Biology and conservation of the unique and diverse halophilic macroinvertebrates of Australian salt lakes
Angus D’Arcy Lawrie A C , Jennifer Chaplin A and Adrian Pinder B
A C , Jennifer Chaplin A and Adrian Pinder B
A Murdoch University, Centre for Sustainable Aquatic Ecosystems, Environmental and Conservation Sciences, 90 South Street, Murdoch, WA 6150, Australia. Email: j.chaplin@murdoch.edu.au
B Western Australia Department of Biodiversity Conservation and Attractions, Kensington, WA 6151, Australia. Email: adrian.pinder@dbca.wa.gov.au
C Corresponding author. Email: anguslawrie@live.com
Marine and Freshwater Research 72(11) 1553-1576 https://doi.org/10.1071/MF21088
Submitted: 22 March 2021 Accepted: 4 June 2021 Published: 2 July 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
This study synthesises information on the biology of the unique and diverse halophilic macroinvertebrates of Australian salt lakes, focusing on gastropods and crustaceans. This information is needed to evaluate and manage the threats posed to these invertebrates by increased periods of drought and secondary salinisation. Most of these species are endemic to Australian salt lakes, and some have adapted to extreme conditions (e.g. salinities >100 g L–1 and pH <5). This study identifies key general findings regarding the taxonomy, ecology and life histories of these invertebrates, such as that many ‘new’ species have been uncovered in the past 20 years, with more likely to come. The study also identifies critical knowledge gaps, such as the need to elucidate the abiotic and biological drivers of the field distributions of species, including why some species are widespread and common whereas other congeneric species are rare or have narrow distributions. Those species that are either restricted to low salinity environments or survive dry periods as aestivating adults (as opposed to desiccation-resistant eggs) are probably the most vulnerable to increasing salinisation and drought. Future work should prioritise the development of a sound taxonomy for all groups, because this is needed to underpin all other biological research.
Keywords: Coxiella, crustaceans, halobionts, halophiles.
Introduction
Salt lakes are defined as enclosed bodies of water with salinity >3 g L–1, although the salinity is usually much greater than this (Williams 1964). Salt lakes are often classed as either athalassic (inland) or coastal (possessing a current or recent connection with the marine environment, including via groundwater; Bayly and Williams 1966).
Australia has a vast number and variety of salt lakes that support unique faunal communities (De Deckker 1983a; Williams 2002). At least in Australia, coastal and athalassic salt lakes are characterised by different faunas. The former mainly contain species that have current or recent marine or estuarine affinities (Timms 2009a, 2010a; Pinder and Quinlan 2015), whereas the latter mainly contain taxa that have evolved in and are restricted to these systems (Bayly 1972). However, some athalassic species occasionally also occur in coastal lakes (Timms 2009a; Pinder and Quinlan 2015). This review focuses on the fauna of Australian athalassic salt lakes but includes data for athalassic species in coastal lakes.
Salt lakes are physically extreme environments, particularly those lakes that are either highly saline or only hold water intermittently (Williams 1985). The physical characteristics of Australian salt lakes are well established and were reviewed recently by Mernagh et al. (2016). Athalassic salt lakes mostly form in locations with semi-arid to arid climates where evaporation exceeds precipitation and drainage is impeded or fully endorheic (Williams 1998a). These lakes vary from expansive playas with long geological records to small, recently formed ponds and wetlands (De Deckker 1983b) that have become saline as a result of the intrusion of saline groundwater. Most Australian salt lakes are alkaline and ionically dominated by NaCl (Bayly and Williams 1966), but some are naturally acidic and can have a pH as low as 3 (Timms 2009b). Some salt lakes hold water permanently but most are either seasonal (usually filling with winter–spring rainfall and drying out over summer–autumn) or episodic (filling only after unseasonal rainfall; Williams 1998a, 1998b).
The terms ‘halotolerant’, ‘halophile’ and ‘halobiont’ are commonly used to classify the aquatic fauna of salt lakes based on their relationship with salinity, although these terms are variously used (Bayly 1972; Williams 1981; Timms 1983; Hammer 1986; Pinder et al. 2002). Herein, ‘halotolerant’ describes biota that predominately occur in fresh water (salinity <3 g L–1) but occasionally occur in waters with salinity up to ~20 g L–1, whereas ‘halophiles’ are biota that occur mostly in athalassic waters (salinity >10 g L–1; Williams 1981). We have not attempted to distinguish between ‘high-salinity’ (sometimes called halobiontic) and ‘low-salinity’ (sometimes called halophilic) taxa due to varying definitions (e.g. Timms 1983 v. Williams 1981 v. Bayly 1972) and because many taxa do not neatly fit into one or the other category. It is acknowledged that our definition neglects some species that occur in salt lakes at salinities >3 g L–1 but typically less than 10 g L–1 that have been described as halophilic elsewhere (e.g. Eocyzicus spp. and Branchinella spp. in Timms 2007, 2014).
Australian salt lakes support unique communities. Some invertebrate groups, particularly crustaceans, are well represented, with many species, genera and even one family restricted to salt lakes (Halse and McRae 2004; Timms 2014). The gastropod Coxiella (which includes the subgenus Coxielladda) is unique to these environments and the only gastropod genus anywhere in the world to consist entirely of halophilic species (Williams and Mellor 1991). Insects are commonly encountered, but, with few exceptions, these are halotolerant rather than halophilic (Williams and Kokkinn 1988; Timms 1993; Pinder et al. 2005). Fish are rare and are mainly found in low-salinity permanent lakes, but may occur in large episodic lakes during heavy filling events when these lakes become connected to refugia such as mound springs supporting fish (e.g. Craterocephalus eyresii (Steindachner) in Lake Eyre and Lake Torrens; Williams and Kokkinn 1988; Williams et al. 1998). Numerous waterbirds opportunistically use salt lakes for feeding and even breeding (Weston 2007; Pedler et al. 2018) and some, such as banded stilts Cladorhynchus leucocephalus (Vieillot), are largely restricted to these lakes (Pedler et al. 2014). These birds are probably important vectors of dispersal for salt lake invertebrates (Green et al. 2008; Sánchez et al. 2012). Australian salt lakes also have distinctive fringing plant communities dominated by genera such as Tecticornia (samphires) and Frankenia (sea heath; Lyons et al. 2004). Salt-tolerant macroalgae such as Characeae and the vascular plants Ruppia and Lepilaena form thick mats in mildly saline waterbodies (Porter 2007; Casanova 2013), and microalgae such as Dunaliella thrive in hypersaline conditions, where they give lakes a characteristic pink hue (Teller 1987).
Salt lakes and their unique biota are threatened globally (Williams 2002). In Australia, reduced rainfall, associated with anthropogenic climate change, is having a widespread and dramatic effect as it causes lakes to fill less often, with shorter hydroperiods (i.e. periods during which water is present) and higher salinities (Hughes 2003; Nielsen and Brock 2009). These changes can have a profound effect on community composition, especially in previously low-salinity or seasonal lakes (Williams 2002; Pinder et al. 2005). Secondary salinisation, agricultural and mining activities, groundwater extraction, diversion of surface flows and pollution also present significant threats to Australian salt lakes and have already deleteriously affected these environments in some areas (Williams 1995; Timms 2005). For example, increased salinisation, changes to hydroperiod and, in some cases, acidification associated with secondary salinisation have diminished the faunal communities of some salt lake environments on the Eyre Peninsula, South Australia (Williams 1984; Timms 2009a) and in the Wheatbelt region of Western Australia (Cale et al. 2004). In addition to expirations, there is a risk that these hydrological changes may lead to the extinction of entire species, at least in Western Australia, without intervention (Halse et al. 2003). Despite the unique biodiversity and apparent threats, very little has been done to assess the conservation status of salt lake invertebrates (Timms et al. 2009).
Conservation planning should be informed by evidence (Sutherland and Wordley 2017). To effectively assess and manage threats to Australian salt lake environments, it is important to synthesise the available information on these environments and use this information to document general trends and highlight critical knowledge gaps. There is, however, no current systematic evaluation of the state of knowledge of the biota of Australian salt lakes. De Deckker (1983b) reviewed the history, chemistry and biota of Australian salt lakes, but our knowledge of these systems has since improved. There have been significant advances in knowledge of the systematics and biology of a range of taxa (Hebert and Wilson 2000; Halse and McRae 2004; Timms 2014) and expanded coverage of some regions (Pinder et al. 2002; Timms et al. 2006; Timms 2008). The study reviews the current state of knowledge of halophilic macroinvertebrates of Australian salt lakes. It focuses on crustaceans and gastropods because they are an important and conspicuous component of these lakes.
The specific aims of this study were to: (1) synthesise knowledge of halophilic crustaceans and gastropods from Australian salt lakes, focussing on information published since De Deckker (1983b); (2) draw attention to important issues in Australian salt-lake conservation; and (3) identify general trends, gaps in knowledge and directions for future research in this area.
Materials and methods
This study reviews what is known about halophilic macroinvertebrates from Australian salt lakes, focusing on data published since De Deckker’s (1983b) review. For quality control, it mainly relies on peer-reviewed studies, but unpublished theses and reports were used when they contained crucial information that was not otherwise available.
Halophilic macroinvertebrates were identified from published ecological studies of Australian salt lakes (Table 1) and from unpublished data held by the Western Australian Department of Biodiversity, Conservation and Attractions (DBCA). The dataset analysed during this study is available from the corresponding author on reasonable request. In all, 79 described halophilic species in 23 genera were identified. Almost all species were either crustaceans or gastropods. Insects were excluded because most species in Australian salt lakes are halotolerant rather than halophilic (Timms 1993; Pinder et al. 2005). Rotifers have also been excluded on the basis that they are microscopic rather than macroscopic (Blinn et al. 2004).

|
Various methods were used to find articles on the biology of the identified halophiles, including searches for keywords ‘salt’, ‘saline’, ‘lake’, ‘wetland’, ‘Australia’, ‘invertebrate’, ‘fauna’, ‘halophile’, ‘halophilic’, ‘halobiont’, ‘crustacean’ and ‘gastropod’ on Scopus and Google Scholar databases. Both databases were last accessed on 1 December 2020.
This review includes salinity data from multiple studies. These studies typically estimated salinity (the sum of all ion concentrations per unit solution) from measurements of conductivity (which has particular utility in Australian salt lakes because most have a homogenous ionic composition; Bayly and Williams 1966) or as gravimetric total dissolved solids (TDS; which may include small but variable amounts of organic matter that can result in exaggerated salinity values; Williams and Sherwood 1994). Salinity data reported from gravimetric determination (TDS) in the source article have been converted to grams per litre of dissolved solutes in this review to facilitate comparisons across different studies. This was done using a correction factor of 0.91, which is based on the highly correlated relationship between salinity estimated using conductivity and salinity estimated from gravimetric methods (Bayly and Williams 1966).
Halophilic biota: Crustacea
Anostraca
Three anostracan genera, Parartemia, Branchinella and Artemia, occur in Australian salt lakes (Pinceel et al. 2013a; Timms 2014).
Parartemia
The biology of Parartemia has been reviewed recently by Timms (2014), so only general points are discussed here.
Taxonomy. Parartemia, the only genus in the family Parartemiidae, is unique to Australia and is composed exclusively of halophilic species (Table 2). With 18 described and one undescribed species (Timms 2014), it is one of the most speciose genera in salt lakes.

|
The taxonomy of Parartemia is relatively advanced compared with that of most other Australian salt lake invertebrates, although a substantial number of species (nine) have only been described in the past 10 years (Table 2) and another species is yet to be described (Timms 2014). The most recent taxonomic assessments of Parartemia are provided by Timms and Hudson (2009), who described four new species from South Australia, and Timms (2010b), who described six new species from Western Australia. The taxonomic work is based on morphology, but Remigio et al. (2001) used variation in the mitochondrial 16S gene to confirm that the eight species described at that time were genetically distinct and as evidence of undiscovered species in Lake MacLeod (GenBank Accession number AY014794) and another in ‘Scadden East’ (GenBank Accession number AY014795) in Western Australia. Whether the undescribed species of Remigio et al. (2001) are populations of species subsequently described by Timms (2010b) is not clear.
Ecology. Species diversity in Parartemia decreases from western to eastern Australia, with Western Australia having 13 described species (10 endemics) compared with 6 (3 endemics) in South Australia and 2 (no endemics) in the eastern states (Table 2). The ubiquity of species varies, ranging from geographically widespread and known from many sites (e.g. Parartemia cylindrifera Linder, Parartemia longicaudata Linder and Parartemia zietziana Sayce) to only known from one or a few sites in a single region (e.g. Parartemia triquetra Timms & Hudson and Parartemia auriciforma Timms & Hudson; Timms et al. 2009). Knowledge of the distributions of some species is inadequate, especially those like P. triquetra and P. auriciforma, which occur in remote areas that have been poorly surveyed (Timms and Hudson 2009; Timms 2010b).
Parartemia species occur mainly in ephemeral and seasonal natural salt lakes, surviving dry periods as drought-resistant cysts (Timms 2012a). They are strong hypoosmotic regulators (Geddes 1981) and the most salt-tolerant of the endemic invertebrates in Australian salt lakes (Timms 2014), but little is known about the physiological basis for this. All 15 species for which relevant data are available have been collected from lakes with salinities >90 g L–1, and 8 of these were from lakes with salinities in excess of 200 g L–1 (Table 2). Laboratory experiments by Manwell (1978) suggested that one species, P. zietziana, was not able to withstand the highest salinities tolerated by Artemia, possibly due to a lesser capacity to produce sufficient haemoglobin to compensate for low oxygen concentrations at very high salinities. Based on field data, the salinity tolerances of Parartemia species are very broad and overlap (Table 2). Nevertheless, species rarely co-occur in the same waterbody and a combination of physical factors, such as water duration, filling pattern, pH and salinity, are suggested to be important in determining species distributions (Timms 2009c; Timms et al. 2009). In addition, Parartemia eggs sink and become bound up in sediments, reducing dispersal ability (Timms et al. 2009). The majority of species live in alkaline waters, but Parartemia mouritzi Timms, Parartemia contracta Linder and Parartemia acidiphila Timms & Hudson are known to inhabit acidic lakes (Conte and Geddes 1988; Timms 2009b, 2010b; Timms and Hudson 2009).
Life history. Sexes are separate for all Parartemia (Timms 2014). Detailed lifecycle and reproductive information is available only for P. zietziana (Marchant and Williams 1977a), which reproduces ovoviviparously, producing multiple cohorts so long as conditions are favourable (Timms 2012b) and uses oviparous reproduction to produce highly resistant cysts when conditions become unfavourable (Geddes 1976; Marchant and Williams 1977b). The cysts of Parartemia laticaudata and Parartemia veronicae have the same stress proteins (p26, artemin and heat shock protein 70) that enable Artemia (cysts) to survive severe desiccation (Clegg and Campagna 2006). Cyst hatching appears to follow a ‘bet-hedging’ strategy, whereby some eggs hatch within hours of a filling event but not all eggs hatch at once (in case the water does not persist long enough to allow reproduction; Timms 2012b).
Trophic ecology. Dietary information is available only for P. zietziana, which selectively feeds on benthic or suspended particles with a high organic content (Marchant and Williams 1977b). It has been assumed that other species feed similarly (Timms 2014). Timms (2012a) suggested that Parartemia mainly feed on organic matter that is resuspended in the water column, which provides limited opportunity for niche diversification based on food resources. Timms (2012a) hypothesised that this may give a species established in a lake a competitive advantage over a species that recently arrives, and hence explain why lakes typically only contain one Parartemia species. Exactly how this may give a resident species a competitive advantage is not clear.
Parartemia are an important food source for waterbirds, such as the banded stilt, that may fly thousands of kilometres to take advantage of booming Parartemia populations (Pedler et al. 2018).
Conservation status. Parartemia is unique among Australian salt lake genera in that its conservation status has been assessed, at least in Western Australia (Timms et al. 2009). The dominant threats to Parartemia are habitat degradation, including acidification and changes to hydrology (especially prolonged inundation) from secondary salinisation (Timms et al. 2009). Parartemia contracta is currently the only species listed by the International Union for Conservation of Nature (as vulnerable), although it is one of the more common species and may not be as at risk as previously thought (Timms et al. 2009). The species that are regarded as at most risk (Parartemia extracta and Parartemia boomeranga) are only known from the Wheatbelt region, where altered hydrology and secondary salinisation is widespread (Halse et al. 2003). Timms et al. (2009) recommended that P. extracta be considered as vulnerable because it has disappeared from many sites and is secure only in lakes in the Jurien Bay area (Fig. 1; Timms 2014; Pinder and Quinlan 2015). Another species, namely P. boomeranga, may be extinct (Timms 2012b) because it has not been recorded recently and all the lakes this species was known to occur in are affected by secondary salinisation (Fig. 1; Timms et al. 2009). Concerns have also been raised for P. mouritzi and Parartemia bicorna, both of which are only known from a few lakes that are currently being affected by either secondary salinisation or mine dewatering discharge (Timms 2014).

|
Artemia
The genus Artemia comprises seven (Rogers 2013) or possibly nine (Naganawa and Mura 2017) species. Although Artemia is widespread and common in hypersaline lakes in most continents, only Artemia franciscana Kellogg and Artemia parthenogenetica Bowen & Sterling are present in Australia (McMaster et al. 2007; Asem et al. 2018). A. franciscana was introduced to Australia by humans to aid in salt production and is overwhelmingly restricted to constructed evaporative ponds (salt works; Timms and Hudson 2009; Asem et al. 2018). A. parthenogenetica may have also been introduced into salt works by humans (McMaster et al. 2007), but its presence in a range of lakes in south-western Australia could be the result of intercontinental bird-mediated dispersal followed by local dispersal (McMaster et al. 2007).
All Artemia species, including the two species found in Australia, have high dispersal capacity because their cysts float and are effectively transported by animals and wind (Timms and Hudson 2009). Despite this, A. franciscana does not seem to be spreading (Timms and Hudson 2009; Asem et al. 2018), but the distribution of A. parthenogenetica is increasing in south-western Australia, where it is mostly colonising degraded salt lakes (McMaster et al. 2007). It is unclear how the further spread of A. parthenogenetica may affect Parartemia species, but currently A. parthenogenetica appears limited to lakes not already occupied by Parartemia (McMaster et al. 2007).
Branchinella
Branchinella, with 40 described species, is the most speciose of the Australian anostracan genera (Timms 2015a, 2015b). Many species have only recently been described (Timms 2015b), with cryptic lineages identified using genetic data (Pinceel et al. 2013b). Only two species, Branchinella buchananensis Geddes and Branchinella simplex Linder, are halophiles; the other species are either strictly freshwater or tolerant of only low levels of salinity (Pinceel et al. 2013a; Timms 2014). Further work is needed to determine whether Branchinella halsei Timms is conspecific with B. simplex, because these taxa are morphologically distinct but molecularly congruent (Pinceel et al. 2013b).
B. buchananensis occurs, usually at salinities of ~15 g L–1 (limit 42 g L–1; Timms 2009c), in Queensland and north-western New South Wales, where it is listed as vulnerable under the Fisheries Management Act 1994 (Timms 2014). B. simplex is known, usually in salinities of ~30 g L–1 (limit 62 g L–1; Timms 2015b), from lakes in central Western Australia, northern South Australia and the southern Northern Territory.
The results of a molecular phylogeographic study suggest that the two halophilic species have evolved from different lineages (Pinceel et al. 2013b). B. buchananensis and four halotolerant species comprise a clade that is estimated to have evolved from a freshwater ancestor somewhere between c. 62 and 23 million years ago, which coincides with increasing availability of temporary saline aquatic habitats in Australia (van de Graaff 1977; Pinceel et al. 2013a).
Notostraca
Triops
Both living notostracan genera, Triops and Lepidurus, occur in Australia (Pinder et al. 2005), although only Triops is known from salt lakes (Timms 2009c). Until recently, Triops was thought to be represented in Australia by a single, widespread and morphologically variable species (Triops australiensis Spencer & Hall; Timms 2012a). However, a recent molecular appraisal has revealed the presence of a range of putative new species and predicted that further diversity would be found once lakes in central and western Australia are adequately surveyed (Murugan et al. 2009; Meusel and Schwentner 2017). Little ecological information is available for halophilic Triops except that undescribed species have been reported from Lake Carey (salinity 11.6–84.3 g L–1, mean 30.3 g L–1 from 10 records; Timms et al. 2006), the Esperance region in Western Australia (salinity 27 and 31 g L–1, 2 records; Timms 2009b), the Paroo wetlands in New South Wales (salinity 0.3–19.3 g L–1; Timms 1993) and Lake Torrens in South Australia (salinity ~16 g L–1; Williams et al. 1998).
Cladocera
The bulk of Cladocera that occur in inland waters in Australia are restricted to fresh water, but three groups have representatives in salt lakes. These groups comprise: (1) six species of Daphniopsis (or Daphnia; see below); (2) two species of Daphnia (Daphnia salinifera Hebert and Daphnia neosalinifera Hebert) from the Daphnia carinata (King) subgenus; and (3) three species of chydorid: Moina baylyi Forró, Moina mongolica Daday and Extremalona timmsi Sinev & Shiel. All species are endemic to Australia except for M. mongolica, which is also known from northern Africa, Europe, the middle-east, Russia and China (He et al. 2001), notwithstanding that molecular data suggest that there are many unrecognised species of Moina (Bekker et al. 2016).
Colbourne et al. (2006) collected D. salinifera from Lake Wyora in Queensland and D. neosalinifera from Colac in Victoria and suggested that these species are usually found at lower salinities than the Australian species of Daphniopsis. However, apart from this, there are virtually no published data on the former two species, and they are not considered any further herein.
Daphniopsis
Taxonomy. Historically, the daphniids in Australian salt lakes were classified in the genus Daphniopsis (Sars) and just one species, Daphniopsis pusilla (Serventy), was recognised. Six species, all supported by genetic data (Hebert et al. 2002), have now been described (Sergeev and Williams 1985; Sergeev 1990a, 1990b; Hebert and Wilson 2000). Furthermore, one of six ephippia morphotypes identified by Kokkinn and Williams (1987), namely ‘morphotype six’ from Lake Eyre South, has not been accounted for by any described species (Hebert and Wilson 2000), and there are reports of undescribed species from salt lakes in the Wheatbelt region in Western Australia (Pinder et al. 2005) and the Eyre Peninsula in South Australia (Timms 2009a). The diversity of Daphniopsis in Australia is high, given that Daphniopsis is represented by a single species in either fresh or saline water in other regions where it occurs (Bayly 1995; Hebert and Wilson 2000; Gibson and Bayly 2007). Because all the described Australian species form a monophyletic group relative to other daphnids (Colbourne et al. 2006), it appears that this group has undergone a significant radiation in Australia.
The taxonomic status of Daphniopsis, specifically its relationship to the genus Daphnia, has long been debated (Schwartz and Hebert 1984). Benzie (2005) concluded that Daphniopsis as a whole is not morphologically or genetically distinct enough to warrant genus status and suggested that this taxon should be subsumed into the Daphnia subgenus Ctenodaphnia. Data from three mitochondrial genes support the view that Daphniopsis is a component of the genus Daphnia (Colbourne et al. 2006). Nevertheless, all the Daphniopsis species that were included in the above genetic study formed a monophyletic group, except for Daphniopsis ephemeralis (Schwartz & Hebert), which is the only representative of this taxon from the Northern Hemisphere. Most recent studies retain the name Daphniopsis (Säwström et al. 2009; Ismail et al. 2010a, 2011a; McCloud et al. 2018; Wang et al. 2019) and this practice has been adopted herein.
Ecology. Given that several species have only been recently described, it is difficult to gauge the reliability of historical accounts of species distributions, although, based on data from recent field records, it appears that south-western Australia has the greatest diversity (Hebert and Wilson 2000). All six described species have been found from at least two sites in this region and two, Daphniopsis wardi (Hebert & Wilson) and Daphniopsis pusilla, are endemic. Historical reports of the latter species from other parts of southern Australia predate the current taxonomy and are due to misidentification (Hebert and Wilson 2000). Another two species, Daphniopsis quadrangulus (Sergeev) and Daphniopsis australis (Sergeev & Williams), predominately occur in lakes in south-eastern Australia, but each has been recorded twice in south-western Australia (Fig. 2a). Daphniopsis truncata (Hebert & Wilson) and Daphniopsis queenslandensis (Sergeev) are common in Western Australia and South Australia, and D. queenslandensis also occurs in New South Wales and Queensland (Fig. 2b).

|
Osmoregulation has been studied in two species of Daphniopsis (D. pusilla and D. australis). Both were determined to be osmoregulators, where the haemolymph is hyperosmotic to the environment at salinities under 8 g L–1, isosmotic in waters with salinity 8–20 g L–1 and hypoosmotic in waters with salinity >20 g L–1 (Aladin and Potts 1995).
All Australian species of Daphniopsis are halophilic. Field salinity data suggest that most species are common in 17–30 g L–1, but nevertheless the salinity ranges of most species are very broad (Table 3). The salinity distribution of one species, D. quadrangulus, is mostly unknown because there are only two reported occurrences of this species (Table 3). Some reports of species from very high salinities should be viewed with caution because they may relate to preserved dead or dying specimens. For example, experimental data indicate that D. australis experiences significant mortality at salinities below 5 and above 33 g L–1 (Ismail et al. 2010a), suggesting that reports of live D. australis at a salinity of 154.1 g L–1 by Sergeev and Williams (1985) are unlikely.
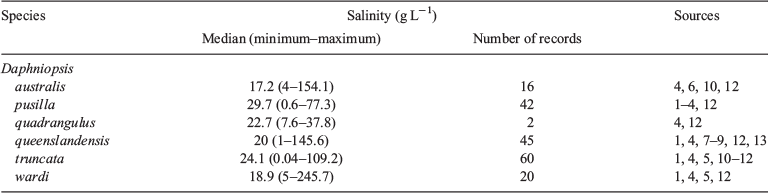
|
Experimental data indicate that growth, reproduction and longevity in D. australis are influenced by both temperature and salinity, but the former is especially important between 16 and 25°C and the latter is especially important at salinities of 17–27 g L–1 (Ismail et al. 2011b). D. australis experienced significant mortality at temperatures above 28°C (Ismail et al. 2010a), which is consistent with field observations that it occurs seasonally in the winter–spring (Campbell 1994). Similarly, D. queenslandensis and D. truncata have been reported to be more abundant in winter–spring than in summer–autumn (Timms 2008, 2009b, 2018), despite minimal differences in salinity between seasons.
Life history. Like most daphniids, Australian Daphniopsis are cyclical parthenogens, although there are interspecific hybrids that reproduce by obligate parthenogenesis (Hebert and Wilson 2000). The switch from parthenogenesis to sexual reproduction and the production of desiccation-resistant ephippial eggs is trigged by unfavourable conditions, possibly one (or a combination) of low food availability, high population density, high salinity and high temperature (Sergeev and Williams 1983; Williams 1986; Ismail et al. 2010b). Detailed lifecycle data are available only for D. australis under laboratory conditions, as described by Ismail et al. (2010b). These data suggest that the parthenogenetic females usually live for 20–30 days, take 6–7 days to reach maturity and produce ~10–12 clutches during their lifetime. The ephippial females have a shorter lifespan and usually produce a maximum of two diapausing eggs. These females are produced under conditions of high population density and can switch from sexual to parthenogenetic reproduction, particularly at low and high food densities in the presence of males whose persistent attempts at mating may stress the females.
Trophic ecology. Little is known about the trophic ecology of Daphniopsis in Australian salt lakes. Based on information for other species of Daphniopsis (Säwström et al. 2009) and Daphnia (Taipale et al. 2012), these species likely consume microalgae and bacteria and are important herbivores in the lake food webs. Ismail et al. (2011a) fed different combinations of three microalgae species commonly used in aquaculture to D. australis and found that the composition of algae in the diet influenced the growth and reproductive output of individuals. Based on detailed observations of swimming behaviour under laboratory conditions, McCloud et al. (2018) developed the following hypotheses for D. australis: (1) males mainly occur in the water column, where they filter feed; (2) parthenogenetic females spend most of their time close to the bottom and mainly rely on benthic resources; and (3) the habits of the ephippial females are intermediate between those of the males and parthenogenetic females. The ostracod Australocypris insularis is known to predate on Daphniopsis (Campbell 1995).
Chydorids
The endemic halophile M. baylyi is widespread in central Australian salt lakes (Timms 2007) and the predominantly Northern Hemisphere M. mongolica has been encountered once in a coastal saline lake near Carnarvon in Western Australia (Fig. 3; Halse et al. 2000a). Very little information is available for M. baylyi other than it is commonly reported at salinities between 2.9 and 60 g L–1 and up to 86.7 g L–1, and is more common during the summer rather than winter filling of lakes (Timms 1987; Williams and Kokkinn 1988; Timms 1993, 2001; Williams et al. 1998). The monotypic E. timmsi is the most recently described halophilic cladoceran from Australia (Sinev and Shiel 2012) and is known only from two acidic salt lakes near Esperance, Western Australia (as Alona spp. in Timms 2009b).

|
Ostracoda
A diverse and heterogeneous range of halophilic ostracods occurs in Australian salt lakes. These ostracods can be divided into two main groups: (1) giant ostracods from the Australian endemic subfamily Mytilocypridinae (herein called giant ostracods); and (2) non-Mytilocypridinae ostracods (herein called small ostracods).
Giant ostracods
The giant ostracods are >3 mm in length and are a conspicuous component of the biota of Australian salt lakes.
Taxonomy. The morphotaxonomy of giant ostracods is relatively well established from the early work of De Deckker (1978, 1981a) and more recent work by Halse and McRae (2004). Halse and McRae (2004) added 2 new genera and 6 new species, bringing the total to 21 described species in 6 genera, although there are uncertainties regarding the status of a few described species (e.g. whether Mytilocypris tasmanica (McKenzie) and Mytilocypris praenuncia (Chapman) should be synonymised; Finston 2000). There are also reports of an undescribed species of Mytilocypris (Pinder et al. 2010; Quinlan et al. 2016) and of Lacrimicypris (L. Bourke, K. Brown, and G. Paczkowska, unpubl. data, 2018), both from Western Australia. Further species are likely to be found, particularly in remote areas (Halse and McRae 2004). Other than for some allozyme data that have helped resolve species questions in Mytilocypris (Finston 2000; Halse and McRae 2004), the current taxonomy of giant ostracods is based on morphological characters, mainly the structure of the hemipenis.
Although some giant ostracod species are known only from fresh or low-salinity waterbodies, most are halophiles (Table 4). These halophiles occur in four of six genera: Australocypris (all seven species), Repandocypris (all two species), Mytilocypris (five of seven species, assuming M. praenuncia and M. tasmanica are distinct) and Trigonocypris (one of two species; Table 4). The broad taxonomic distribution of halophilic, low-salinity and freshwater forms suggests that halophily has probably evolved more than once in the Mytilocypridinae.
Ecology. Other than for some taxonomic and distribution information, very little is known about the ecology of giant ostracods, even for Australocypris, which is a very diverse, widespread and abundant component of the salt lake ecosystems.
The highest diversity of halophilic giant ostracods is in Western Australia (Table 4), particularly for Australocypris, which shows a marked decrease in species richness and endemism from west to east (Table 4). Most halophilic Mytilocypris species occur broadly across Australia, although M. praenuncia–tasmanica (see above) has only been reported from south-eastern Australia (Table 4). Repandocypris has one species only known from Western Australia (Repandocypris austinensis Halse & McRae) and another only known from South Australia (Repandocypris gleneagles Halse & McRae; Table 4). The halophilic species of Trigonocypris (Trigonocypris globulosa (De Deckker)) is found in Western Australia, South Australia, New South Wales and Queensland (Table 4). Most halophilic species of giant ostracod are broadly distributed, although a few seem to have a very narrow range (Halse and McRae 2004). Australocypris mongerensis Halse & McRae is only known from a small mildly saline claypan next to Lake Monger in Western Australia (Halse and McRae 2004). However, knowledge of species distributions, particularly for those species first described by Halse and McRae (2004) like A. mongerensis, is likely to be incomplete.
With the exception of A. mongerensis, the halophilic species of giant ostracod have been collected from a broad and overlapping range of salinities (Table 4). Eight species have been recorded from salinities >100 g L–1 (Table 4). On average, Mytilocypris species tend to occur in a lower and narrower range of salinities than Australocypris species (Table 4). However, the salinity distribution of Mytilocypris mytiloides (Brady) is very broad and extends into highly saline water (Table 4). Both species of Repandocypris have only ever been found at salinities >40 g L–1 (Table 4), although they are only known from a few sites (Halse and McRae 2004). The salinity range of T. globulosa is broad and exceeds 100 g L–1. Based on data for M. praenuncia, halophilic giant ostracods are osmoregulators (Aladin and Potts 1996).
All giant ostracods occur in neutral or slightly alkaline waters, but the distributions of A. insularis (Chapman), Australocypris bennetti Halse & McRae and M. mytiloides extend into acidic waters and all have been collected from waters with a pH <4 (Cale et al. 2004; Halse and McRae 2004; Timms 2009b). It is not known how these ostracods can survive in such acidic conditions, which are expected to dissolve the calcite carapace (De Deckker 2002).
Life history. All giant ostracods are dioecious and sexually reproducing (De Deckker 1977, 1983a). Giant ostracods produce desiccation-resistant eggs (Halse and McRae 2004), which re-establish populations after unfavourable or dry conditions (De Stasio 1989) and likely facilitate dispersal through attachment to or ingestion by waterbirds (De Deckker 1977; Green et al. 2008). Specific information on the life history, reproduction and dispersal of giant ostracods is mainly restricted to species of Mytilocypris (De Deckker 1977; Martens 1985; Finston 2000, 2002, 2004, 2007).
Trophic ecology. Giant ostracods tend to actively swim in the water column (De Deckker 1983a). This, and their large size, is likely related to the general absence of fish predators in the salt lakes or temporary freshwater waterbodies they inhabit (De Deckker 1983a). The diet of giant ostracods is poorly understood, although at least A. insularis consumes detritus and is also planktivorous and capable of significantly reducing the abundance of Calamoecia, Daphniopsis and Diacypris in microcosm experiments (Campbell 1995).
Small ostracods
Taxonomy. Most of the small ostracods in Australian salt lakes belong to the genera Diacypris and Reticypris. These genera contain seven and five described species respectively (Table 5), all of which are halophilic (De Deckker 1981b). They comprise the subfamily Diacypridinae, which is endemic to Australia, and have no known recent freshwater or marine ancestors (De Deckker 1981b). The taxonomy of Diacypridinae has not been addressed since De Deckker (1981a, 1981c), and the extent to which it captures the biodiversity of this group is not clear. There are reports of multiple undescribed species of Diacypris and Reticypris, predominately from Western Australia (Williams 1984; Williams and Kokkinn 1988; Timms 1993; Pinder et al. 2005; Quinlan et al. 2016). Species diagnoses are based on the structure of the hemipenis (see fig. 13 in De Deckker 1981c for Diacypris). Some characters, such as variation in the carapace shape and size, are ecophenotypic at least in several Diacypris and Reticypris species (De Deckker 1981c, 1981d).

|
Other small halophilic ostracods from Australian salt lakes are the endemic Platycypris baueri (Herbst), Patcypris outback Halse & Martens and Trilocypris horwitzi Halse & Martens, which are all in the cosmopolitan subfamily Cyprinotinae (De Deckker and Geddes 1980; De Deckker 1981b; Karanovic 2012; Halse and Martens 2019). In addition, Billcypris davisae Halse & Martens has been collected from Lake Lefroy and Lake Cowan in the Goldfields region of Western Australia (Halse and Martens 2019). These are both large hypersaline lakes and this species is likely halophilic, although no information on the salinity at the time of its collection is available (Halse and Martens 2019). The endemic species Cyprideis australiensis Hartmann and Leptocythere lacustris De Deckker have also been reported from Australian salt lakes but, because these species have marine ancestries, they lack desiccation-resistant eggs and so are restricted to lakes with permanent water (De Deckker 1983a; Pinder et al. 2002; Schön et al. 2017). A few species of Cyprinotus, most notably Cyprinotus cingalensis Brady, which is widely distributed in south-east Asia, are found in Australian salt lakes (Karanovic 2008), but usually in low-salinity waters, and are more halotolerant than halophilic (Timms 1993, 2009b; Pinder et al. 2002).
Ecology. Knowledge of the biology of the small halophilic ostracods from Australian salt lakes is rudimentary. Two of the Cyprinotinae species (P. outback and T. horwitzi) have only recently been described (Halse and Martens 2019) and thus far reported from only one or a small number of sites in Western Australia. These species are not considered in the following discussion. Understanding the ecology of Diacypris and Reticypris is complicated by the fact that these taxa are not identified to species level in many studies (Timms 1998, 2018; Timms et al. 2006).
All described Diacypris and Reticypris species, as well as P. baueri, have broad and overlapping geographic distributions (Table 5). All these species are known from both Western Australia and South Australia, except for Reticypris herbsti McKenzie (known from South Australia but not Western Australia) and Reticypris pinguis De Deckker (known from Western Australia but not South Australia; Table 5). Some of these species also occur in other states, the exact details of which vary from species to species (Table 5). Many species, such as Diacypris phoxe De Deckker, R. herbsti, Reticypris kurdimurka De Deckker, R. pinguis and Reticypris walbu De Deckker, are patchily distributed (i.e. reported from a small number of sites that are typically separated by hundreds or even thousands of kilometres; Fig. 4).

|
Diacypris and Reticypris species generally have broad and overlapping salinity tolerances, although some species are more commonly found at higher salinities than others (Fig. 5). For example, Diacypris spinosa De Deckker is more common at lower salinities (median 14.7 g L–1) than Diacypris whitei (Herbst) (median 69.6 g L–1), Diacypris fodiens (Herbst) (median 73.6 g L–1) and R. herbsti (median 115.1 g L–1; Fig. 5; De Deckker and Geddes 1980; Pinder et al. 2005). Other species, like Diacypris compacta (Herbst) and P. baueri, occur across a broad range of salinities, sometimes in excess of 200 g L–1 (Fig. 5; Williams et al. 1990). D. spinosa is an osmoregulator (Aladin and Potts 1996), and the other small halophilic ostracods are probably likewise.
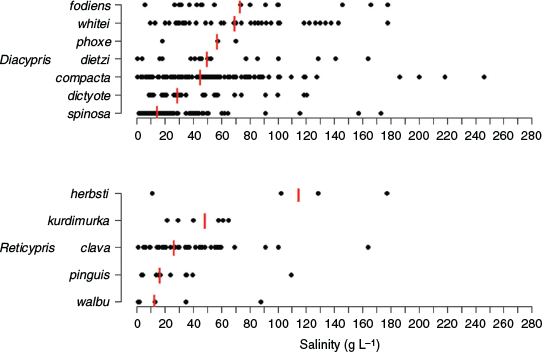
|
It is often said that dispersal of inland ostracods, including halophilic small and giant species, is mediated by birds (De Deckker 1977; Halse 2002). This is supported by the fact that ostracod eggs have been hatched from the faeces of waterbirds that frequent the temporary wetlands in arid Australia, although no information on the species hatched is available (Green et al. 2008).
Copepoda
Copepods are widespread and common in Australian salt lakes, with three orders, namely Calanoida (three species), Cyclopoida (five species) and Harpaticoida (i.e. Mesochra baylyi Hamond), represented.
Calanoida
Taxonomy. All three halophilic calanoids that occur in Australian salt lakes belong to the genus Calamoecia. The taxonomy of these species (Calamoecia salina (Nicholls), Calamoecia clitellata Bayly and Calamoecia trilobata Halse & McRae) has been unchanged since Bayly (1962), except for the description of C. trilobata (Halse and McRae 2001). Other Calamoecia occur in Australian freshwater environments (Bayly and Boxshall 2009). Mitochondrial 16S and nuclear 28S gene data do not support suggestions, based on morphological data (Bayly 1962), that the salt lake and freshwater species should be placed in different genera (Adamowicz et al. 2010). In addition, these gene data suggest that C. clitellata colonised salt lakes via fresh water (Adamowicz et al. 2010) rather than directly from the marine environment, as has been suggested previously (Maly 1996).
Ecology. Both C. clitellata and C. salina occur throughout mainland southern Australia; the former is also found in Tasmania (Fig. 6). By contrast, C. trilobata is endemic to Western Australia (Fig. 6a).
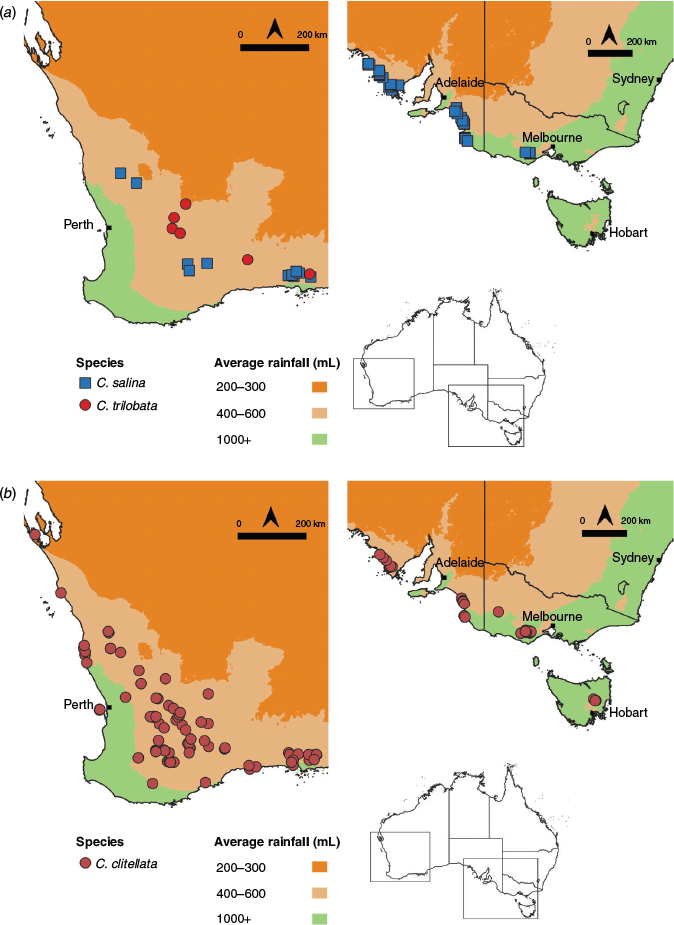
|
Despite being osmoconformers (Bayly 1972), these calanoids are often reported from high salinities; for example, C. clitellata and C. salina have been reported from salinities up to 132 and 195 g L–1 respectively (De Deckker and Geddes 1980; Timms 2009b). Both C. salina and C. clitellata are often found in the same lake, with C. salina succeeding C. clitellata at higher salinities, which is attributable to its higher hatching salinity (61.4–82.9 g L–1) than that of C. clitellata (20–71 g L–1; Geddes 1976). Less information is available for C. trilobata, which has only been reported from acidic salt lakes (pH 2.96–6.3) and primarily occurs at salinities of ~31–63 g L–1 (Pinder et al. 2005; Timms 2009b). Some records of C. trilobata at salinities of 160 and 240 g L–1 are probably of dead, brine-preserved individuals (Halse and McRae 2001) and therefore overestimate the upper salinity tolerance of this species (Halse and McRae 2001).
Life history. Calamoecia, like most salt lake crustaceans, produces two types of eggs: (1) subitaneous eggs that maintain populations during favourable conditions; and (2) resistant resting eggs that allow populations to persist through unfavourable environmental conditions (Whitehead 2005). These resting eggs are likely important for dispersal. Waterbirds are considered the most likely vector for dispersal (Whitehead 2005), although water or human movement may also be important (Maly et al. 1997). Population genetic data for C. clitellata suggest that gene flow is restricted between regions and drainage basins, and sometimes even on fine spatial scales, and therefore populations are typically isolated and self-sustaining (Whitehead 2005). However, occasional dispersal may have significant effects on evolutionary timescales, resulting in secondary contact between highly divergent lineages (Whitehead 2005).
Trophic ecology. Apart from reports that Calamoecia feed on microalgae (Whitehead 2005) and are preyed upon by A. insularis and potentially other giant ostracods (Campbell 1995), little is known of the trophic ecology of the salt lake calanoids.
Cyclopoidea
In Australian salt lakes, cyclopoids are represented by three endemic species from two genera, as well as the cosmopolitan halophiles Apocyclops dengizicus (Lepeshkin) and Pescecyclops arnaudi (Sars) (Anufriieva 2015). However, the taxonomy of these copepods is confused, due, in part, to a series of name changes (Table 6) and to reports of a significant number of undescribed halophilic species from throughout Australia (De Deckker and Geddes 1980; Brock and Shiel 1983; Williams and Kokkinn 1988; Williams et al. 1990; Timms 1993; Timms and Boulton 2001; Pinder et al. 2005). It is impossible to meaningfully review the biology of these species until the taxonomy is resolved.
|
Harpacticoidea
The harpacticoid genera Mesochra and Schizopera occur in Australian salt lakes, but only one described species, M. baylyi, is known to be halophilic (Hamond 1971; De Deckker and Geddes 1980; Brock and Shiel 1983). Reports of other harpacticoid species from Australian salt lakes are either of undescribed species or of marine and estuarine species that have colonised permanent or semi-permanent coastal salt lakes (Bayly 1970; Hamond 1971; Timms 1993, 2001, 2009b; Pinder et al. 2005). M. baylyi is widely distributed in southern Australia (south-western Western Australia, south-eastern South Australia, Victoria and Tasmania) and has been reported in waters with salinities up to 129 g L–1, but is typically reported in waters with salinities below 25 g L–1 (De Deckker and Geddes 1980; De Deckker and Williams 1982; Pinder et al. 2005). It is one of only a few halophilic species suggested to have colonised salt lakes directly from the marine environment (Williams 1981).
Isopoda
The oniscoid Haloniscus searlei Chilton is the only described halophilic isopod that occurs in Australia, although recent molecular phylogenetic analyses have suggested that the diversity of this genus is underappreciated (Guzik et al. 2019). The putative new species (revealed by molecular data) are mostly from groundwater, freshwater or semiterrestrial environments, but at least one is from salt lakes (Cooper et al. 2008; Stringer et al. 2019).
H. searlei sensu lato is geographically widespread, occurring in seasonal and permanent salt lakes in Western Australia, South Australia, Victoria and Tasmania (Williams 1983; Guzik et al. 2019). It is a powerful osmoregulator commonly found in salinities from 3.6 to 161 g L–1 (Williams 1983). It primarily grazes on diatoms, but has been noted to occasionally consume chironomid larvae (Blinn et al. 1989). This species is unusual among halophilic crustaceans because it has colonised the salt lakes via a terrestrial rather than freshwater ancestor (Ellis and Williams 1970). Related to this is the fact that it survives dry lake periods as an adult rather than with resistant eggs (Williams 1983) and appears unable to colonise lakes that only fill episodically (Williams and Kokkinn 1988; Timms 2008).
Halophilic biota: Gastropoda
Coxiella
All halophilic gastropods in Australian salt lakes belong to the endemic genus Coxiella, the only gastropod genus in the world to consist exclusively of halophilic species. Coxiella is currently placed within the family Pomatiopsidae, which mainly contains freshwater species (Wilke 2019). However, molecular phylogenetic analysis suggests that Coxiella and Tomichia, a morphologically similar genus from Africa that includes freshwater, brackish and halophilic species, may merit recognition as a separate family (Tomichiidae; Wilke et al. 2013). Despite the ubiquity and abundance of Coxiella in Australian salt lakes, the genus is poorly studied (Williams and Mellor 1991; Pinder et al. 2002).
Taxonomy
The taxonomy of Coxiella has effectively remained unchanged since it was reviewed by Macpherson (1957), who listed 10 species. Kendrick (1978) added one further species (Coxiella roeae), known only from fossil material from Western Australia. Coxiella badgerensis (Johnston) has since been synonymised with Coxiella striata (Reeve) (Smith 1979), and Coxiella molesta Iredale and Coxiella minima Macpherson have not been collected since they were initially described (for a discussion about C. molesta, see Bayly and Williams 1966). Currently, nine extant species in the two subgenera Coxiella (eight species) and Coxielladda (one species) are recognised (Davis 1979; Wilke 2019), with seven species having been collected in recent decades. The discussion below focuses on these seven species.
Current species descriptions are based on external shell and operculum characters. Subsequent studies have found single specimens that exhibit the diagnostic morphological characteristics of more than one species (De Deckker and Geddes 1980; Williams and Mellor 1991) or have suggested the existence of undescribed species (Pinder et al. 2005; Timms 2009b). In addition, operculum characters have been found to vary with salinity (De Deckker and Geddes 1980) and size (Williams and Mellor 1991). Currently, researchers are unable to confidently identify Coxiella material to species level (Williams and Mellor 1991; Pinder et al. 2002; Timms 2009b). Owing to this inadequate taxonomy, all species-level information in this group, included that presented herein, must be considered tentative.
Ecology
Coxiella species are mostly found in southern Australia, including Tasmania, but also occur in some parts of central and southern Queensland (Williams and Mellor 1991; Timms 1998). Western Australia is the most diverse area for Coxiella, with six described species, including four that are endemic to this region: Coxiella striatula (Menke), Coxiella exposita (Iredale), Coxiella glabra Macpherson and Coxiella pyrrhostoma (Cox) (Table 7). Coxiella glauerti Macpherson occurs in both Western Australia and South Australia, whereas C. striata occurs in South Australia, Victoria and Tasmania, but not Western Australia (Table 7). Coxielladda gilesi (Angas) has been reported from remote inland lakes in Western Australia, South Australia and Queensland (Macpherson 1957; Timms 1998).

|
The geographical ranges of some Coxiella species overlap, but different species rarely appear to occur in the same waterbody, although there are reports of C. striata and C. glauerti co-occurring in lakes on the Eyre Peninsula (Timms 2009a). Weston (2007) identified four Coxiella species from bird faecal samples collected from a single lake in Western Australia, but the species identifications are questionable.
Coxiella are osmoconformers (Williams and Mellor 1991). Most field records of active individuals are from a salinity range between 10 and 70 g L–1; the lower and upper recorded ranges are 0.3 and 130 g L–1 (Geddes et al. 1981; Timms 1983; Williams et al. 1990; Williams and Mellor 1991; Pinder et al. 2005). These field data cannot always be accurately ascribed to particular species due to taxonomic uncertainties. Experimental data indicate that the adults of one species (possibly C. striata) from Lake Tallinga in South Australia have a broad salinity tolerance, with LD50 limits of 2 and 95.5 g L–1 for gradual acclimation, and 6 and 83 g L–1 for a direct transfer (Williams and Mellor 1991). The extent to which Coxiella species vary in salinity tolerance is not clear, but some (e.g. C. glauerti) seem to occur at higher salinities than others (e.g. C. striata; Timms 2009a; Timms et al. 2014).
Life history
The life histories of Coxiella species are poorly known. Species are dioecious and reproduction is sexual and probably iteroparous (Williams and Mellor 1991). Individuals are long-lived (compared with most halophilic invertebrates), possibly for as long as 2 years (Williams and Mellor 1991). Related to this, Coxiella, like H. searlei, aestivate as adults during dry lake periods in areas of high humidity (e.g. under plant or algal mats) or in plates of densely packed individuals to reduce desiccation (Williams 1985; Timms et al. 2014). Laboratory experiments suggest that the Coxiella from Lake Tallinga can aestivate for at least 2 months with little mortality (Williams and Mellor 1991), whereas aestivating specimens of C. striata have been reanimated from lakes known to have been dry for at least 3 months (De Deckker and Geddes 1980). The total length of time that individuals can survive aestivated, and whether this varies among species, is not known. However, it must be limited because Coxiella are found in permanent and seasonal lakes, but not in episodic ones (Williams 1998a).
Trophic ecology
The diets of Coxiella species have not been investigated directly, but it has been suggested that they feed on benthic algae or detritus (De Deckker 1982; Bayly 1993). Coxiella are known to be consumed by native fish (e.g. Galaxias maculatus (Jenyns), Atherinosoma microstoma (Günther), Pseudogobius olorum (Sauvage) and Philypnodon grandiceps (Krefft); Chessman and Williams 1987; Becker and Laurenson 2007) and introduced fish (e.g. Cyprinus carpio Linnaeus; Khan 2003) in some waterbodies. They are also an important food source for some waterbirds, such as the hooded plover Thinornis rubricollis in south-western Australia, where Coxiella has been found to make up to 90% of the diet (Weston 2007).
Discussion
This study synthesises available information on the halophilic macroinvertebrates of Australian salt lakes, focusing on crustaceans and gastropods. The results show that our understanding of the taxonomy and diversity of some groups has improved markedly since the main previous review of these organisms (De Deckker 1983b), as has our knowledge of aspects of the ecology and life histories of a small number of species, although many knowledge gaps remain. This progress and these gaps, and their conservation implications, are discussed below.
Unique biodiversity
This review highlights the evolutionary distinctiveness of the halophilic macroinvertebrates in Australian salt lakes. The fauna is dominated by crustaceans and molluscs, of which 1 family (Parartemiidae), 2 subfamilies (Diacypridinae and Mytilocypridinae), 11 genera (Parartemia, Extremalona, Australocypris, Repandocypris, Diacypris, Reticypris, Platycypris, Patcypris, Trilocypris, Meridiecyclops and Coxiella) and 74 of the 79 described species are endemic to these lakes. These high levels of endemism are likely due to a long association between the fauna and Australian salt lake environments or their precursors (De Deckker 1981b; Williams 1981). The number of macroscopic halophilic invertebrate species in Australian salt lakes is also higher than in lakes elsewhere in the world (Alonso 1990; Bayly 1993; Williams et al. 1995; De Los Rios-Escalante and Amarouayache 2016). In addition, many components of the Australian fauna are capable of tolerating extreme levels of salinity, and some have other unusual characteristics. For example, although ostracod species are usually absent from environments with pH <5 (because a lower pH inhibits formation of the calcite carapace; Ruiz et al. 2013), several species of ostracod occur in highly acidic salt lakes (Timms 2009b). By virtue of its distinctiveness, the invertebrate biodiversity of Australian salt lakes is of high conservation value.
Taxonomy
What we do not know, we cannot protect [Martens and Savatenalinton 2011].
In the past 20 years, considerable progress has been made regarding the discovery and description of species in a few groups of Australian salt lake invertebrates, notably Parartemia and giant (Mytilocypridinae) ostracods (Halse and McRae 2004; Timms and Hudson 2009; Timms 2010b). However, the taxonomy of other groups is much less advanced and in some cases (e.g. Coxiella gastropods and cyclopoid copepods) the current taxonomy does not allow consistent and accurate species identifications (Williams and Mellor 1991; Pinder et al. 2002). Many new species, and sometimes even new genera, have been discovered in those groups that have been subject to recent taxonomic revision, and more biodiversity likely remains to be discovered, especially in poorly studied groups and regions.
Molecular data have substantially changed our understanding of the diversity of many groups, such as Triops (Meusel and Schwentner 2017), Daphniopsis (Hebert and Wilson 2000), Parartemia (Remigio et al. 2001), Mytilocypris (Finston 2000, 2007), Branchinella (Pinceel et al. 2013a) and Haloniscus (Guzik et al. 2019). Nevertheless, the discovery and identification of Australian salt lake invertebrates is still overwhelmingly reliant on morphotaxonomy. Using molecular and morphological data to document the full extent of the diversity of halophilic invertebrates of Australian salt lakes is essential to progress our understanding and conservation of these ecosystems.
Ecology
The species diversity of halophilic invertebrates is highest in Western Australian semi-arid and arid regions. Therefore, this part of Australia is an important region for the conservation of these invertebrates, especially because the salt lakes here are experiencing exceptionally high levels of disturbance due to climate change, secondary salinisation and mining (Halse et al. 2003; Timms 2005; Nielsen and Brock 2009).
Conservation planning and assessment requires a sound knowledge of species’ distributions and how these are changing through time (Cardoso et al. 2011). This point is illustrated by attempts to assess the conservation status of Parartemia species, where concerns about P. contracta have decreased through time as more intensive sampling has revealed a range of additional populations while concerns about P. extracta have increased because temporal sampling suggests that the number of populations and the geographic range of this species are limited and shrinking (Timms et al. 2009; Pinder and Quinlan 2015).
Our knowledge of the geographic distribution of most halophilic invertebrates is incomplete. This problem is particularly acute for those species that are difficult to identify because either the existing taxonomy is incomplete or because they cannot be reliably identified using morphological characters (e.g. Coxiella). It is also problematic for species that are rare or occur in remote locations. In addition, some older distributional data may be misleading because they are based on outdated taxonomy (e.g. D. pusilla). Obtaining a comprehensive inventory of the distribution of halophilic invertebrates in Australian salt lakes is a challenging prospect, partly because there are many lakes that either occur in remote locations or hold water infrequently. Surveying some sites may benefit from a flexible sampling approach, such as raising individuals from egg banks (Timms 2012b). Analysis of environmental DNA in dry sediments could also become an important survey tool but requires a database of reference sequences and a well-developed taxonomy to be effective (Cristescu and Hebert 2018).
The available evidence indicates that most of the halophilic invertebrates in Australia are osmoregulators (e.g. Parartemia, giant and small ostracods, Daphniopsis), but some are osmoconformers (e.g. calanoid copepods and Coxiella). Regardless, most species appear to have broad and overlapping salinity tolerances and many are able to tolerate extremely high salinities, as described above. However, the bulk of information on salinity tolerances in these invertebrates is derived from scattered field data. Such data do not necessarily indicate the optimal conditions for reproduction or completing the lifecycle, which may be much more restrictive than the conditions adults can survive (Hammer 1986). Those species that can only reproduce or complete their lifecycle at low salinity, or within a narrow salinity range, are seemingly more vulnerable to increases or changes in the salinity of the lakes.
Lake Corangamite, a large permanent lake in western Victoria, provides a clear example of how increasing salinity can have a big effect on resident organisms. Due to the diversion of the main inflow creek dating back to the 1960s, water levels in this lake have fallen and salinity has increased, resulting in a change in the biota from one characteristic of moderate salinity (35–>50 g L–1) to one of higher salinity (50–100 g L–1; Williams 1995). Associated with this, populations of halophilic species have been lost from the lake (e.g. C. striata). Some salt lakes in central Western Australia and elsewhere are being subjected to the discharge of highly saline waste water from mining activities (Timms 2005; Timms et al. 2006). How these lakes are being affected by increased salt loads is unclear (Timms 2005) but, based on the Lake Corangamite example, community changes and local extinctions are possible.
Two interesting features of the distribution of the halophilic invertebrates are that: (1) some species are much more common and broadly distributed than others, even congeners; and (2) congeneric species rarely occur in the same lake, even though many have overlapping geographic and salinity distributions (see above). It is currently unknown whether the common and widespread species have relatively broader ecological tolerances, greater dispersal capacity or both (Williams 1984). The factors controlling the distribution of halophilic invertebrates are likely to be complex. The effects of salinity are correlated with other chemical and physical changes; for example, the availability of dissolved oxygen decreases as salinity (and temperature) increases (Williams 1998a). Thus, although the ecological tolerances of species are usually measured in terms of salinity, this is an oversimplification. Abiotic factors alone are unlikely to explain why congeneric species are rarely found in the same lake, although more intensive sampling and better taxonomy may lead to more examples of co-occurrence. Understanding the relative importance of various abiotic and biotic factors in controlling the distribution of the halophilic invertebrates is critical to predicting how species and communities will respond to perturbations and for developing effective interventions (Nielsen and Brock 2009).
Life history
Detailed life history information is typically available for one species for each of the main types (e.g. ostracod, cladoceran, gastropod) of halophilic macroinvertebrate in Australia. This information indicates that these invertebrates’ life histories are fundamentally similar to those of their relatives from other environments (Ellis and Williams 1970; Finston 2002; Ismail et al. 2011b). Most species have a rapid life cycle, with short-lived individuals. Multiple generations may be produced while environmental conditions remain favourable and desiccation-resistant eggs are used to survive dry or unfavourable conditions (Timms 2014). By contrast, H. searlei and Coxiella species survive dry or unfavourable periods as adults (Williams 1983; Williams and Mellor 1991). The total length of time that the aestivating adults can remain viable is not known, but those of C. striata can survive for at least 3 months (De Deckker and Geddes 1980) and those of H. searlei for at least 1 month depending on humidity (Williams 1983). This time is probably minimal compared with the duration that desiccation-resistant eggs of other taxa may remain viable (Williams and Kokkinn 1988). Consequently, Coxiella species and, particularly, H. searlei are excluded from waterbodies that dry for extended periods (Williams and Kokkinn 1988), and therefore seem especially vulnerable to a drying climate. Understanding the long-term viability and hatching cues for desiccation-resistant eggs, or the equivalent for the aestivating adults, and how these vary among species is crucial for assessing the capacity of species to survive as the climate dries.
It is generally believed that halophilic invertebrates in Australia mainly disperse via desiccation-resistant eggs (when present) and that aquatic birds and, to a lesser extent, wind and water flow are important dispersal vectors (Finston 2002; Green et al. 2008). Nevertheless, other than for population genetic studies of the copepod C. clitellata (Whitehead 2005) and several species of Mytilocypris ostracod (Finston 2002), the amount or pattern of dispersal in these invertebrates is unknown. Although it is unlikely that the amount of dispersal is enough to directly affect the population dynamics of a species, if the rate of local extinction is high, a species will need to have a ‘reasonable’ chance of dispersing into a suitable habitat in order to persist in the landscape.
Biological interactions
Information on biological interactions, such as predation and competition, in Australian salt lake communities is rudimentary (Timms 2021). This makes it difficult to assess the resilience of these systems to the loss of a species. Some waterbirds are known to prey on the invertebrates (Weston 2007; Pedler et al. 2018), although comprehensive data on the relationships between these predators and prey are lacking. It is possible that declines in the abundance and availability of the invertebrates (e.g. if salt lakes are dry for longer periods of time and are more saline) will affect the population dynamics of these birds (Senner et al. 2018). Fish predation may also be important at certain times in some waterbodies (Chessman and Williams 1987; Becker and Laurenson 2007), although fish are absent from most of the Australian salt lakes, which are typically either ephemeral or highly saline (De Deckker 1983b).
Competitive exclusion (Timms 2012a) may explain why congeneric species in a range of taxa (e.g. Parartemia, Australocypris and Coxiella) rarely co-occur in a single lake, although there is currently no evidence to support that competitive exclusion is more important than niche differentiation or dispersal limitation. The effect of the recent spread of A. parthenogenetica, and the potential (although not yet observed) spread of A. franciscana, on the native halophilic invertebrates is difficult to predict without detailed information on the ecological requirements of the native species (McMaster et al. 2007). However, it is worth noting that A. franciscana has out-competed and replaced native Artemia species on other continents (Ruebhart et al. 2008).
Conclusion
A very diverse and unique range of halophilic invertebrates inhabit Australian salt lakes. These invertebrates are threatened by a range of processes, the most significant of which are increasing periods of extensive drought and secondary salinisation over large parts of the Australian landscape. Populations, and potentially species, have already become extinct (Williams 1995; Timms et al. 2009; Timms 2012b) and, without further study, more may be lost without notice (Halse et al. 2003). It is currently difficult to properly evaluate the consequences of the threats due to critical gaps in our knowledge of the biology of the invertebrates. Future studies of Australian halophilic invertebrates should prioritise the following:
-
documenting the full extent of biodiversity in these invertebrates; this is the most critical gap to be filled because a sound taxonomy is needed to underpin all other biological research
-
developing a better understanding of species’ distributions and community composition, particularly in remote regions
-
ascertaining species’ tolerances to salinity and other physicochemical parameters across all stages of their lifecycle
-
elucidating the long-term viability and hatching cues for desiccation-resistant eggs, and the equivalent for aestivating adults, and how these vary among species
-
elucidating the abiotic and biotic factors that control species’ distributions, including why congeneric species rarely occur in the same lake despite overlapping geographic and salinity distributions
-
determining the patterns and mechanisms of dispersal and how these may influence the capacity of species to persist in the landscape
-
elucidating the biological relationships between species, including how the spread of Artemia in Australian salt lakes will affect native species
-
on a more holistic level, documenting the levels of anthropogenic stressors, including pollution and mining activities, on salt lake environments and identifying and preserving any key habitats that may serve as refugia for Australian halophilic invertebrates, particularly in the face of a drying climate.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Angus Lawrie was supported by a Commonwealth Supported Research Training Program while conducting this research.
Acknowledgements
The authors thank Brian Timms for graciously supplying some raw data, for general advice and for commenting on an earlier version of the manuscript. The authors also thank Patrick De Deckker and an anonymous reviewer for their insight and generous advice, which has helped improve the manuscript. The authors also thank Mahabubur Rahman for information about giant ostracods.
References
Adamowicz, S. J., Menu-Marque, S., Halse, S. A., Topan, J. C., Zemlak, T. S., Hebert, P. D., and Witt, J. D. (2010). The evolutionary diversification of the Centropagidae (Crustacea, Calanoida): a history of habitat shifts. Molecular Phylogenetics and Evolution 55, 418–430.| The evolutionary diversification of the Centropagidae (Crustacea, Calanoida): a history of habitat shifts.Crossref | GoogleScholarGoogle Scholar | 20005299PubMed |
Aladin, N., and Potts, W. (1995). Osmoregulatory capacity of the Cladocera. Journal of Comparative Physiology – B. Biochemical, Systemic, and Environmental Physiology 164, 671–683.
| Osmoregulatory capacity of the Cladocera.Crossref | GoogleScholarGoogle Scholar |
Aladin, N., and Potts, W. (1996). The osmoregulatory capacity of the Ostracoda. Journal of Comparative Physiology – B. Biochemical, Systemic, and Environmental Physiology 166, 215–222.
| The osmoregulatory capacity of the Ostracoda.Crossref | GoogleScholarGoogle Scholar |
Alonso, M. (1990). Anostraca, Cladocera and Copepoda of Spanish saline lakes. Hydrobiologia 197, 221–231.
| Anostraca, Cladocera and Copepoda of Spanish saline lakes.Crossref | GoogleScholarGoogle Scholar |
Angas, G. (1877). Descriptions of a new species of Bulimus from Western Australia, and of a Paludinella from Lake Eyre, South Australia. Proceedings of the Zoological Society of London 170–171.
Anufriieva, E. V. (2015). Do copepods inhabit hypersaline waters worldwide? A short review and discussion. Chinese Journal of Oceanology and Limnology 33, 1354–1361.
| Do copepods inhabit hypersaline waters worldwide? A short review and discussion.Crossref | GoogleScholarGoogle Scholar |
Asem, A., Eimanifar, A., Li, W., Wang, P.-Z., Brooks, S. A., and Wink, M. (2018). Phylogeography and population genetic structure of an exotic invasive brine shrimp, Artemia Leach, 1819 (Crustacea: Anostraca), in Australia. Australian Journal of Zoology 66, 307–316.
| Phylogeography and population genetic structure of an exotic invasive brine shrimp, Artemia Leach, 1819 (Crustacea: Anostraca), in Australia.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. (1962). Additions to the inland water genus Calamoecia (Copepoda: Calanoida). Marine and Freshwater Research 13, 252–264.
| Additions to the inland water genus Calamoecia (Copepoda: Calanoida).Crossref | GoogleScholarGoogle Scholar |
Bayly, I. (1970). Further studies on some saline lakes of south-east Australia. Marine and Freshwater Research 21, 117–130.
| Further studies on some saline lakes of south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. (1972). Salinity tolerance and osmotic behavior of animals in athalassic saline and marine hypersaline waters. Annual Review of Ecology and Systematics 3, 233–268.
| Salinity tolerance and osmotic behavior of animals in athalassic saline and marine hypersaline waters.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. (1976). The plankton of Lake Eyre. Marine and Freshwater Research 27, 661–665.
| The plankton of Lake Eyre.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. (1993). The fauna of athalassic saline waters in Australia and the Altiplano of South America: comparisons and historical perspectives. Hydrobiologia 267, 225–231.
| The fauna of athalassic saline waters in Australia and the Altiplano of South America: comparisons and historical perspectives.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. A. (1995). Distinctive aspects of the zooplankton of large lakes in Australasia, Antarctica and South America. Marine and Freshwater Research 46, 1109–1120.
| Distinctive aspects of the zooplankton of large lakes in Australasia, Antarctica and South America.Crossref | GoogleScholarGoogle Scholar |
Bayly, I. A., and Boxshall, G. A. (2009). An all-conquering ecological journey: from the sea, calanoid copepods mastered brackish, fresh, and athalassic saline waters. Hydrobiologia 630, 39–47.
| An all-conquering ecological journey: from the sea, calanoid copepods mastered brackish, fresh, and athalassic saline waters.Crossref | GoogleScholarGoogle Scholar |
Bayly, I., and Williams, W. D. (1966). Chemical and biological studies on some saline lakes of south-east Australia. Marine and Freshwater Research 17, 177–228.
| Chemical and biological studies on some saline lakes of south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Becker, A., and Laurenson, L. J. (2007). Seasonal and diel comparisons of the diets of four dominant fish species within the main channel and flood-zone of a small intermittently open estuary in south-eastern Australia. Marine and Freshwater Research 58, 1086–1095.
| Seasonal and diel comparisons of the diets of four dominant fish species within the main channel and flood-zone of a small intermittently open estuary in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Bekker, E. I., Karabanov, D. P., Galimov, Y. R., and Kotov, A. A. (2016). DNA barcoding reveals high cryptic diversity in the North Eurasian Moina species (Crustacea: Cladocera). PLoS One 11, e0161737.
| DNA barcoding reveals high cryptic diversity in the North Eurasian Moina species (Crustacea: Cladocera).Crossref | GoogleScholarGoogle Scholar | 27992559PubMed |
Benzie, J. (2005). The genus Daphnia (including Daphniopsis) (Anomopoda: Daphniidae). In ‘Guides to the Identification of the Microinvertebrates of the Continental Waters of the World’. (Ed. H. Dumont.) Vol. 21, pp. 32–38 (Backhuys Publishers: Leiden, Netherlands.)
Blinn, D., Blinn, S., and Bayly, I. (1989). Feeding ecology of Haloniscus searlei Chilton, an Oniscoid Isopod living in Athalassic Saline Waters. Marine and Freshwater Research 40, 295–301.
| Feeding ecology of Haloniscus searlei Chilton, an Oniscoid Isopod living in Athalassic Saline Waters.Crossref | GoogleScholarGoogle Scholar |
Blinn, D., Halse, S., Pinder, A., and Shiel, R. (2004). Diatom and micro-invertebrate communities and environmental determinants in the western Australian wheatbelt: a response to salinization. Hydrobiologia 528, 229–248.
| Diatom and micro-invertebrate communities and environmental determinants in the western Australian wheatbelt: a response to salinization.Crossref | GoogleScholarGoogle Scholar |
Brock, M. A., and Shiel, R. (1983). The composition of aquatic communities in saline wetlands in Western Australia. Hydrobiologia 105, 77–84.
| The composition of aquatic communities in saline wetlands in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cale, D., Halse, S., and Walker, C. (2004). Wetland monitoring in the Wheatbelt of south-west Western Australia: site descriptions, waterbird, aquatic invertebrate and groundwater data. Conservation Science Western Australia 5, 20–136.
Campbell, C. E. (1994). Seasonal zooplankton fauna of salt evaporation basins in South Australia. Marine and Freshwater Research 45, 199–208.
| Seasonal zooplankton fauna of salt evaporation basins in South Australia.Crossref | GoogleScholarGoogle Scholar |
Campbell, C. E. (1995). The influence of a predatory ostracod, Australocypris insularis, on zooplankton abundace and species composition in a saline lake. Hydrobiologia 302, 229–239.
| The influence of a predatory ostracod, Australocypris insularis, on zooplankton abundace and species composition in a saline lake.Crossref | GoogleScholarGoogle Scholar |
Cardoso, P., Erwin, T. L., Borges, P. A., and New, T. R. (2011). The seven impediments in invertebrate conservation and how to overcome them. Biological Conservation 144, 2647–2655.
| The seven impediments in invertebrate conservation and how to overcome them.Crossref | GoogleScholarGoogle Scholar |
Casanova, M. T. (2013). Lamprothamnium in Australia (Characeae, Charophyceae). Australian Systematic Botany 26, 268–290.
| Lamprothamnium in Australia (Characeae, Charophyceae).Crossref | GoogleScholarGoogle Scholar |
Chessman, B., and Williams, W. D. (1987). A note on the diet of Galaxias maculatus (Jenyns) (Pisces, Salmoniformes, Galaxiidae) in a closed saline lake in western Victoria. Bulletin of the Australian Society for Limnology 11, 43–46.
Clegg, J. S., and Campagna, V. (2006). Comparisons of stress proteins and soluble carbohydrate in encysted embryos of Artemia franciscana and two species of Parartemia. Comparative Biochemistry and Physiology – B. Biochemistry & Molecular Biology 145, 119–125.
| Comparisons of stress proteins and soluble carbohydrate in encysted embryos of Artemia franciscana and two species of Parartemia.Crossref | GoogleScholarGoogle Scholar |
Colbourne, J., Wilson, C., and Hebert, P. (2006). The systematics of Australian Daphnia and Daphniopsis (Crustacea: Cladocera): a shared phylogenetic history transformed by habitat-specific rates of evolution. Biological Journal of the Linnean Society. Linnean Society of London 89, 469–488.
| The systematics of Australian Daphnia and Daphniopsis (Crustacea: Cladocera): a shared phylogenetic history transformed by habitat-specific rates of evolution.Crossref | GoogleScholarGoogle Scholar |
Conte, F. P., and Geddes, M. C. (1988). Acid brine shrimp: metabolic strategies in osmotic and ionic adaptation. Hydrobiologia 158, 191–200.
| Acid brine shrimp: metabolic strategies in osmotic and ionic adaptation.Crossref | GoogleScholarGoogle Scholar |
Cooper, S. J., Saint, K. M., Taiti, S., Austin, A. D., and Humphreys, W. F. (2008). Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia. Invertebrate Systematics 22, 195–203.
| Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cotton, B. C. (1942). Australian Gastropoda of the families Hydrobiidae, Assimineidae and Acmeidae. Transactions of the Royal Society of South Australia 66, 124–129.
Cox, J. C. (1868). ‘A Monograph of Australian Land Shells.’ (W. Maddock: Sydney, NSW, Australia.)
Cristescu, M. E., and Hebert, P. D. (2018). Uses and misuses of environmental DNA in biodiversity science and conservation. Annual Review of Ecology, Evolution, and Systematics 49, 209–230.
| Uses and misuses of environmental DNA in biodiversity science and conservation.Crossref | GoogleScholarGoogle Scholar |
Davis, G. (1979). ‘Origin and Evolution of the Gastropod Family Pomatiopsidae, with Emphasis on the Mekong River Triculinae.’ (Allen Press: Lawrence, KS, USA.)
De Deckker, P. (1977). The distribution of the ‘Giant’ ostracods Family: Cyprididae (Baird 1845) endemic to Australia. In ‘Sixth International Ostracod Symposium’. pp. 285–294. (Junk: The Hague, Netherlands.)
De Deckker, P. (1978). Comparative morphology and review of mytilocyprinid ostracods (Family Cyprididae). Australian Journal of Zoology Supplementary Series 26, 1–62.
| Comparative morphology and review of mytilocyprinid ostracods (Family Cyprididae).Crossref | GoogleScholarGoogle Scholar |
De Deckker, P. (1979). Ostracods from the mound springs area between Strangways and Curdimurka, South Australia. Transactions of the Royal Society of South Australia 103, 155–168.
De Deckker, P. (1981a). Taxonomy and ecology notes of some ostracods from Australian inland waters. Transactions of the Royal Society of South Australia 105, 91–138.
De Deckker, P. (1981b). Ostracods of athalassic saline lakes. Hydrobiologia 81, 131–144.
| Ostracods of athalassic saline lakes.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P. (1981c). Taxonomic notes on some Australian ostracods with description of new species. Zoologica Scripta 10, 37–55.
| Taxonomic notes on some Australian ostracods with description of new species.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P. (1981d). On Reticypris pinguis De Decker sp. nov. Stereo-Atlas of Ostracod Shells 8, 93–100.
De Deckker, P. (1982). Holocene ostracods, other invertebrates and fish remains from cores of four maar lakes in southeastern Australia. Proceedings of the Royal Society of Victoria 94, 183–220.
De Deckker, P. (1983a). Notes on the ecology and distribution of non-marine ostracods in Australia. Hydrobiologia 106, 223–234.
| Notes on the ecology and distribution of non-marine ostracods in Australia.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P. (1983b). Australian salt lakes: their history, chemistry, and biota – a review. Hydrobiologia 105, 231–244.
| Australian salt lakes: their history, chemistry, and biota – a review.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P. (2002). Ostracod palaeoecology. In ‘The Ostracoda: Applications in Quaternary Research’. (Eds J. Holmes and A. Chivas.) Vol. 131, pp. 121–134. (American Geophysical Union: Washington, DC, USA.)
| Crossref |
De Deckker, P., and Geddes, M. (1980). Seasonal fauna of ephemeral saline lakes near the Coorong Lagoon, South Australia. Marine and Freshwater Research 31, 677–699.
| Seasonal fauna of ephemeral saline lakes near the Coorong Lagoon, South Australia.Crossref | GoogleScholarGoogle Scholar |
De Deckker, P., and Williams, W. D. (1982). Chemical and biological features of Tasmanian salt lakes. Marine and Freshwater Research 33, 1127–1132.
| Chemical and biological features of Tasmanian salt lakes.Crossref | GoogleScholarGoogle Scholar |
De Los Rios-Escalante, P., and Amarouayache, M. (2016). Crustacean zooplankton assemblages in Algerian saline lakes: a comparison with their Chilean Altiplano counterparts. Crustaceana 89, 1485–1500.
| Crustacean zooplankton assemblages in Algerian saline lakes: a comparison with their Chilean Altiplano counterparts.Crossref | GoogleScholarGoogle Scholar |
De Stasio, B. T. (1989). The seed bank of a freshwater crustacean: copepodology for the plant ecologist. Ecology 70, 1377–1389.
| The seed bank of a freshwater crustacean: copepodology for the plant ecologist.Crossref | GoogleScholarGoogle Scholar |
Doupe, R., and Horwitz, P. (1995). The value of macroinvertebrate assemblages for determining priorities in wetland rehabilitation: a case study from Lake Toolibin, Western Australia. Journal of the Royal Society of Western Australia 78, 33–38.
Edward, D. (1983). Inland waters of Rottnest Island. Journal of the Royal Society of Western Australia 66, 41–47.
Ellis, P., and Williams, W. (1970). The biology of Haloniscus searlei Chilton, an oniscoid isopod living in Australian salt lakes. Marine and Freshwater Research 21, 51–70.
| The biology of Haloniscus searlei Chilton, an oniscoid isopod living in Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Fiers, F. (2001). Meridiecyclops, gen. nov., a new cyclopid genus (Crustacea: Copepoda: Cyclopidae) from southern Australia. Invertebrate Systematics 15, 893–908.
| Meridiecyclops, gen. nov., a new cyclopid genus (Crustacea: Copepoda: Cyclopidae) from southern Australia.Crossref | GoogleScholarGoogle Scholar |
Finston, T. (2000). Morphology and molecules conflict to confound species boundaries in salt lake ostracodes of the genus Mytilocypris (Crustacea: Ostracoda). Australian Journal of Zoology 48, 393–409.
| Morphology and molecules conflict to confound species boundaries in salt lake ostracodes of the genus Mytilocypris (Crustacea: Ostracoda).Crossref | GoogleScholarGoogle Scholar |
Finston, T. (2002). Geographic patterns of population genetic structure in Mytilocypris (Ostracoda: Cyprididae): interpreting breeding systems, gene flow and history in species with differing distributions. Molecular Ecology 11, 1931–1946.
| Geographic patterns of population genetic structure in Mytilocypris (Ostracoda: Cyprididae): interpreting breeding systems, gene flow and history in species with differing distributions.Crossref | GoogleScholarGoogle Scholar | 12296938PubMed |
Finston, T. (2004). Effect of a temporally heterogeneous environment on size and shape of the giant ostracods Mytilocypris (Ostracoda: Cyprididae) from Australian salt lakes. Marine and Freshwater Research 55, 499–507.
| Effect of a temporally heterogeneous environment on size and shape of the giant ostracods Mytilocypris (Ostracoda: Cyprididae) from Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Finston, T. (2007). Size, shape and development time are plastic traits in salt lake ostracods of the Mytilocypris mytiloides (Ostracoda: Cyprididae) species complex. Marine and Freshwater Research 58, 511–518.
| Size, shape and development time are plastic traits in salt lake ostracods of the Mytilocypris mytiloides (Ostracoda: Cyprididae) species complex.Crossref | GoogleScholarGoogle Scholar |
Geddes, M. (1973). A new species of Parartemia (Anostraca) from Australia. Crustaceana 25, 5–12.
| A new species of Parartemia (Anostraca) from Australia.Crossref | GoogleScholarGoogle Scholar |
Geddes, M. (1976). Seasonal fauna of some ephemeral saline waters in western Victoria with particular reference to Parartemia zietziana Sayce (Crustacea: Anostraca). Marine and Freshwater Research 27, 1–22.
| Seasonal fauna of some ephemeral saline waters in western Victoria with particular reference to Parartemia zietziana Sayce (Crustacea: Anostraca).Crossref | GoogleScholarGoogle Scholar |
Geddes, M. (1981). The brine shrimps Artemia and Parartemia: comparative physiology and distribution in Australia. Hydrobiologia 81–82, 169–179.
| The brine shrimps Artemia and Parartemia: comparative physiology and distribution in Australia.Crossref | GoogleScholarGoogle Scholar |
Geddes, M., De Deckker, P., Williams, W. D., Morton, D., and Topping, M. (1981). On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia 81–82, 201–222.
| On the chemistry and biota of some saline lakes in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Gibson, J. A., and Bayly, I. A. (2007). New insights into the origins of crustaceans of Antarctic lakes. Antarctic Science 19, 157–164.
| New insights into the origins of crustaceans of Antarctic lakes.Crossref | GoogleScholarGoogle Scholar |
Green, A. J., Jenkins, K., Bell, D., Morris, P., and Kingsford, R. (2008). The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshwater Biology 53, 380–392.
Guzik, M. T., Stringer, D. N., Murphy, N. P., Cooper, S. J., Taiti, S., King, R. A., Humphreys, W. F., and Austin, A. D. (2019). Molecular phylogenetic analysis of Australian arid-zone oniscidean isopods (Crustacea: Haloniscus) reveals strong regional endemicity and new putative species. Invertebrate Systematics 33, 556–574.
Halse, S. (1981). Faunal assemblages of some saline lakes near Marchagee, Western Australia. Marine and Freshwater Research 32, 133–142.
| Faunal assemblages of some saline lakes near Marchagee, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, S. (2002). Diversity of Ostracoda (Crustacea) in inland waters of Western Australia. Internationale Vereinigung für theoretische und angewandte Limnologie, Verhandlungen 28, 914–918.
| Diversity of Ostracoda (Crustacea) in inland waters of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, S. A., and Martens, K. (2019). Four new genera and five new species of ‘Heterocypris’ from Western Australia (Crustacea, Ostracoda, Cyprinotinae). European Journal of Taxonomy 493, 1–35.
| Four new genera and five new species of ‘Heterocypris’ from Western Australia (Crustacea, Ostracoda, Cyprinotinae).Crossref | GoogleScholarGoogle Scholar |
Halse, S., and McRae, J. (2001). Calamoecia trilobata n sp (Copepoda: Calanoida) from salt lakes in southwestern Australia. Journal of the Royal Society of Western Australia 84, 5–11.
Halse, S., and McRae, J. (2004). New genera and species of ‘giant’ ostracods (Crustacea: Cyprididae) from Australia. Hydrobiologia 524, 1–52.
| New genera and species of ‘giant’ ostracods (Crustacea: Cyprididae) from Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, S., Shiel, R., and Williams, W. (1998). Aquatic invertebrates of Lake Gregory, northwestern Australia, in relation to salinity and ionic composition. Hydrobiologia 381, 15–29.
| Aquatic invertebrates of Lake Gregory, northwestern Australia, in relation to salinity and ionic composition.Crossref | GoogleScholarGoogle Scholar |
Halse, S., Shiel, R., Storey, A., Edward, D., Lansbury, I., Cale, D., and Harvey, M. (2000a). Aquatic invertebrates and waterbirds of wetlands and rivers of the southern Carnarvon Basin, Western Australia. Records of the Western Australian Museum 61, 217–265.
| Aquatic invertebrates and waterbirds of wetlands and rivers of the southern Carnarvon Basin, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Halse, S., Pearson, G., McRae, J., and Shiel, R. (2000b). Monitoring aquatic invertebrates and waterbirds at Toolibin and Walbyring Lakes in the Western Australian wheatbelt. Journal of the Royal Society of Western Australia 83, 17–28.
Halse, S., Ruprecht, J., and Pinder, A. (2003). Salinisation and prospects for biodiversity in rivers and wetlands of south-west Western Australia. Australian Journal of Botany 51, 673–688.
| Salinisation and prospects for biodiversity in rivers and wetlands of south-west Western Australia.Crossref | GoogleScholarGoogle Scholar |
Hammer, U. T. (1986). ‘Saline Lake Ecosystems of the World.’ (W. Junk: Dordrecht, Netherlands.)
Hamond, R. (1971). The Australian species of Mesochra (Crustacea: Harpacticoida), with a comprehensive key to the genus. Australian Journal of Zoology Supplementary Series 19, 1–32.
| The Australian species of Mesochra (Crustacea: Harpacticoida), with a comprehensive key to the genus.Crossref | GoogleScholarGoogle Scholar |
He, Z., Qin, J., Wang, Y., Jiang, H., and Wen, Z. (2001). Biology of Moina mongolica (Moinidae, Cladocera) and perspective as live food for marine fish larvae. Hydrobiologia 457, 25–37.
| Biology of Moina mongolica (Moinidae, Cladocera) and perspective as live food for marine fish larvae.Crossref | GoogleScholarGoogle Scholar |
Hebert, P. D., and Wilson, C. C. (2000). Diversity of the genus Daphniopsis in the saline waters of Australia. Canadian Journal of Zoology 78, 794–808.
| Diversity of the genus Daphniopsis in the saline waters of Australia.Crossref | GoogleScholarGoogle Scholar |
Hebert, P. D., Remigio, E. A., Colbourne, J. K., Taylor, D. J., and Wilson, C. C. (2002). Accelerated molecular evolution in halophilic crustaceans. Evolution 56, 909–926.
| Accelerated molecular evolution in halophilic crustaceans.Crossref | GoogleScholarGoogle Scholar | 12093027PubMed |
Hughes, L. (2003). Climate change and Australia: trends, projections and impacts. Austral Ecology 28, 423–443.
| Climate change and Australia: trends, projections and impacts.Crossref | GoogleScholarGoogle Scholar |
Iredale, T. (1943). A basic list of the fresh water Mollusca of Australia. Australian Zoologist 10, 188–230.
Ismail, H. N., Qin, J. G., and Seuront, L. (Eds) (2010a). Thermal and halo tolerance of a brackish cladoceran Daphniopsis australis (Sergeev & Williams). New Oceanography Research Developments: Marine Chemistry, Ocean Floor Analyses and Marine Phytoplankton. (Nova Science Publisher: New York, NY, USA.)
Ismail, H. N., Qin, J. G., Seuront, L., and Adams, M. (2010b). Impacts of male and food density on female performance in the brackish cladoceran Daphniopsis australis. Hydrobiologia 652, 277–288.
| Impacts of male and food density on female performance in the brackish cladoceran Daphniopsis australis.Crossref | GoogleScholarGoogle Scholar |
Ismail, H. N., Qin, J. G., and Seuront, L. (2011a). Dietary responses of the brackish cladoceran Daphniopsis australis fed on different algal species. Journal of Experimental Marine Biology and Ecology 409, 275–282.
| Dietary responses of the brackish cladoceran Daphniopsis australis fed on different algal species.Crossref | GoogleScholarGoogle Scholar |
Ismail, H. N., Qin, J. G., and Seuront, L. (2011b). Regulation of life history in the brackish cladoceran, Daphniopsis australis (Sergeev and Williams, 1985) by temperature and salinity. Journal of Plankton Research 33, 763–777.
| Regulation of life history in the brackish cladoceran, Daphniopsis australis (Sergeev and Williams, 1985) by temperature and salinity.Crossref | GoogleScholarGoogle Scholar |
Karanovic, I. (2008). Three interesting Cyprididae (Ostracoda) from Western Australia. Records of the Western Australian Museum 24, 267–287.
| Three interesting Cyprididae (Ostracoda) from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Karanovic, I. (2012). Superfamily Cypridoidea Baird 1845 (Suborder Cypridocopina Jones 1901). In ‘Recent Freshwater Ostracods of the World’. pp. 175–564. (Springer: Berlin, Germany.)
Karanovic, T., Eberhard, S., and Murdoch, A. (2011). A cladistic analysis and taxonomic revision of Australian Metacyclops and Goniocyclops, with description of four new species and three new genera (Copepoda, Cyclopoida). Crustaceana 84, 1–67.
| A cladistic analysis and taxonomic revision of Australian Metacyclops and Goniocyclops, with description of four new species and three new genera (Copepoda, Cyclopoida).Crossref | GoogleScholarGoogle Scholar |
Kendrick, G. W. (1978). New species of fossil nonmarine molluscs from Western Australia and evidence of late Quaternary climatic change in the Shark Bay district. Journal of the Royal Society of Western Australia 60, 49–60.
Khan, T. A. (2003). Dietary studies on exotic carp (Cyprinus carpio) from two lakes of western Victoria, Australia. Aquatic Sciences 65, 272–286.
| Dietary studies on exotic carp (Cyprinus carpio) from two lakes of western Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Kokkinn, M. J., and Williams, W. D. (1987). Is ephippial morphology a useful taxonomic descriptor in the Cladocera? An examination based on a study of Daphniopsis (Daphniidae) from Australian salt lakes. Hydrobiologia 145, 67–73.
| Is ephippial morphology a useful taxonomic descriptor in the Cladocera? An examination based on a study of Daphniopsis (Daphniidae) from Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Linder, F. (1941). Contributions to the morphology and the taxonomy of the Branchiopoda Anostraca. Zoologische Beiträge aus Uppsala 20, 101–302.
Lyons, M., Gibson, N., Keighery, G., and Lyons, S. (2004). Wetland flora and vegetation of the Western Australian Wheatbelt. Records of the Western Australian Museum 67, 39–89.
| Wetland flora and vegetation of the Western Australian Wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Macpherson, J. (1957). A review of the genus Coxiella Smith, 1894, sensu lato. Western Australian Naturalist 5, 191–204.
Maly, E. J. (1996). A review of relationships among centropagid copepod genera and some species found in Australasia. Crustaceana 69, 727–733.
| A review of relationships among centropagid copepod genera and some species found in Australasia.Crossref | GoogleScholarGoogle Scholar |
Maly, E. J., Halse, S. A., and Maly, M. P. (1997). Distribution and incidence patterns of Boeckella, Calamoecia, and Hemiboeckella (Copepoda: Calanoida) in Western Australia. Marine and Freshwater Research 48, 615–621.
| Distribution and incidence patterns of Boeckella, Calamoecia, and Hemiboeckella (Copepoda: Calanoida) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Manwell, C. (1978). Haemoglobin in the Australian anostracan Parartemia zietziana: evolutionary strategies of conformity vs regulation. Comparative Biochemistry and Physiology – A. Physiology 59, 37–44.
| Haemoglobin in the Australian anostracan Parartemia zietziana: evolutionary strategies of conformity vs regulation.Crossref | GoogleScholarGoogle Scholar |
Marchant, R., and Williams, W. D. (1977a). Population dynamics and production of a brine shrimp, Parartemia zietziana Sayce (Crustacea: Anostraca), in two salt lakes in western Victoria, Australia. Marine and Freshwater Research 28, 417–438.
| Population dynamics and production of a brine shrimp, Parartemia zietziana Sayce (Crustacea: Anostraca), in two salt lakes in western Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Marchant, R., and Williams, W. D. (1977b). Field measurements of ingestion and assimilation for the Australian brine shrimp Parartemia zietziana Sayce (Crustacea: Anostraca). Australian Journal of Ecology 2, 379–390.
| Field measurements of ingestion and assimilation for the Australian brine shrimp Parartemia zietziana Sayce (Crustacea: Anostraca).Crossref | GoogleScholarGoogle Scholar |
Martens, K. (1985). Effects of temperature and salinity on postembryonic growth in Mytilocypris henricae (Chapman) (Crustacea, Ostracoda). Journal of Crustacean Biology 5, 258–272.
| Effects of temperature and salinity on postembryonic growth in Mytilocypris henricae (Chapman) (Crustacea, Ostracoda).Crossref | GoogleScholarGoogle Scholar |
Martens, K., and Savatenalinton, S. (2011). A subjective checklist of the recent, free-living, non-marine Ostracoda (Crustacea). Zootaxa 2855, 1–79.
| A subjective checklist of the recent, free-living, non-marine Ostracoda (Crustacea).Crossref | GoogleScholarGoogle Scholar |
McCloud, C. L., Ismail, H. N., and Seuront, L. (2018). Cue hierarchy in the foraging behaviour of the brackish cladoceran Daphniopsis australis. Journal of Oceanology and Limnology 36, 2050–2060.
| Cue hierarchy in the foraging behaviour of the brackish cladoceran Daphniopsis australis.Crossref | GoogleScholarGoogle Scholar |
McMaster, K., Savage, A., Finston, T., Johnson, M. S., and Knott, B. (2007). The recent spread of Artemia parthenogenetica in Western Australia. Hydrobiologia 576, 39–48.
| The recent spread of Artemia parthenogenetica in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Menke, K. (1843). ‘Molluscorum Novae Hollandiae Specimen.’ (Libraria Aulica Hahniana: Hannover, Germany.)
Mernagh, T., Bastrakov, E., Jaireth, S., de Caritat, P., English, P., and Clarke, J. (2016). A review of Australian salt lakes and associated mineral systems. Australian Journal of Earth Sciences 63, 131–157.
| A review of Australian salt lakes and associated mineral systems.Crossref | GoogleScholarGoogle Scholar |
Meusel, F., and Schwentner, M. (2017). Molecular and morphological delimitation of Australian Triops species (Crustacea: Branchiopoda: Notostraca) – large diversity and little morphological differentiation. Organisms, Diversity & Evolution 17, 137–156.
| Molecular and morphological delimitation of Australian Triops species (Crustacea: Branchiopoda: Notostraca) – large diversity and little morphological differentiation.Crossref | GoogleScholarGoogle Scholar |
Morton, D., and Bayly, I. (1977). Studies on the ecology of some temporary freshwater pools in Victoria with special reference to microcrustaceans. Marine and Freshwater Research 28, 439–454.
| Studies on the ecology of some temporary freshwater pools in Victoria with special reference to microcrustaceans.Crossref | GoogleScholarGoogle Scholar |
Murugan, G., Obregón-Barboza, H., Maeda-Martínez, A. M., and Timms, B. V. (2009). Co-occurrence of two tadpole shrimp, Triops cf. australiensis (Branchiopoda: Notostraca), lineages in middle Paroo, north-western New South Wales, with the first record of Triops hermaphrodites for the Australian continent. Australian Journal of Zoology 57, 77–84.
| Co-occurrence of two tadpole shrimp, Triops cf. australiensis (Branchiopoda: Notostraca), lineages in middle Paroo, north-western New South Wales, with the first record of Triops hermaphrodites for the Australian continent.Crossref | GoogleScholarGoogle Scholar |
Naganawa, H., and Mura, G. (2017). Two new cryptic species of Artemia (Branchiopoda, Anostraca) from Mongolia and the possibility of invasion and disturbance by the aquaculture industry in East Asia. Crustaceana 90, 1679–1698.
| Two new cryptic species of Artemia (Branchiopoda, Anostraca) from Mongolia and the possibility of invasion and disturbance by the aquaculture industry in East Asia.Crossref | GoogleScholarGoogle Scholar |
Nielsen, D. L., and Brock, M. A. (2009). Modified water regime and salinity as a consequence of climate change: prospects for wetlands of Southern Australia. Climatic Change 95, 523–533.
| Modified water regime and salinity as a consequence of climate change: prospects for wetlands of Southern Australia.Crossref | GoogleScholarGoogle Scholar |
Pedler, R., Ribot, R., and Bennett, A. (2014). Extreme nomadism in desert waterbirds: flights of the banded stilt. Biology Letters 10, 20140547.
| Extreme nomadism in desert waterbirds: flights of the banded stilt.Crossref | GoogleScholarGoogle Scholar | 25319819PubMed |
Pedler, R. D., Ribot, R. F., and Bennett, A. T. (2018). Long‐distance flights and high‐risk breeding by nomadic waterbirds on desert salt lakes. Conservation Biology 32, 216–228.
| Long‐distance flights and high‐risk breeding by nomadic waterbirds on desert salt lakes.Crossref | GoogleScholarGoogle Scholar | 28981964PubMed |
Pinceel, T., Brendonck, L., Larmuseau, M. H., Vanhove, M. P., Timms, B. V., and Vanschoenwinkel, B. (2013a). Environmental change as a driver of diversification in temporary aquatic habitats: does the genetic structure of extant fairy shrimp populations reflect historic aridification? Freshwater Biology 58, 1556–1572.
| Environmental change as a driver of diversification in temporary aquatic habitats: does the genetic structure of extant fairy shrimp populations reflect historic aridification?Crossref | GoogleScholarGoogle Scholar |
Pinceel, T., Vanschoenwinkel, B., Waterkeyn, A., Vanhove, M. P., Pinder, A., Timms, B. V., and Brendonck, L. (2013b). Fairy shrimps in distress: a molecular taxonomic review of the diverse fairy shrimp genus Branchinella (Anostraca: Thamnocephalidae) in Australia in the light of ongoing environmental change. Hydrobiologia 700, 313–327.
| Fairy shrimps in distress: a molecular taxonomic review of the diverse fairy shrimp genus Branchinella (Anostraca: Thamnocephalidae) in Australia in the light of ongoing environmental change.Crossref | GoogleScholarGoogle Scholar |
Pinder, A., and Quinlan, K. (2015). Aquatic invertebrate communities of wetlands along the Jurien coast of Western Australia. Journal of the Royal Society of Western Australia 98, 69–88.
Pinder, A. M., Halse, S. A., Shiel, R. J., Cale, D. J., and McRae, J. M. (2002). Halophile aquatic invertebrates in the wheatbelt region of south-western Australia. Internationale Vereinigung für theoretische und angewandte Limnologie, Verhandlungen 28, 1687–1694.
| Halophile aquatic invertebrates in the wheatbelt region of south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Pinder, A. M., Halse, S. A., McRae, J. M., and Shiel, R. J. (2005). Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 543, 1–24.
| Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity.Crossref | GoogleScholarGoogle Scholar |
Pinder, A., Halse, S., Shiel, R., and McRae, J. (2010). An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia. Records of the Western Australian Museum 78, 205–246.
| An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Pinder, A., Quinlan, K., Cale, D., and Leung, A. (2012). Aquatic invertebrates of the Hutt River and Hutt Lagoon catchments, Western Australia. Journal of the Royal Society of Western Australia 95, 29–51.
Porter, J. L. (2007). Contrasting emergence patterns of Lamprothamnium macropogon (Characeae, Charophyceae) and Ruppia tuberosa (Potamogetonaceae) from arid-zone saline wetlands in Australia. Charophytes 1, 19–27.
Quinlan, K., Pinder, A., Coppen, R., and Jackson, J. (2016). An opportunistic survey of aquatic invertebrates in the Goldfields region of Western Australia. Conservation Science Western Australia 10, 1–21.
Reeve, L. (1842) ‘Conchologia systematica, or Complete System of Conchology: in which the Lepades and Conchiferous Mollusca are Described and Classified according to their Natural Organization and Habits.’ (Wiley and Putnam: New York, NY, USA.)
Remigio, E., Hebert, P., and Savage, A. (2001). Phylogenetic relationships and remarkable radiation in Parartemia (Crustacea: Anostraca), the endemic brine shrimp of Australia: evidence from mitochondrial DNA sequences. Biological Journal of the Linnean Society. Linnean Society of London 74, 59–71.
| Phylogenetic relationships and remarkable radiation in Parartemia (Crustacea: Anostraca), the endemic brine shrimp of Australia: evidence from mitochondrial DNA sequences.Crossref | GoogleScholarGoogle Scholar |
Rogers, D. C. (2013). Anostraca catalogus (Crustacea: Branchiopoda). The Raffles Bulletin of Zoology 61, 525–546.
Ruebhart, D. R., Cock, I. E., and Shaw, G. R. (2008). Invasive character of the brine shrimp Artemia franciscana Kellogg 1906 (Branchiopoda: Anostraca) and its potential impact on Australian inland hypersaline waters. Marine and Freshwater Research 59, 587–595.
| Invasive character of the brine shrimp Artemia franciscana Kellogg 1906 (Branchiopoda: Anostraca) and its potential impact on Australian inland hypersaline waters.Crossref | GoogleScholarGoogle Scholar |
Ruiz, F., Abad, M., Bodergat, A., Carbonel, P., Rodríguez-Lázaro, J., González-Regalado, M., Toscano, A., García, E., and Prenda, J. (2013). Freshwater ostracods as environmental tracers. International Journal of Environmental Science and Technology 10, 1115–1128.
| Freshwater ostracods as environmental tracers.Crossref | GoogleScholarGoogle Scholar |
Sánchez, M. I., Hortas, F., Figuerola, J., and Green, A. J. (2012). Comparing the potential for dispersal via waterbirds of a native and an invasive brine shrimp. Freshwater Biology 57, 1896–1903.
| Comparing the potential for dispersal via waterbirds of a native and an invasive brine shrimp.Crossref | GoogleScholarGoogle Scholar |
Säwström, C., Karlsson, J., Laybourn-Parry, J., and Granéli, W. (2009). Zooplankton feeding on algae and bacteria under ice in Lake Druzhby, East Antarctica. Polar Biology 32, 1195–1202.
| Zooplankton feeding on algae and bacteria under ice in Lake Druzhby, East Antarctica.Crossref | GoogleScholarGoogle Scholar |
Sayce, O. (1903). The phyllopods of Australia, including a description of some new genera and species. Proceedings of the Royal Society of Victoria 15, 224–261.
Schön, I., Halse, S., and Martens, K. (2017). Cyprideis (Crustacea, Ostracoda) in Australia. Journal of Micropalaeontology 36, 31–37.
| Cyprideis (Crustacea, Ostracoda) in Australia.Crossref | GoogleScholarGoogle Scholar |
Schwartz, S. S., and Hebert, P. D. (1984). Subgeneric distinction in the genus Daphnia: a new diagnostic trait. Transactions of the American Microscopical Society 103, 341–346.
| Subgeneric distinction in the genus Daphnia: a new diagnostic trait.Crossref | GoogleScholarGoogle Scholar |
Senner, N. R., Moore, J. N., Seager, S. T., Dougill, S., Kreuz, K., and Senner, S. E. (2018). A salt lake under stress: relationships among birds, water levels, and invertebrates at a Great Basin saline lake. Biological Conservation 220, 320–329.
| A salt lake under stress: relationships among birds, water levels, and invertebrates at a Great Basin saline lake.Crossref | GoogleScholarGoogle Scholar |
Sergeev, V. N. (1990a). The ephippial female of a new species of Daphniopsis Sars, 1903 (Anomopoda, Daphniidae) from Queensland, Australia. Crustaceana 59, 146–155.
| The ephippial female of a new species of Daphniopsis Sars, 1903 (Anomopoda, Daphniidae) from Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Sergeev, V. N. (1990b). A new species of Daphniopsis (Crustacea: Anomopoda: Daphniidae) from Australian salt lakes. Hydrobiologia 190, 1–7.
| A new species of Daphniopsis (Crustacea: Anomopoda: Daphniidae) from Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Sergeev, V. N., and Williams, W. D. (1983). Daphniopsis pusilla Serventy (Cladocera: Daphniidae), an important element in the fauna of Australian salt lakes. Hydrobiologia 100, 293–300.
| Daphniopsis pusilla Serventy (Cladocera: Daphniidae), an important element in the fauna of Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Sergeev, V. N., and Williams, W. D. (1985). Daphniopsis australis nov. sp. (Crustacea: Cladocera), a further daphniid in Australian salt lakes. Hydrobiologia 120, 119–128.
| Daphniopsis australis nov. sp. (Crustacea: Cladocera), a further daphniid in Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Sinev, A. Y., and Shiel, R. J. (2012). Extremalona timmsi gen. nov., sp. nov., a new cladoceran (Cladocera: Anomopoda: Chydoridae) from an acid saline lake in southwest Western Australia. Journal of Natural History 46, 2845–2864.
| Extremalona timmsi gen. nov., sp. nov., a new cladoceran (Cladocera: Anomopoda: Chydoridae) from an acid saline lake in southwest Western Australia.Crossref | GoogleScholarGoogle Scholar |
Smith, B. J. (1979). ‘Field Guide to the Non-Marine Molluscs of South Eastern Australia.’ (Australian National University Press: Canberra, ACT, Australia.)
Stringer, D. N., King, R. A., Taiti, S., Guzik, M. T., Cooper, S. J., and Austin, A. D. (2019). Systematics of Haloniscus Chilton, 1920 (Isopoda: Oniscidea: Philosciidae), with description of four new species from threatened Great Artesian Basin springs in South Australia. Journal of Crustacean Biology 39, 651–668.
| Systematics of Haloniscus Chilton, 1920 (Isopoda: Oniscidea: Philosciidae), with description of four new species from threatened Great Artesian Basin springs in South Australia.Crossref | GoogleScholarGoogle Scholar |
Sutherland, W. J., and Wordley, C. F. (2017). Evidence complacency hampers conservation. Nature Ecology & Evolution 1, 1215–1216.
| Evidence complacency hampers conservation.Crossref | GoogleScholarGoogle Scholar |
Taipale, S. J., Brett, M. T., Pulkkinen, K., and Kainz, M. J. (2012). The influence of bacteria-dominated diets on Daphnia magna somatic growth, reproduction, and lipid composition. FEMS Microbiology Ecology 82, 50–62.
| The influence of bacteria-dominated diets on Daphnia magna somatic growth, reproduction, and lipid composition.Crossref | GoogleScholarGoogle Scholar | 22564190PubMed |
Tang, D., and Knott, B. (2009). Freshwater cyclopoids and harpacticoids (Crustacea: Copepoda) from the Gnangara Mound region of Western Australia. Zootaxa 2029, 1–70.
| Freshwater cyclopoids and harpacticoids (Crustacea: Copepoda) from the Gnangara Mound region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Teller, J. T. (1987). The pink colour of lakes, with an example from Australia. Journal of Arid Environments 12, 101–103.
| The pink colour of lakes, with an example from Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1983). A study of benthic communities in some shallow saline lakes of western Victoria, Australia. Hydrobiologia 105, 165–177.
| A study of benthic communities in some shallow saline lakes of western Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1987). Limnology of Lake Buchanan, a tropical saline lake, and associated pools, of North Queensland. Marine and Freshwater Research 38, 877–884.
| Limnology of Lake Buchanan, a tropical saline lake, and associated pools, of North Queensland.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1993). Saline lakes of the Paroo, inland New South Wales, Australia. Hydrobiologia 267, 269–289.
| Saline lakes of the Paroo, inland New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (1998). A study of Lake Wyara, an episodically filled saline lake in southwest Queensland, Australia. International Journal of Salt Lake Research 7, 113–132.
| A study of Lake Wyara, an episodically filled saline lake in southwest Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2001). A study of the Werewilka Inlet of the saline Lake Wyara, Australia – a harbour of biodiversity for a sea of simplicity. Hydrobiologia 466, 245–254.
| A study of the Werewilka Inlet of the saline Lake Wyara, Australia – a harbour of biodiversity for a sea of simplicity.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2005). Salt lakes in Australia: present problems and prognosis for the future. Hydrobiologia 552, 1–15.
| Salt lakes in Australia: present problems and prognosis for the future.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2007). The biology of the saline lakes of central and eastern inland of Australia: a review with special reference to their biogeographical affinities. Hydrobiologia 576, 27–37.
| The biology of the saline lakes of central and eastern inland of Australia: a review with special reference to their biogeographical affinities.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2008). The ecology of episodic saline lakes of inland eastern Australia, as exemplified by a ten year study of the Rockwell-Wombah Lakes of the Paroo. Proceedings of the Linnean Society of New South Wales 129, 1–16.
Timms, B. V. (2009a). Biodiversity of large branchiopods of Australian saline lakes. Current Science 96, 74–80.
Timms, B. V. (2009b). Study of the saline lakes of the Esperance Hinterland, Western Australia, with special reference to the roles of acidity and episodicity. Natural Resources and Environmental Issues 15, 215–224.
Timms, B. V. (2009c). A study of the salt lakes and salt springs of Eyre Peninsula, South Australia. Hydrobiologia 626, 41–51.
| A study of the salt lakes and salt springs of Eyre Peninsula, South Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2010a). Blue Lagoon, South Australia: a closed marine lake harbouring potential invaders of continental saline lakes? Internationale Vereinigung für theoretische und angewandte Limnologie, Verhandlungen 30, 1425–1428.
| Blue Lagoon, South Australia: a closed marine lake harbouring potential invaders of continental saline lakes?Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2010b). Six new species of the brine shrimp Parartemia Sayce 1903 (Crustacea: Anostraca: Artemiina) in Western Australia. Zootaxa 2715, 1–35.
| Six new species of the brine shrimp Parartemia Sayce 1903 (Crustacea: Anostraca: Artemiina) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2012a). An appraisal of the diversity and distribution of large branchiopods (Branchiopoda: Anostraca, Laevicaudata, Spinicaudata, Cyclestherida, Notostraca) in Australia. Journal of Crustacean Biology 32, 615–623.
| An appraisal of the diversity and distribution of large branchiopods (Branchiopoda: Anostraca, Laevicaudata, Spinicaudata, Cyclestherida, Notostraca) in Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2012b). An identification guide to the brine shrimps (Crustacea: Anostraca: Artemiina) of Australia. Museum Victoria Science Reports 16, 1–36.
| An identification guide to the brine shrimps (Crustacea: Anostraca: Artemiina) of Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2014). A review of the biology of Australian halophilic anostracans (Branchiopoda: Anostraca). Journal of Biological Research 21, 21.
| A review of the biology of Australian halophilic anostracans (Branchiopoda: Anostraca).Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2015a). A new species of the fairy shrimp Branchinella (Crustacea: Anostraca: Thamnocephalidae) from western New South Wales, Australia. Proceedings of the Linnean Society of New South Wales 137, 37–43.
Timms, B. V. (2015b). A revised identification guide to the fairy shrimps (Crustacea: Anostraca: Anostracina) of Australia. Museum Victoria Science Reports 19, 1–44.
| A revised identification guide to the fairy shrimps (Crustacea: Anostraca: Anostracina) of Australia.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2018). On the influence of season and salinity on the phenology of invertebrates in Australian saline lakes, with special reference to those of the Paroo in the semiarid inland. Journal of Oceanology and Limnology 36, 1907–1916.
| On the influence of season and salinity on the phenology of invertebrates in Australian saline lakes, with special reference to those of the Paroo in the semiarid inland.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V. (2021). Drivers restricting biodiversity in Australian saline lakes: a review. Marine and Freshwater Research 72, 462–468.
| Drivers restricting biodiversity in Australian saline lakes: a review.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V., and Boulton, A. J. (2001). Typology of arid-zone floodplain wetlands of the Paroo River (inland Australia) and the influence of water regime, turbidity, and salinity on their aquatic invertebrate assemblages. Archiv für Hydrobiologie 153, 1–27.
| Typology of arid-zone floodplain wetlands of the Paroo River (inland Australia) and the influence of water regime, turbidity, and salinity on their aquatic invertebrate assemblages.Crossref | GoogleScholarGoogle Scholar |
Timms, B. V., and Hudson, P. (2009). The brine shrimps (Artemia and Parartemia) of South Australia, including descriptions of four new species of Parartemia (Crustacea: Anostraca: Artemiina). Zootaxa 2248, 47–68.
| The brine shrimps (Artemia and Parartemia) of South Australia, including descriptions of four new species of Parartemia (Crustacea: Anostraca: Artemiina).Crossref | GoogleScholarGoogle Scholar |
Timms, B. V., Datson, B., and Coleman, M. (2006). The wetlands of the Lake Carey catchment, northeast Goldfields of Western Australia, with special reference to large branchiopods. Journal of the Royal Society of Western Australia 89, 175–183.
Timms, B. V., Pinder, A. M., and Campagna, V. S. (2009). The biogeography and conservation status of the Australian endemic brine shrimp Parartemia (Crustacea, Anostraca, Parartemiidae). Conservation Science Western Australia 7, 413–427.
Timms, B. V., Coleman, P., and Cooper, J. (2014). Seagull Lake, Western Eyre Peninsula, South Australia: a saline lake to benefit from climate change? Transactions of the Royal Society of South Australia 138, 161–180.
| Seagull Lake, Western Eyre Peninsula, South Australia: a saline lake to benefit from climate change?Crossref | GoogleScholarGoogle Scholar |
van de Graaff, W. E. (1977). Relict early Cainozoic drainages in arid Western Australia. Zeitschrift für Geomorphologie 21, 379–400.
Wang, M., Zhao, W., Wei, J., Wang, S., and Xie, X. (2019). Acute effects of UVB radiation on the survival, growth, development, and reproduction of Daphniopsis tibetana Sars (Crustacea: Cladocera). Environmental Science and Pollution Research International 26, 10916–10925.
| Acute effects of UVB radiation on the survival, growth, development, and reproduction of Daphniopsis tibetana Sars (Crustacea: Cladocera).Crossref | GoogleScholarGoogle Scholar | 30783928PubMed |
Weston, M. (2007). The foraging and diet of non-breeding hooded plovers Thinornis rubricollis in relation to habitat type. Journal of the Royal Society of Western Australia 90, 89–95.
Whitehead, A. L. (2005). The effects of isolation and environmental heterogeneity on intraspecific variation in Calamoecia clitellata, a salt lake-inhabiting copepod. Ph.D. Thesis, University of Western Australia.
Wilke, T. (2019). Pomatiopsidae Stimpson, 1865. In ‘Freshwater Mollusks of the World: a Distribution Atlas’. (Eds C. Lydeard and K. S. Cummings.) pp. 126–130. (John Hopkins University Press: Baltimore, MD, USA.)
Wilke, T., Haase, M., Hershler, R., Liu, H.-P., Misof, B., and Ponder, W. (2013). Pushing short DNA fragments to the limit: phylogenetic relationships of ‘hydrobioid’ gastropods (Caenogastropoda: Rissooidea). Molecular Phylogenetics and Evolution 66, 715–736.
| Pushing short DNA fragments to the limit: phylogenetic relationships of ‘hydrobioid’ gastropods (Caenogastropoda: Rissooidea).Crossref | GoogleScholarGoogle Scholar | 23142112PubMed |
Williams, W. D. (1964). A contribution to lake typology in Victoria, Australia. Internationale Vereinigung für theoretische und angewandte Limnologie, Verhandlungen 15, 158–168.
| A contribution to lake typology in Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1981). The limnology of saline lakes in Western Victoria. Hydrobiologia 81–82, 233–259.
| The limnology of saline lakes in Western Victoria.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1983). On the ecology of Haloniscus searlei (Isopoda, Oniscoidea), an inhabitant of Australian salt lakes. Hydrobiologia 105, 137–142.
| On the ecology of Haloniscus searlei (Isopoda, Oniscoidea), an inhabitant of Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1984). Chemical and biological features of salt lakes on the Eyre Peninsula, South Australia, and an explanation of regional differences in the fauna of Australian salt lakes. Internationale Vereinigung für theoretische und angewandte Limnologie, Verhandlungen 22, 1208–1215.
| Chemical and biological features of salt lakes on the Eyre Peninsula, South Australia, and an explanation of regional differences in the fauna of Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1985). Biotic adaptations in temporary lentic waters, with special reference to those in semi-arid and arid regions. Hydrobiologia 125, 85–110.
| Biotic adaptations in temporary lentic waters, with special reference to those in semi-arid and arid regions.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1986). Daphniopsis pusilla, a cladoceran of Australian salt lakes: recognition and ecological data for palaeolimmologists. Palaeogeography, Palaeoclimatology, Palaeoecology 54, 305–316.
| Daphniopsis pusilla, a cladoceran of Australian salt lakes: recognition and ecological data for palaeolimmologists.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1995). Lake Corangamite, Australia, a permanent saline lake: conservation and management issues. Lakes and Reservoirs: Research and Management 1, 55–64.
| Lake Corangamite, Australia, a permanent saline lake: conservation and management issues.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (1998a). Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 381, 191–201.
| Salinity as a determinant of the structure of biological communities in salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D. (Ed.) (1998b) ‘Guidelines of Lake Management. Vol. 6. Management of Inland Saline Waters.’ (International Lake Environment Committee Foundation: Kusatu, Japan.)
Williams, W. D. (2002). Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025. Environmental Conservation 29, 154–167.
| Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., and Kokkinn, M. J. (1988). The biogeographical affinities of the fauna in episodically filled salt lakes: a study of Lake Eyre South, Australia. Hydrobiologia 158, 227–236.
| The biogeographical affinities of the fauna in episodically filled salt lakes: a study of Lake Eyre South, Australia.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., and Mellor, M. W. (1991). Ecology of Coxiella (Mollusca, Gastropoda, Prosobranchia), a snail endemic to Australian salt lakes. Palaeogeography, Palaeoclimatology, Palaeoecology 84, 339–355.
| Ecology of Coxiella (Mollusca, Gastropoda, Prosobranchia), a snail endemic to Australian salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., and Sherwood, J. E. (1994). Definition and measurement of salinity in salt lakes. International Journal of Salt Lake Research 3, 53–63.
| Definition and measurement of salinity in salt lakes.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., Boulton, A., and Taaffe, R. (1990). Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197, 257–266.
| Salinity as a determinant of salt lake fauna: a question of scale.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., Carrick, T. R., Bayly, I. A., Green, J., and Herbst, D. B. (1995). Invertebrates in salt lakes of the Bolivian Altiplano. International Journal of Salt Lake Research 4, 65–77.
| Invertebrates in salt lakes of the Bolivian Altiplano.Crossref | GoogleScholarGoogle Scholar |
Williams, W. D., De Deckker, P., and Shiel, R. J. (1998). The limnology of Lake Torrens, an episodic salt lake of central Australia, with particular reference to unique events in 1989. Hydrobiologia 384, 101–110.
| The limnology of Lake Torrens, an episodic salt lake of central Australia, with particular reference to unique events in 1989.Crossref | GoogleScholarGoogle Scholar |



