected by peripheral anthropogenic activities (Pen et al. 2000). Prior to 1951, Leschenault Inlet was dominantly brackish (<30 psu) over wintertime. After ‘The Cut’ was first opened (Fig. 1), the salinity concentration has varied between hypersaline in summer to brackish in winter, the latter being due to the heavy inflow of fresh water from rain and Collie River discharge (Dürr and Semeniuk 2000). This results in salinity stratification due to the difference in density between a top freshwater layer and variably saline water below a depth of 2 m. Waves generated by wind, and storms in mid-July to mid-August, are mainly responsible for the mixing of the water column and the disturbance of bottom sediments (Charteris and Deeley 2000; Li et al. 2007). Water temperatures are subject to seasonal and daily fluctuations. In summer, the maximum water temperature can reach 25°C; in winter, it can fall to 14°C (Brearley 2005). A previous study conducted in Leschenault Inlet between October 1986 and March 1987 (austral summer) showed that water salinity varied from 33.5 to 45.0‰ and that oxygen concentrations fluctuated between 5.7 and 10.8 mg L–1, although the water depths at which the measurements were taken were not mentioned (Wurm and Semeniuk 2000, tables 2, 3). The salinity of the Collie River recorded in August 2005 (austral winter) at a sample site with the presently identified T. hadai-like morphotype varied from 0.4‰ at a water depth of 0.3 m to 32.1‰ at a depth of 2 m, showing distinct stratification (Site C3 in Ostrognay and Haig 2012). At this site, in April 2006, the oxygen concentration varied between 90.5% saturation at a water depth of 0.3 m and 100.2% at a water depth of 2.2 m (Ostrognay and Haig 2012). No data are available to demonstrate similar winter stratification in Leschenault Inlet.
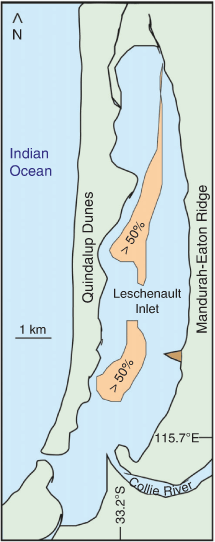
The estuarine sediment is mainly marine mud, calcareous and quartz sand and organic detritus. The distribution of mud was mapped by Wurm and Semeniuk (2000, fig. 4) based on limited sample points. An area of >50% mud occurs along the central axis of the Inlet (Fig. 1, 3) at a depth of ~4–5 m. On the western side, erosion of the Quindalup Dunes provides sand that progrades into the estuary. The quartz–carbonate sand is heterogeneously distributed along the shoreline (Semeniuk and Meagher 1981).
|
Materials and methods
Sample localities
Kitazato and Matsushita (1996) indicated that T. hadai is more prolific between autumn and winter. Therefore, the Leschenault Inlet and Collie River were sampled on three different dates at the beginning of the 2020 austral winter: 22 May 2020 (sunny, clear weather), 4 June 2020 (sunny, clear weather) and 18 June 2021 (high tide, before rainfall). The Leschenault Inlet was sampled using a kayak at 12 localities (see Table S1 of the Supplementary material) along the eastern side of the middle channel, ~700 m from the shoreline (Fig. 1, 2a, c). The samples were collected via a small pipe dredge (Fig. 2b). At each sample location, global positioning system (GPS) coordinates were taken (Table S1). The tethered pipe dredge was thrown from the kayak and retrieved, providing a shallow scoop of the surface mud. The collected mud was transferred into a labelled flat, shallow, plastic container and covered with estuarine water.
Sample processing and analysis
In the laboratory, the samples were left to settle and then examined under a stereomicroscope to observe the living Trochammina. These were picked and mounted on a cardboard slide. To obtain additional specimens, the mud fraction was decanted and the resultant sand residue examined. To illustrate the species, successive images were taken under reflected light using a biological microscope at a magnification of 10×, moving the specimen through the plane of focus. The resulting images were stacked and rendered using Helicon focus software (Helicon Soft). Scanning electron micrographs were taken of the umbilical sides and apertures of selected specimens. The specimens were dried at room temperature, mounted in required orientations using carbon tape on standard metal stubs, and coated with 10 nm of platinum before imaging. Samples and isolated specimens are curated in the Earth Science Museum at the University of Western Australia (Table S1).
DNA extraction, PCR amplification and sequencing
Single-cell DNA extractions were carried out for six specimens of the Trochammina from Leschenault Inlet (Fig. S1 of the Supplementary material). Living specimens mounted on cardboard slides and dried at room temperature were sent by express mail (over 5 days) to the University of Geneva, Switzerland at ambient temperatures and extracted upon arrival using guanidine lysis (buffer following the protocol detailed in Pawlowski 2000). The 3′ fragment of the small subunit ribosomal RNA SSU rDNA gene was amplified by semi-nested polymerase chain reaction (PCR) using primer pairs s14F3 (ACGCAMGTGTGAAACTTG)–s20r (GACGGGCGGTGTGTACAA) for the first amplification and s14F1 (AAGGGCACCACAAGAACGC)–s20r for the second amplification. This fragment represents the standard barcoding fragment in foraminifera (Pawlowski and Holzmann 2014). In total, 35 and 25 cycles were performed for the initial and the semi-nested PCR respectively, with an annealing temperature of 50°C for the first and 52°C for the second. The amplified PCR products were purified using a High Pure PCR Cleanup Micro Kit (Sigma–Aldrich). Sequencing reactions were performed using a BigDye Terminator cycle sequencing kit (ver. 3.1, Applied Biosystems). The newly acquired sequences were deposited in the EMBL/GenBank database (isolate and accession numbers are given in Table S2).
The obtained sequences were added to an existing database using the Muscle automatic alignment option as implemented in Seaview (ver. 4.3.3, see http://doua.prabi.fr/software/seaview; Gouy et al. 2010). The alignment of partial SSU rDNA sequences consists of 51 sequences, of which 6 were obtained for the present study. The alignment contains 1237 sites, with A, C, G and T nucleotide frequencies of 0.25, 0.19, 0.22 and 0.34 respectively.
A phylogenetic tree was constructed using maximum likelihood phylogeny (PhyML, ver. 3.0, see http://www.atgc-montpellier.fr/phyml/) as implemented in ATGC: PhyML (Guindon et al. 2010). An automatic model selection by smart model selection in PhyMl (SMS; Lefort et al. 2017) based on the Akaike information criterion (AIC) was used, resulting in a general time reversible + gamma distributed rate variation among sites + proportion of invariable sites (GTR+G+I) nucleotide substitution model being selected for the analysis. The initial tree is based on improved neighbour-joining (BIONJ; Gascuel 1997). Bootstrap values (BV) are based on 100 replicates.
Results
Morphological comparison to T. hadai
The Western Australian Trochammina specimens are morphologically similar to T. hadai. The holotype of T. hadai came from a water depth of 65 m off the Shinano River, Niigata Prefecture, Japan (Uchio 1962, pp. 387, 388, pl. 18, fig. 9a–c). The specimens in the present study (Fig. 4, 5) have a spiral side that is slightly convex, usually with a flat initial coil. They are strongly inflated on the umbilical side with a depressed umbilicus. Three whorls are present with four to six chambers, usually five, in the last whorl. The maximum test diameter is 0.17–0.55 mm in Leschenault Inlet and 0.37–0.48 mm in Collie River. The holotype of T. hadai has a similar test profile and has three whorls with five chambers in the last whorl; its maximum diameter is 0.52 mm. The wall of the Western Australian specimens is non-calcareous, agglutinated with clear angular quartz between 0.02 and 0.07 mm in size and rare black grains in a matrix of much finer quartz (Fig. 4–7). On the wall surface, quartz grains are positioned with flat surfaces tangential to the wall surface. The large grains do not overlap at the surface and the intervening spaces are filled by a matrix of smaller grains (Fig. 7a). In cross-section, the wall is a layer of closely packed grains that is several grains thick (15–20 µm), and in some sections large flatter quartz grains are positioned at the test surface (Fig. 7b). A similar wall structure is evident in the Japanese specimens of T. hadai (see Matsushita and Kitazato 1990, pl. 2; Nomura and Seto 1992, fig. 14, numbers 3a–c; Tsujimoto et al. 2006, fig. 6, numbers 7a–c). The test does not react with 2% HCl but disaggregates in bleach, indicating an organic cement. The colour of live specimens varies between light reddish-grey and reddish-brown (following the Munsell Soil Color Charts, see https://munsell.com/, accessed 4 November 2021). The holotype of T. hadai was described as reddish-brown to yellowish-brown (Uchio 1962, p. 387) but the mineralogical composition of the test and cement were not described. In the Western Australian assemblages, the aperture is a low arch. Its position varies from umbilical (e.g. Fig. 6b) to umbilical–extraumbilical, but only reaching to between half and three-quarters of the way to the periphery (e.g. Fig. 6h) to extraumbilical with a similar range to the periphery (e.g. Fig. 6d, e, o). In most specimens, a thin apertural lip borders the upper margin of the aperture. It is usually a very narrow ledge (e.g. Fig. 6p). Uchio (1962, p.388, pl.18, fig. a, b) described the aperture of the holotype of T. hadai as ‘an arched slit at the base of the apertural face of the last chamber’ and illustrated a very narrow lip in the hand-drawn figures.

|

|

|

|
In addition to Uchio’s (1962) description of the type assemblage, morphological variation in Japanese T. hadai has been described and illustrated in Lake Hamana, Lake Nakaumi, Osaka Bay, Lake Saroma and Lake Kugushi (Matsushita and Kitazato 1990, p. 714, pl. 1, l a–c; Nomura and Seto 1992, p. 235, fig. 14, 3a, b, ?4a–c; Takata et al. 2006, p. 58, pl. 1, fig. l a–c; Tsujimoto et al. 2006, p 150, fig. 6, 7a–c, not 8a–c; Nomura and Kawano 2011, p. 50, fig. 9, 10). Matsushita and Kitazato (1990) bred T. hadai from Lake Hamana under controlled laboratory conditions. They found four different morphotypes based on the height of coiling and noted that test morphology varied with the seasons. In the Japanese specimens, there are usually four to five chambers in the final whorl (sometimes as many as seven) and an aperture that varies from an umbilical to extraumbilical position.
In a previous study of foraminifers from Leschenault Inlet (Revets 2000), T. hadai-like morphotypes were identified as Paratrochammina challengeri Brönnimann & Whittaker (1988, p. 43, 44, fig. 16 H–K) and Paratrochammina simplissima (Cushman & McCulloch, 1948; a new name for Trochammina pacifica var. simplex Cushman & McCulloch, 1939, p. 104, pl. 11, fig. 4 a–c). However, P. challengeri differs from T. hadai by its very large globigeriniform test (up to 2 mm diameter), and as such does not conform to the Leschenault specimens. P. simplissima also differs from T. hadai by its less inflated periphery and its more rapid increase in chamber size in the last whorl. The specimens recorded by Revets (2000) fall within the range of variability observed here for T. hadai. Ostrognay and Haig (2012, p. 143, fig. 6, 8–10) recorded the species in the Collie River as Paratrochammina sp. The present study found umbilical–extraumbilical variation in the apertural position and some variation in the apertural lip (Fig. 6). Because of this, the species is referred to Trochammina Parker & Jones, 1859 with type species, Trochammina inflata (Montagu, 1808; neotype designated by Brönnimann and Whittaker 1984). There seems to be no substantial difference between apertural shape, position and lip in Western Australian populations of T. hadai and T. inflata that would warrant a different generic assignment, although molecular comparisons outlined in the phylogenetic analysis presented below indicate that Trochammina is polyphyletic.
McGann et al. (2012, fig. 2, 2A–3C) illustrated the species from California and then, in Brazil, Eichler et al. (2018, pl. l A–K) described and illustrated T. hadai. In the North American assemblages, T. hadai has two to three whorls with usually five chambers in the last whorl that increase gradually in size, and an aperture that varies between umbilicate to extraumbilicate. In the Brazilian assemblages from São Paulo, T. hadai is mostly flat on its spiral side and has four strongly inflated chambers in the last whorl that increase proportionally in size as added. A specimen illustrated by Eichler et al. (2018, pl. 1, fig. a–c) has a narrow-arched aperture that is centred in the umbilicus and extends to an extraumbilical position. The aperture is bordered by a narrow lip. The initial whorls of the illustrated specimens in Eichler et al. (2018) have a flat initial coil on the spiral side. In one specimen (Eichler et al. 2018, pl. 1, fig. h), the last whorl is deflected slightly to the umbilical side, giving the appearance of a high trochospiral test. Faria et al. (2021) identified Ammoglobigerina globigeriniformis (Parker & Jones, 1865) as an invasive species in an estuary in southern Brazil south of São Paulo and indicated that it was the same as T. hadai identified previously in Brazil and California (see records discussed above). The specimen illustrated by Faria et al. (2021, fig. 2) seems morphologically closer to the type specimen of T. hadai (see above) than to the original specimens of A. globigeriniformis illustrated by Parker and Jones (1865, pl. 15, fig. 46, 47, pl. 17, fig. 96–98) and to the lectotype of this species illustrated by Loeblich and Tappan (1964, fig. 173, numbers 2a–c). We believe that T. hadai and A. globigeriniformis are separate species based on comparison of the type material.
Phylogenetic analysis
The phylogenetic tree is presented in Fig. 8. It contains 47 sequences of agglutinated foraminifera and is rooted in Reophacidae (R. scorpiurus, R. spiculifer, R. curtus, R. pilulifer-arenulacea). The obtained sequences cluster with T. hadai, supported by a BV of 100%. T. hadai is part of a well-supported clade (BV 86%) that contains Srinivasania sundarbanensis, Eggerelloides scaber and T. pacifica. Three other clades are present in the tree. One consists of Bigenerina sp., Textularia gramen, Siphoniferoides sp. and Textularia agglutinans, strongly supported by a BV of 96%. A second clade contains Arenoparrella mexicana, Entzia macrescens, Haplophragmoides wilberti and Balticammina macrescens, but is not supported by BV. A third clade, also without BV support, contains Ammobaculites sp., Ammotium pseudocassis and Cribrostomoides spp. Several species are branching separately: Cyrea spp., Liebusella goesi, Spiroplectammina sp., Trochammina sp. and T. inflata. Species represented by more than one sequence are all supported by a BV of 100%.

|
Comparison to modern Trochammina known from other Western Australian estuaries
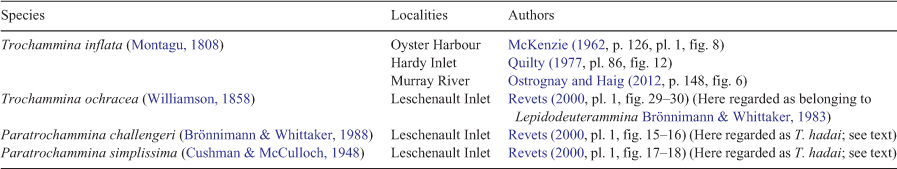
In south-west Australian estuaries and inlets, trochamminid species referred to as T. inflata, Trochammina ochracea, P. challengeri and P. simplissima have been recognised (Table 1). T. inflata is the type species of Trochammina and is distinguished from T. hadai by its smooth, finely agglutinated wall and five to six chambers in the last whorl that are ovoid rather than globular in peripheral view. T. inflata is commonly found in abundance in marshes surrounding estuaries in Western Australia; by contrast, T. hadai seems confined to water deeper than 3 m and lives in estuarine mud. The species referred to by Revets (2000) as T. ochracea is a distinctive species with a compressed trochospire and multiple chambers in the last whorl (10 chambers in his figured specimen) and is better placed in Lepidodeuterammina. The Paratrochammina species identified by Revets (2000) in Leschenault Inlet are discussed above and are here considered to fall within the range of variability of T. hadai.
|
Comparison to modern estuarine Trochammina known from elsewhere in Australia
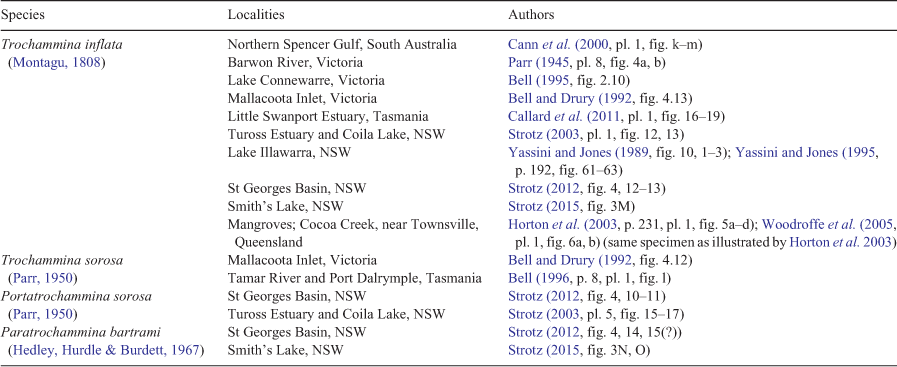
Studies in other parts of Australia have shown that T. inflata is widespread in Australian estuaries (Table 2). Other trochamminids illustrated include morphotypes attributed to Trochammina sorosa Parr (also referred to as Portatrochammina), Paratrochammina stonei and Paratrochammina bartrami Hedley, Hurdle & Burdett, 1967 (Table 2). T. hadai has not been recorded. T. sorosa was originally described from Maria Island, Tasmania, in 122–155 m deep water. Unlike T. hadai, it has a distinctly elevated spiral side, including the first whorl (Parr 1950, pl. 5, fig. 15–17). The inflated globular chambers resemble those of T. hadai. The aperture was not illustrated, but was described as a ‘narrow opening at the base of the last chamber’ (Parr 1950). The specimens illustrated by Bell and Drury (1992) and Bell (1996) also show a conical spiral side with inflated chambers, but the aperture was not illustrated. The New South Wales species referred to as Portatrochammina sorosa lacks the inflated chambers of Parr’s (1950) species and does not belong within Trochammina. Specimens referred to P. stonei and P. bartrami have compressed chambers, in contrast with the inflated chambers of T. hadai.
|
Although not illustrated, the record of ‘Trochammina globigeriformis’ by Narayan and Pandolfi (2010, table 1) from Moreton Bay in the Brisbane region of Queensland is intriguing. T. globigeriniformis (= type species of Ammoglobigerina; see Loeblich and Tappan 1987, p. 120) usually has just over three chambers in the last whorl and is mainly found in deep water. Its record in shallow Moreton Bay needs re-evaluation. The species recorded by Narayan and Pandolfi (2010) in the vicinity of the Port of Brisbane may represent T. sorosa or T. hadai.
Habitat in Leschenault Inlet and Collie River
T. hadai and Ammonia haigi Hayward & Holzmann are the dominant species in the Leschenault Inlet samples. Also present are Ammobaculites sp., Ammonia cf. ariakensis Akimoto, Ammonia cf. quiltyi Hayward & Holzmann, Cornuspira planorbis Schultze, Elphidium cf. advenum (Cushman), Elphidium cf. excavatum (Terquem), Elphidium gunteri Cole, Nonion subturgidum (Cushman), Quinqueloculina cf. littoralis (Collins) and Spiroloculina sp. In the Collie River sample with rare T. hadai, A. haigi is dominant and is accompanied by Elphidium cf. crispum (Linnaeus), E. cf. excavatum, E. gunteri, Elphidium cf. simplex Cushman and Quinqueloculina spp., including Q. cf. littoralis, Q. milletti (Wiesner) and Q. cf. seminula (Linnaeus).
At the sampled localities in Leschenault Inlet and Collie River, marine mud forms the substrate of the middle boat channel at a water depth of between 3 and 5 m. It was in this mud that live T. hadai were found. Observations under the microscope suggest that the sediment surface layer consists of clotted mud that is flocculent (Fig. 9). As Manning et al. (2011) noted, flocculation plays a major role in mud sedimentation processes within estuaries. Below the flocculent layer is a denser, muddy sand with abundant ovoid faecal pellets with a mean length of 400 μm and, in places, fine to medium quartz.
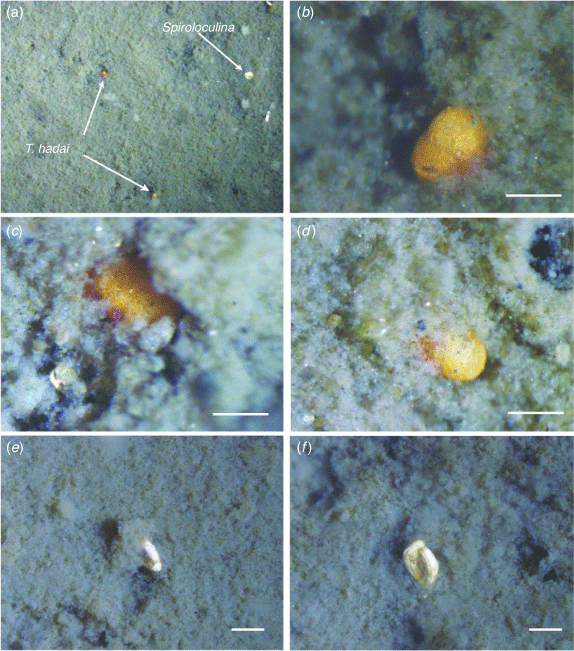
|
Live foraminifers at the top of the flocculent layer include scattered T. hadai (Fig. 9a, d) and Spiroloculina sp. (Fig. 9a, e, f). T. hadai are easily recognisable by their bright pinkish-red to reddish-brown colour. The specimens were found living with the periphery of the test vertical and the aperture downward in the mud. Only one or two chambers of the last whorl were visible at the surface of the flocculent layer (Fig. 9b–d). Furthermore, live Spiroloculina sp. were also vertically suspended at the top of the flocculent layer with the aperture facing down (Fig. 9a, e, f). Trochammina and Spiroloculina were the only live foraminifers observed at the surface of the mud. The mode of life of these imperforate species, with the aperture facing downwards in the flocculent mud layer, differs from that suggested by Lipps (1983) for many foraminifers, which apparently keep their apertures above the sediment–water interface and protrude their pseudopodia into the free water column for suspension feeding.
The faecal pellets may belong to Palaemonetes australis Dakin, 1915, a shrimp first described in Western Australian estuaries, in the lower part of the Swan River. Its occurrence has been widely recorded in estuaries all along the south-west of Western Australian (Boulton and Knott 1984). P. australis can cope with radical salinity changes, varying from acid fresh water to full salt water, as part of seasonal variation (Bray 1976). In the flocculent layer of the mud, small shrimps were observed to reconstruct their burrows shortly after the mud was disturbed.
Discussion
Species identification and distribution in Australia
As demonstrated above, the Japanese T. hadai shares close morphological features with the species recorded here from Leschenault Inlet and the mouth of the Collie River. In recent years, molecular analysis has increasingly been used to confirm the identification of species from distant areas. The molecular signature of the Western Australian specimens corresponds to that of T. hadai from Japan and San Francisco (Fig. 8). The life mode of T. hadai described by Matsushita and Kitazato (1990) from specimens in Lake Hamana in Japan seems similar to that of Western Australian morphotypes. In Japan, T. hadai was shown to live mostly in the top centimetre of sediment on the floor of Lake Hamana in water depths <10 m. In Leschenault Inlet, T. hadai live partly submerged in sediment on the top of the flocculent marine mud in water that is ~5 m deep. In Shinano Bay, Japan, Uchio (1962) found the holotype of the species at a greater water depth (65 m), but the specimen may have been transported downslope out of the river during spring and summer freshwater outflow. High maritime activity by vessels navigating in and out of the river may also have contributed to its wide distribution into deeper waters adjacent to the river mouth. Because of close morphological similarity, a consistent molecular signature and ecological equivalence, the Western Australian morphotypes are identified as T. hadai.
T. hadai from Leschenault Inlet and the mouth of the Collie River has not been recorded elsewhere in south-west Australia, including Oyster Harbour and Kalgan River (McKenzie 1962), Hardy Inlet (Quilty 1977), Swan Estuary (Quilty and Hosie 2006) and the Murray and Serpentine rivers (Ostrognay and Haig 2012). It is absent from the Young River and Stokes Inlet, Jerdancuttup River, Phillips River, Wellstead Estuary, Hay and Denmark rivers, Franklin River, Walpole Inlet, Vasse River, Harvey and Peel inlets and the Canning, Moore, Irwin, Greenough, Chapman, Murchison and Gascoyne Rivers (D. W. Haig, J. H. Parker and C. M. Tremblin, pers. obs.; for locations, see Brearley 2005). Furthermore, the species has not been recorded in eastern Australian estuaries, although the record of a possibly similar species from Moreton Bay near Brisbane Port (Narayan and Pandolfi 2010) requires further investigation. Because it has not been recorded elsewhere in the estuaries and inlets around Australia, T. hadai is considered to be invasive in the Leschenault Inlet and Collie River.
Possible means of introduction
The continuous passage of marine vessels coming to Australia for commercial purposes may be a vector for the introduction of exotic species. Anthropogenic introductions are primarily due to cargo de-ballasting at the port of arrival (Firestone and Corbett 2005; Bailey 2015). Ballast water ensures the stability of the ship in transit. The vessel takes in ballast water as it unloads its cargo and discharges ballast water as it loads cargo. In Australia, 85% of ballast water discharged comes from the Asian region, of which more than 50% originates from Japanese ports (Kerr 1994). Japanese companies are the major exporters of woodchip from Australia. They use small vessels with shallow draughts for easy mobility and navigation in shallow water up to a depth of ~20 m (Hutchings 1992). Because of this, they are more likely to disturb and take in bottom sediment when loading ballast water.
T. hadai had been introduced into Leschenault Inlet and Collie River by at least May 1999 when samples were collected for the study of Revets (2000), in which he identified morphotypes that the present study considers to be T. hadai. No previous sampling for foraminifers in this estuarine system has been reported. The original estuarine mouth has been altered by the building of Bunbury Port. In 1837, Bunbury Harbour was one of the major stations, after Fremantle and Albany, involved in pelagic or shore-based whaling activities in south-west Australia (Gibbs 1995, 2000). The whale quest became financially lucrative when the local fisheries were supported by American and French whale ships in the late 1850s. A period of large exportation to the US of the prolific catch was reported between 1867 and 1869. However, growing conflicts between the Western Australian settlers and American whalers in Bunbury in the 1870s caused the foreign vessels to cease activity in Bunbury and to pursue whaling in King George Sound in Albany until 1888 (Gibbs 2000). Between 1945 and 1975, considerable changes were made to the Bunbury coastline for the new port. The original open mouth of the estuary was closed (‘The Plug’) in 1951 when the new entrance to Leschenault Inlet (‘The Cut’) was opened (Fig. 1; Brearley 2005, fig. 4-4). From the 1970s to the present day, Bunbury Port has become a worldwide maritime hub exporting coal, woodchip, plantation timber and agricultural engines (Agriculture Fisheries and Forestry Australia and Invest Australia 2003; Australian Bureau of Agricultural and Resource Economics and Sciences 2016). Japan is the dominant importer of woodchip from Bunbury (Agriculture Fisheries and Forestry Australia and Invest Australia 2003). The Hoyo Maru in 1982 was the first Japanese vessel recorded in Bunbury Harbour to transport woodchip back to Japan.
To confirm the mode of introduction of T. hadai into the estuarine system adjacent to Bunbury port, two aspects for further study should be pursued: (1) coring of the bottom sediment in the estuary at sites where T. hadai is now abundant in order to establish the time of introduction of the species; and (2) sampling the ballast water and associated sediment from incoming ships in Bunbury Harbour to detect live foraminifers. Representatives of T. hadai will be readily identifiable in the sediment cores under microscopic examination and it should be possible to determine the date of introduction of the species using 204Pb analysis of unaltered carbonate shells or organic plant material. Because T. hadai and other foraminifers are of sand size, they will be easily identified in ballast sediment.
This study demonstrates that foraminiferal species can be included among the invasive marine taxa in Australian estuaries. The presence of T. hadai may indicate a Japanese origin, presumably transported in ballast water sediment. T. hadai may also provide an indicator, identified by simple microscopic examination, for the detection of other more elusive exotic species derived from Japanese estuaries.
Data availability
Samples and isolated foraminiferal specimens are curated in the Earth Science Museum at the University of Western Australia (Table S1). The newly acquired molecular sequences were deposited in the EMBL/GenBank database (isolate and accession numbers are given in Table S2; photographs of specimens sequenced are shown in Fig. S1).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The authors thank Jenny Bevan and Eckart Håkansson for assistance in the field and for discussions on the localities; Mary McGann and Patricia Eichler for very useful comments on our assemblages and on the American occurrences; and Kailah Thorne for curatorial assistance. Jenny Bevan and Eckart Håkansson kindly reviewed an earlier draft of the manuscript. The Oceans Graduate School at the University of Western Australia is thanked for facilitating the voluntary research internship of Clément Tremblin and the honorary research position of David Haig in his retirement. The authors acknowledge the facilities, and the scientific and technical assistance of Microscopy Australia at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by the University and state and commonwealth governments. The authors are grateful to Associate Editor Daniel Roelke and the two anonymous reviewers for their useful comments and suggestions that have helped improve the manuscript.
References
Agriculture Fisheries and Forestry Australia and Invest Australia (2003). Report of Australian Forest Products Industry. Australian Government, Canberra, ACT, Australia.Australian Bureau of Agricultural and Resource Economics and Sciences (2016). Report to Forest and Wood Products Australia. Australian Government, Canberra, ACT, Australia.
Bailey, S. A. (2015). An overview of thirty years of research on ballast water as a vector for aquatic invasive species to freshwater and marine environments. Aquatic Ecosystem Health & Management 18, 261–268.
| An overview of thirty years of research on ballast water as a vector for aquatic invasive species to freshwater and marine environments.Crossref | GoogleScholarGoogle Scholar |
Bax, N., Williamson, A., Aguero, M., Gonzalez, E., and Geeves, W. (2003). Marine invasive alien species: a threat to global biodiversity. Marine Policy 27, 313–323.
| Marine invasive alien species: a threat to global biodiversity.Crossref | GoogleScholarGoogle Scholar |
Bell, K. N. (1995). Foraminiferans from Lake Connewarre, Victoria. Victorian Naturalist 112, 228–234.
Bell, K. N. (1996). Foraminifera faunas of the River Tamar and Port Dalrymple, Tasmania: a preliminary survey. Records of the Queen Victoria Museum Launceston 102, 1–25.
Bell, K. N., and Drury, S. R. (1992). Foraminiferal fauna of Mallacoota Inlet, East Gippsland, Victoria. Victorian Naturalist 109, 7–16.
Bolton, T. F., and Graham, W. M. (2004). Morphological variation among populations of an invasive jellyfish. Marine Ecology Progress Series 278, 125–139.
| Morphological variation among populations of an invasive jellyfish.Crossref | GoogleScholarGoogle Scholar |
Boulton, A. J., and Knott, B. (1984). Morphological and electrophoretic studies of the Palaemonidae (Crustacea) of the Perth Region, Western Australia. Australian Journal of Marine and Freshwater Research 35, 769–783.
| Morphological and electrophoretic studies of the Palaemonidae (Crustacea) of the Perth Region, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bray, D. M. (1976). A review of two Western Australian shrimps of the genus Palaemonetes, P. australis Dakin 1915 and P. atrinubes sp. nov. (Decapoda, Palaemonidae). Records of the Western Australian Museum 4, 65–84.
Brearley, A. (2005). ‘Ernest Hodgkin’s Swanland: Estuaries and Coastal Lagoons of Southwestern Australia.’ (University of Western Australia Press: Perth, WA, Australia.)
Brönnimann, P., and Whittaker, J. E. (1983). Deuterammina (Lepidodeuterammina) subgen. nov., and a redescription of Rotalina ochracea Williamson (Protozoa: Foraminiferida). Bulletin of the British Museum, Natural History (Zoology) 45, 233–238.
Brönnimann, P., and Whittaker, J. E. (1984). A neotype for Trochammina inflata (Montagu) (Protozoa: Foraminiferida) with notes on the wall structure. Bulletin of the British Museum (Natural History) Zoology 46, 311–315.
Brönnimann, P., and Whittaker, J. E. (1988). ‘The Trochamminacea of the “Discovery” Reports.’ (British Museum (Natural History): London, UK.)
Callard, S. L., Gehrels, W. R., Morrison, B. V., and Grenfell, H. R. (2011). Suitability of saltmarsh foraminifera as proxy indicators of sea-level in Tasmania. Marine Micropaleontology 79, 121–131.
| Suitability of saltmarsh foraminifera as proxy indicators of sea-level in Tasmania.Crossref | GoogleScholarGoogle Scholar |
Cann, J. H., Belperio, A. P., and Murray-Wallace, C. V. (2000). Late Quaternary paleosealevels and paleoenvironments inferred from foraminifera, Northern Spencer Gulf, South Australia. Journal of Foraminiferal Research 30, 29–53.
| Late Quaternary paleosealevels and paleoenvironments inferred from foraminifera, Northern Spencer Gulf, South Australia.Crossref | GoogleScholarGoogle Scholar |
Ceccherelli, G., Piazzi, L., and Cinelli, F. (2000). Response of the non-indigenous Caulerpa racemosa (Forsskål) J. Agardh to the native seagrass Posidonia oceanica (L.) Delile: effect of density of shoots and orientation of edges of meadows. Journal of Experimental Marine Biology and Ecology 243, 227–240.
| Response of the non-indigenous Caulerpa racemosa (Forsskål) J. Agardh to the native seagrass Posidonia oceanica (L.) Delile: effect of density of shoots and orientation of edges of meadows.Crossref | GoogleScholarGoogle Scholar |
Charteris, A., and Deeley, D. (2000). Hydrodynamics of Leschenault Inlet, Western Australia. Journal of the Royal Society of Western Australia 83, 251–254.
Cushman, J. A., and McCulloch, I. (1939). A report on some arenaceous Foraminifera. Reports of the Allan Hancock Pacific Expeditions 6, 1–113.
Cushman, J. A., and McCulloch, I. (1948). Three new names for recent Pacific Foraminifera. Contributions from the Cushman Laboratory for Foraminiferal Research 24, 76.
Dakin, W. (1915). Fauna of West Australia. IV Palaemonetes australis sp. n., being the first record of the genus in Australia. Proceedings of the Zoological Society of London 1915, 571–574.
Dias, P. J., Muñoz, J., Huisman, J. M., and McDonald, J. I. (2015). Biosecurity monitoring of Harmful Algal Bloom (HAB) species in Western Australian waters: first confirmed record of Alexandrium catenella (Dinophyceae). BioInvasions Records 4, 233–241.
| Biosecurity monitoring of Harmful Algal Bloom (HAB) species in Western Australian waters: first confirmed record of Alexandrium catenella (Dinophyceae).Crossref | GoogleScholarGoogle Scholar |
Dürr, V., and Semeniuk, T. A. (2000). Long term spatial dynamics of polycheates in Leschenault Inlet estuary. Journal of the Royal Society of Western Australia 83, 463–474.
Edelist, D., Rilov, G., Golani, D., Carlton, J. T., and Spanier, E. (2013). Restructuring the sea: profound shifts in the world’s most invaded marine ecosystem. Diversity & Distributions 19, 69–77.
| Restructuring the sea: profound shifts in the world’s most invaded marine ecosystem.Crossref | GoogleScholarGoogle Scholar |
Eichler, P., McGann, M., Rodrigues, A. R., Mendonça, A., Amorim, A., Bonetti, C., Cordeiro de Farias, C., Mello e Sous, S. H., Vital, H., and Praxedes Gome, M. (2018). The occurrence of the invasive foraminifera Trochammina hadai Uchio in Flamengo Inlet, Ubatuba, São Paulo State, Brazil. Micropaleontology 64, 391–402.
Faria, L., De Andrade Freshse, F., Vinicius Trento Occhi, T., Maichak de Carvalho, B., Vincente Pupo, D., Trevisan Disaro, S., and Ricardo Simoes Vitule, J. (2021). Occurrence of non-native species in a subtropical coastal River, in Southern Brazil. Acta Limnologica Brasiliensia 33, e101.
| Occurrence of non-native species in a subtropical coastal River, in Southern Brazil.Crossref | GoogleScholarGoogle Scholar |
Firestone, J., and Corbett, J. J. (2005). Coastal and port environments: international legal and policy responses to reduce ballast water introductions of potentially invasive species. Ocean Development and International Law 36, 291–316.
| Coastal and port environments: international legal and policy responses to reduce ballast water introductions of potentially invasive species.Crossref | GoogleScholarGoogle Scholar |
Gascuel, O. (1997). BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Molecular Biology and Evolution 14, 685–695.
| BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data.Crossref | GoogleScholarGoogle Scholar | 9254330PubMed |
Gibbs, M. (1995). ‘The Historical Archaeology of Shore-based Whaling in Western Australia 1836–1879.’ (Centre for Archaeology, Department of Anthropology University of Western Australia, Perth, WA, Australia.)
Gibbs, M. (2000). Conflicts and commerce: American whalers and the Western Australian colonies 1826–1888. The Great Circle 22, 3–23.
Gouy, M., Guindon, S., and Gascuel, O. (2010). SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27, 221–224.
| SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building.Crossref | GoogleScholarGoogle Scholar | 19854763PubMed |
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307–321.
| New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0.Crossref | GoogleScholarGoogle Scholar | 20525638PubMed |
Haig, D. W. (2020). Ammobaculites (Foraminifera): living fossils in southern Western Australian estuaries. Journal of the Royal Society of Western Australia 103, 57–77.
Hedley, R. H., Hurdle, C. M., and Burdett, I. D. J. (1967). The marine fauna of New Zealand: intertidal foraminifera of the Corallina officinalis zone. New Zealand Oceanographic Institute, Wellington, Memoir 38, 1–86.
Horton, B. P., Larcombe, P., Woodroffe, S. A., Whittaker, J. E., Wright, M. R., and Wynn, C. (2003). Contemporary foraminiferal distributions of a mangrove environment, Great Barrier Reef coastline, Australia: implications for sea-level reconstructions. Marine Geology 198, 225–243.
| Contemporary foraminiferal distributions of a mangrove environment, Great Barrier Reef coastline, Australia: implications for sea-level reconstructions.Crossref | GoogleScholarGoogle Scholar |
Hubertz, E. D., and Cahoon, L. B. (1999). Short-term variability of water quality parameters in two shallow estuaries of North Carolina. Estuaries 22, 814–823.
| Short-term variability of water quality parameters in two shallow estuaries of North Carolina.Crossref | GoogleScholarGoogle Scholar |
Hunt, C. E., and Behrens Yamada, S. (2003). Biotic resistance experienced by an invasive crustacean in a temperate estuary. Biological Invasions 5, 33–43.
| Biotic resistance experienced by an invasive crustacean in a temperate estuary.Crossref | GoogleScholarGoogle Scholar |
Hutchings, P. (1992). Ballast water introductions of exotic marine organisms into Australia. Marine Pollution Bulletin 25, 196–199.
| Ballast water introductions of exotic marine organisms into Australia.Crossref | GoogleScholarGoogle Scholar |
Kerr, S. B. (1994). Ballast water – ports and shipping study. Australian Quarantine and Inspection Services, Ballast water Research series, report number 5, Australian Government Publishing, Canberra, ACT, Australia.
Kitazato, H., and Matsushita, S. (1996). Laboratory observations of sexual and asexual reproduction of Trochammina hadai Uchio. Transactions and Proceedings of the Paleontological Society of Japan, N.S. 182, 454–466.
Lanfranconi, A., Hutton, M., Brugnoli, E., and Muniz, P. (2009). New record of the alien mollusc Rapana venosa (Valenciennes 1846) in the Uruguayan coastal zone of Rio de la Plata. Pan-American Journal of Aquatic Sciences 4, 216–221.
Lefort, V., Longueville, J. E., and Gascuel, O. (2017). SMS: smart model selection in PhyML. Molecular Biology and Evolution 34, 2422–2424.
| SMS: smart model selection in PhyML.Crossref | GoogleScholarGoogle Scholar | 28472384PubMed |
Li, M., Zhong, L., Boicourt, W. C., Zhang, S., and Zhang, D. L. (2007). Hurricane induced destratification and restratification in a partially mixed estuary. Journal of Marine Research 65, 169–192.
| Hurricane induced destratification and restratification in a partially mixed estuary.Crossref | GoogleScholarGoogle Scholar |
Lipps, J. H. (1983). Biotic interactions in benthic Foraminifera. In ‘Biotic Interactions in Recent and Fossil Benthic Communities’. pp. 331–376. (Plenum Publishing Corporation, New York, NY, USA.)
Loeblich, A. R., and Tappan, H. (1964). Sarcodina chiefly ‘Thecamoebians’ and Foraminiferida. In ‘Treatise on Invertebrate Paleontology, Part C, Protista 2’. (Ed. R.C. Moore.) pp. 1–900. (Geological Society of America and University of Kansas Press.)
Loeblich, A. R., and Tappan, H. (1987). Foraminiferal Genera and their Classification. (Van Nostrand Reinhold Company, New York, NY, USA.)
Manning, A. J., Baugh, J. V., Soulsby, R. L., Spearman, J. R., and Whitehouse, J. S. (2011). Cohesive sediment flocculation and the application to settling flux modelling. In ‘Sediment Transport’. pp. 91–116. (IntechOpen.)
| Crossref |
Matsushita, S., and Kitazato, H. (1990). Seasonality in the Benthic Foraminiferal Community and the Life History of Trochammina hadai Uchio in Hamana Lake, Japan. In ‘Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera’. (Eds C. Hemleben, M. A. Kaminski, W. Kuhnt, and D. B. Scott.) NATO ASI Series C: Mathematical and Physical Sciences, vol. 327, pp. 695–715 (Springer: Dordrecht, Netherlands.)
| Crossref |
McArthur, W. M., and Bettenay, E. (1974). Development and Distribution of soils of the Swan Coastal Plain, W.A. Soil Publication 16. Commonwealth Scientific and Industrial Research Organization, Melbourne, Vic., Australia.
McGann, M., Sloan, D., and Cohen, A. N. (2000). Invasion by a Japanese marine microorganism in western North America. Hydrobiologia 421, 25–30.
| Invasion by a Japanese marine microorganism in western North America.Crossref | GoogleScholarGoogle Scholar |
McGann, M., Grossman, E. E., Takesue, R. K., Penttila, D., Walsh, J. P., and Corbett, R. (2012). Arrival and expansion of the invasive Foraminifera Trochammina hadai Uchio in Padilla Bay, Washington. Northwest Science 86, 9–26.
| Arrival and expansion of the invasive Foraminifera Trochammina hadai Uchio in Padilla Bay, Washington.Crossref | GoogleScholarGoogle Scholar |
McKenzie, K. G. (1962). A record of Foraminifera from Oyster Harbour, near Albany, Western Australia. Journal of the Royal Society of Western Australia 45, 117–133.
Montagu, G. (1808). ‘Supplement to Testacea Britannica with Additional Plates.’ (Woolmer: Exeter, UK.)
Murray, J. W. (1991). ‘Ecology and Palaeoecology of Benthic Foraminifera.’ (Longman Scientific and Technical: Harlow, UK.)
Narayan, Y. R., and Pandolfi, J. M. (2010). Benthic foraminiferal assemblages from Moreton Bay, South-East Queensland, Australia: applications in monitoring water and substrate quality in subtropical estuarine environments. Marine Pollution Bulletin 60, 2062–2078.
| Benthic foraminiferal assemblages from Moreton Bay, South-East Queensland, Australia: applications in monitoring water and substrate quality in subtropical estuarine environments.Crossref | GoogleScholarGoogle Scholar | 20696442PubMed |
Nomura, R., and Kawano, S. (2011). Foraminiferal assemblages response to anthropogenic influence and parallel to decadal sea-level changes over the last 70 years in Lake Kugushi, Fukui Prefecture, southwest Japan. Quaternary International 230, 44–56.
| Foraminiferal assemblages response to anthropogenic influence and parallel to decadal sea-level changes over the last 70 years in Lake Kugushi, Fukui Prefecture, southwest Japan.Crossref | GoogleScholarGoogle Scholar |
Nomura, R., and Seto, K. (1992). Benthic Foraminifera from brackish Lake Nakanoumi, San-In District, Southwestern Honshu, Japan. In ‘Centenary of Japanese Micropaleontology’. (Eds K. Ishizaki and T. Saito.) pp. 227–240. (Terra Scientific Publishing Company: Tokyo, Japan.)
Ostrognay, D. B., and Haig, D. W. (2012). Foraminifera from microtidal rivers with large seasonal salinity variation, southwest Western Australia. Journal of the Royal Society of Western Australia 95, 137–153.
Parker, J. (2009). Taxonomy of Foraminifera from Ningaloo Reef, Western Australia. Memoirs of the Association of Australasian Palaeontologists 36, 1–810.
Parker, W. K., and Jones, T. R. (1859). On the nomenclature of the Foraminifera. II. On the species enumerated by Walker and Montagu. Annals & Magazine of Natural History 4, 333–351.
| On the nomenclature of the Foraminifera. II. On the species enumerated by Walker and Montagu.Crossref | GoogleScholarGoogle Scholar |
Parker, W. K., and Jones, T. R. (1865). On some Foraminifera from the North Atlantic and Arctic Oceans, including David Straits and Baffin’s Bay. Philosophical Transactions of the Royal Society 155, 325–441.
| On some Foraminifera from the North Atlantic and Arctic Oceans, including David Straits and Baffin’s Bay.Crossref | GoogleScholarGoogle Scholar |
Parr, W. J. (1945). Recent Foraminifera from Barwon Heads, Victoria. Proceedings of the Royal Society of Victoria 56, 189–218.
Parr, W. J. (1950). Foraminifera. B.A.N.Z. Antarctic Research Expedition 1929–1931, Ser. B (Zoology, Botany) Reports 5, 232–392.
Patris, S., Martin, L. E., Bell, L. J., and Dawson, M. N. (2019). Expansion of an introduced sea anemone population, and its associations with native species in a tropical marine lake (Jellyfish Lake, Palau). Frontiers of Biogeography 11, e41048.
| Expansion of an introduced sea anemone population, and its associations with native species in a tropical marine lake (Jellyfish Lake, Palau).Crossref | GoogleScholarGoogle Scholar |
Pawlowski, J. (2000). Introduction to the molecular systematics of Foraminifera. Micropaleontology 46, 1–12.
Pawlowski, J., and Holzmann, M. (2014). A plea for DNA barcoding of Foraminifera. Journal of Foraminiferal Research 44, 62–67.
| A plea for DNA barcoding of Foraminifera.Crossref | GoogleScholarGoogle Scholar |
Pawlowski, J., Bolivar, I., Fahrni, J. F., De Vergas, C., Gouy, M., and Zaninetti, L. (1997). Extreme differences in rates of molecular evolution of Foraminifera revealed by comparison of ribosomal DNA sequences and the fossil record. Molecular Biology and Evolution 14, 498–505.
| Extreme differences in rates of molecular evolution of Foraminifera revealed by comparison of ribosomal DNA sequences and the fossil record.Crossref | GoogleScholarGoogle Scholar | 9159927PubMed |
Pen, L., Semeniuk, V., and Semeniuk, C. A. (2000). Peripheral wetland habitats and vegetation of Leschanult Inlet estuary. Journal of the Royal Society of Western Australia 83, 293–316.
Quilty, P. G. (1977). Foraminifera of Hardy Inlet, southwestern Australia. Journal of the Royal Society of Western Australia 59, 79–90.
Quilty, P. G., and Hosie, G. (2006). Modern Foraminifera, Swan River estuary, Western Australia: Distribution and controlling factors. Journal of Foraminiferal Research 36, 291–314.
| Modern Foraminifera, Swan River estuary, Western Australia: Distribution and controlling factors.Crossref | GoogleScholarGoogle Scholar |
Revets, S. A. (2000). Foraminifera of Leschenault Inlet. Journal of the Royal Society of Western Australia 83, 365–375.
Russell, D. J., Thuesen, P. A., and Thomson, F. E. (2012). A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations. Fish Biology and Fisheries 22, 533–554.
| A review of the biology, ecology, distribution and control of Mozambique tilapia, Oreochromis mossambicus (Peters 1852) (Pisces: Cichlidae) with particular emphasis on invasive Australian populations.Crossref | GoogleScholarGoogle Scholar |
Scott, D. B., Tobin, R., Schafer, C. T., and Medioli, F. S. (2001). ‘Monitoring in Coastal Environments using Foraminifera and Thecamoebian Indicators.’ (Cambridge University Press.)
Semeniuk, V., and Meagher, T. D. (1981). The geomorphology and surface processes of the Australind–Leschenault Inlet coastal area. Journal of the Royal Society of Western Australia 64, 33–51.
Semeniuk, V., Cresswell, D. I., and Wurm, P. A. S. (1989). The Quindalup Dunes: the regional system, physical framework and vegetation habitats. Journal of the Royal Society of Western Australia 71, 2–47.
Simpson, T. S., Wernberg, T., and McDonald, J. I. (2016). Distribution and localised effects of the invasive Didemnum perlicidum (Monniot 1983) in an urban estuary. PLoS ONE 11, e0154201.
| Distribution and localised effects of the invasive Didemnum perlicidum (Monniot 1983) in an urban estuary.Crossref | GoogleScholarGoogle Scholar | 27144600PubMed |
Smale, D. A., and Childs, S. (2012). The occurrence of a widespread marine invader Didemnum perlucidum (Tunicata, Ascidiacea) in Western Australia. Biological Invasions 14, 1325–1330.
| The occurrence of a widespread marine invader Didemnum perlucidum (Tunicata, Ascidiacea) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Smith, J. E., Hunter, C. L., and Smith, C. M. (2002). Distribution and reproduction characteristics of non-indigenous and invasive marine algae in the Hawaiian Islands. Pacific Science 56, 299–315.
| Distribution and reproduction characteristics of non-indigenous and invasive marine algae in the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Strotz, L. C. (2003). Holocene Foraminifera from Tuross Estuary and Coila Lake, South Coast, New South Wales: A preliminary study. Proceedings of the Linnean Society of New South Wales 124, 163–182.
Strotz, L. C. (2012). Foraminiferal fauna and biotopes of a barrier estuary system, St Georges Basin, New South Wales, Australia. Journal of Foraminiferal Research 42, 369–382.
| Foraminiferal fauna and biotopes of a barrier estuary system, St Georges Basin, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Strotz, L. C. (2015). Spatial patterns and diversity of foraminifera from an intermittently closed and open lagoon, Smith Lake, Australia. Estuarine, Coastal and Shelf Science 164, 340–352.
| Spatial patterns and diversity of foraminifera from an intermittently closed and open lagoon, Smith Lake, Australia.Crossref | GoogleScholarGoogle Scholar |
Takata, H., Takayasu, K., and Hasegawa, S. (2006). Foraminifera in an organic-rich, brackish-water lagoon, Lake Saroma, Hokkaido, Japan. Journal of Foraminiferal Research 36, 44–60.
| Foraminifera in an organic-rich, brackish-water lagoon, Lake Saroma, Hokkaido, Japan.Crossref | GoogleScholarGoogle Scholar |
Tsujimoto, A., Nomura, R., Yasushi, M., and Yoshikawa, S. (2006). Benthic foraminiferal assemblages in Osaka Bay, southwestern Japan: faunal changes over the last 50 years. Paleontological Research 10, 141–161.
| Benthic foraminiferal assemblages in Osaka Bay, southwestern Japan: faunal changes over the last 50 years.Crossref | GoogleScholarGoogle Scholar |
Uchio, T. (1962). Influence of the River Shinano on Foraminifera and sediment grain size distributions. Publications of the Seto Marine Biological Laboratory 10, 363–392.
| Influence of the River Shinano on Foraminifera and sediment grain size distributions.Crossref | GoogleScholarGoogle Scholar |
Wells, F. E., McDonald, J. I., and Huisman, J. M. (2009). Introduced marine species in Western Australia. (Department of Fisheries, Perth, WA, Australia.) Available at http://www.fish.wa.gov.au/Documents/occasional_publications/fop057.pdf
Williamson, W. C. (1858). ‘On the Recent Foraminifera of Great Britain.’ (The Ray Society: London, UK.)
Woodroffe, S. H., Horton, B. P., Larcombe, P., and Whittaker, J. E. (2005). Intertidal mangrove foraminifera from the central Great Barrier Reef shelf, Australia: Implications for sea-level reconstruction. Journal of Foraminiferal Research 35, 259–270.
| Intertidal mangrove foraminifera from the central Great Barrier Reef shelf, Australia: Implications for sea-level reconstruction.Crossref | GoogleScholarGoogle Scholar |
Wurm, P. A. S., and Semeniuk, V. (2000). The Leschenault Inlet Estuary: physical features and habitats for benthic fauna. Journal of the Royal Society of Western Australia 83, 229–250.
Yassini, I., and Jones, B. G. (1989). Estuarine foraminiferal communities in Lake Illawarra. Proceedings of the Linnean Society of New South Wales 110, 229–266.
Yassini, I., and Jones, B. G. (1995). ‘Recent Foraminifera and Ostracoda from Estuarine and Shelf Environments on the Southern Coast of Australia.’ (The University of Wollongong Press: Wollongong, NSW, Australia.)




