Sea-surface temperature used to predict the relative density of giant Pacific octopuses (Enteroctopus dofleini) in intertidal habitats of Prince William Sound, Alaska
D. ScheelAlaska Pacific University, 4101 University Drive, Anchorage, AK 99508, USA. Email: dscheel@alaskapacific.edu
Marine and Freshwater Research 66(10) 866-876 https://doi.org/10.1071/MF14197
Submitted: 4 July 2014 Accepted: 10 November 2014 Published: 1 April 2015
Journal Compilation © CSIRO Publishing 2015 Open Access CC BY-NC-ND
Abstract
Productivity linked to upwelling strength is an important environmental factor affecting the production and dynamics of octopus populations. This often takes the form of a negative relationship between octopus abundance and sea-surface temperatures (SST). Enteroctopus dofleini (giant Pacific octopuses) is caught as by-catch in several fisheries, but management for octopuses is data-poor. Visual surveys (in Prince William Sound (PWS) and Puget Sound) showed significant negative correlations of octopus counts with winter SST over the previous 30 months in the waters of eastern Gulf of Alaska, as expected on the basis of life-history parameters. In PWS, local octopus densities varied more than six-fold during the study, and correlations with SST accounted for 48–61% of the variance in counts. Octopus by-catch datasets were not similarly significantly correlated with SST. The negative correlation with SST suggests that octopus populations are influenced by factors regulating marine productivity during larval stages of life history far from the site of recruitment to benthic habitats. Targeted visual surveys for E. dofleini may be more predictable than by-catch statistics, and may be better estimators of variation in octopus abundance.
Additional keywords: environmental variability, population, Puget Sound, recruitment.
Introduction
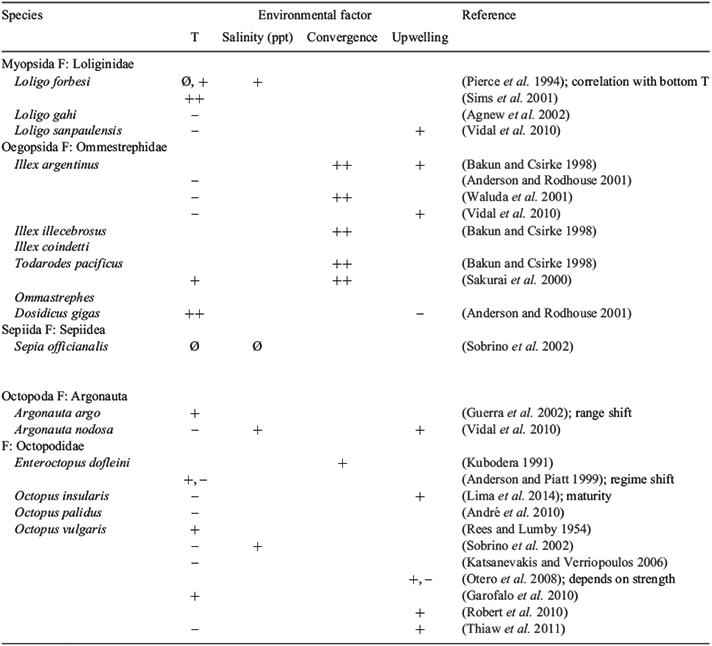
Octopoda life histories are linked to environmental factors. The most commonly reported environmental factors that affect cephalopod abundance and population dynamics are temperature, currents and upwelling. Kubodera (1991) reported concentrations of paralarvae in convergence zones in the north Pacific; and octopuses appear to be sensitive to reduced salinity. However, octopuses are influenced more by productivity linked to upwelling strength, so that population sizes are often negatively associated with temperatures (Table 1).
The common life-history characteristics of coleoid cephalopods dictate that the abundance of most species will be highly variable. The abundant coleoid cephalopods are mostly short-lived (3 months to 5 years, commonly 1–2-year life cycles) and physiologically efficient terminal spawners (Boyle and Boletzky 1996). One outcome of this life-cycle is that the annual fluctuations in biomass are sensitive to changes in ocean currents, which are influenced by ocean temperatures. Squid, cuttlefish and octopus populations often are correlated with oceanographic parameters (Table 1). Many authors have argued that this combination of life-history traits results in a standing biomass composed of recruits from a single cohort, and thus maximally responsive to environmental changes because of short life span and high conversion efficiency (e.g. Boyle and Boletzky 1996; O’Dor 1998). The classic example has become that of Ommestraphid squids such as Todarodes pacificus (e.g. Sakurai et al. 2000) and Illex illecebrosus (e.g. Hendrickson 2004), which have similar 1 year-long life histories in western boundary currents of the world’s oceans (Bakun and Csirke 1998; Sakurai et al. 2000; Rodhouse 2008).
Productivity of Octopods species have typically been linked to upwelling events that regulate local marine productivity. Examples include the concentration of Argonauta nodosa and other cephalopods in southern Brazil upwelling (Vidal et al. 2010), and the correlation of recruitment of Octopus vulgaris to indices of upwelling in waters off north-western Africa, Morocco and Spain (Table 1). However, there are also some exceptions. Argonauta and octopus were associated with mild conditions such as warm winters and unusual spatial patterns of warm-water currents (Garstang 1900; Rees and Lumby 1954; Okutani and Kawaguchi 1983; Guerra et al. 2002; Demicheli et al. 2006). The factors affecting planktonic survival and recruitment may include the relative contribution to these correlations of physiological effects on growth rates and settlement, as well as food-web-mediated effects (Bakun and Csirke 1998; Dawe and Brodziak 1998; Waluda et al. 2001; Katsanevakis and Verriopoulos 2006; André et al. 2010). However, the lack of a strong correlation between local octopus abundance and local food supply (e.g. Garstang 1900; Scheel et al. 2007) may be typical of those octopus populations with oceanographically driven recruitment.
The giant Pacific octopus (Enteroctopus dofleini) is an important north Pacific cephalopod comprising by weight and number a large proportion of cephalopod catch throughout its range. Its biology has been reviewed by Hartwick (1983b) and Hartwick and Barriga (1997). E. dofleini is a muscular benthic octopus occurring along the Pacific Rim from the continental shelf waters of California north to Alaska and west to Japan. The species is long-lived for a coleoid cephalopod, with an estimated life span of 3–5 years (Hartwick 1983a). Distinct seasonality in spawning or hatching has not been identified (Conners et al. 2012a). Females lay 17 000 to 100 000 small eggs (6–8-mm length each) suspended from a hard surface in clusters of several hundred eggs each. After laying eggs, females cease to feed, tend their eggs, and die about the time the eggs hatch. Eggs hatch 5–7 months post-spawning (but up to 1.5 years, Hartwick 1983b; Kubodera 1991). Paralarvae are neustonic, feeding and growing in the plankton near the surface until ~2–4 g in size (spending 2–3 months or more in the plankton, Hartwick 1983a). Egg and paralarval life stages may therefore have a combined duration of as little as 7 months but up to 21 months. Benthic juveniles occupy rocky habitats from the intertidal to 1000 m (Scheel 2002; Jorgensen 2009) where they typically shelter in rocky dens and feed on a wide diversity of primarily Crustacea and Mollusca benthic prey (Vincent et al. 1998; Scheel and Anderson 2012). Ontogenic migrations have been reported (Hartwick 1983a; Hartwick and Barriga 1997) but are poorly understood, although larger animals move more than smaller individuals (Scheel and Bisson 2012).
The potential for oceanographic influence on the early life stages of E. dofleini recruiting into rocky habitats is considerable. However, the link between oceanographic conditions and post-recruitment benthic abundance has not previously been explored for this species. In Prince William Sound, Alaska, the giant Pacific octopus is common in intertidal and shallow subtidal habitats, and its density fluctuates over time (Scheel et al. 2007). Recruitment into the Sound may occur through wind-forced flushing with Gulf of Alaska water from the Alaska coastal current (e.g. Cooney et al. 2001). Thus, it is reasonable to consider the role of oceanographic conditions in the Alaska coastal current for their influence on octopus density in Prince William Sound.
That mild winters influence octopus recruitment has been advanced to explain population outbreaks of Octopus vulgaris in British waters (Rees and Lumby 1954), whereas the 3–5-year life span of E. dofleini suggests the population at any time might be influenced over more than one winter. I examined sea-surface temperatures (SST) of areas to the south, east and west of Prince William Sound. To the north the region is bounded by land. These areas of the north Gulf of Alaska lie in the northward and westward flowing Alaska coastal current as it diverges from the North Pacific Current off southern British Columbia and Washington State towards Puget Sound, and as it turns northward towards Prince William Sound. I expected SST in these areas to correlate with the density of octopuses recorded during visual surveys in both Puget Sound and Prince William Sound (Scheel 2002; Scheel et al. 2007). I predicted that correlations of temperature with octopus density would increase to the south and east (upstream in the predominant flow), and would be highest for SST in winter months, during which conditions might have the greatest effect on paralarval survival and subsequent recruitment to benthic habitats. In this way, I attempt to identify correlations of oceanography with octopus density, and to establish whether these make biological sense in light of known life history, and as suggested by the literature on environmental regulation of cephalopod populations. This retrospective may be useful in formulating hypotheses of specific mechanisms that link octopus population variability with environmental drivers.
I also report on the correspondence to these ideas of data obtained from other visual survey and by-catch datasets available for north Pacific octopuses over approximately the same time period. I examine datasets with different methodologies (see Materials and methods) and biases. By-catch data on octopuses may under-sample rocky habitats (e.g. trawl sampling) and be size-biased. Very small octopuses are not retained in pot or trawl mesh (e.g. Laidig et al. 1995), whereas small pot and trawl methods under-sample larger octopuses (e.g. Reuter et al. 2010). By contrast, visual censuses detect both smaller and larger octopuses, and can census rocky habitats avoided by bottom gear. Even on soft sediments, visual censuses detect higher octopus densities than do trawls (Laidig et al. 1995; Katsanevakis and Verriopoulos 2004). The present study deliberately includes data from diverse sampling methods. I expect that SST will better predict the abundance indexes from visual censuses, because of fewer size and habitat sampling biases inherent in this method.
Materials and methods
Study sites and population trends
I used four independent datasets created by different methods that provide indexes of octopus abundance, and compared them with publically available data on SST. Only the first, summer beach surveys in Prince William Sound, counted the number of octopuses in a known sampling area, providing an estimate of local density. The second (Gulf of Alaska) is a biomass estimate from by-catch data. The third (autumn pot-fishing survey) and fourth (winter diver survey) are both analysed as count-per-unit-effort. I consider all four measures as indexes of relative abundance.
Early summer beach-walk surveys, Prince William Sound, Alaska
The local density of giant Pacific octopus, Enteroctopus dofleini, was assessed during early summer low-tide beach-walk surveys. No other octopus species were found or counted. Surveys were conducted at four beaches in Prince William Sound (PWS) most years from 1995 to 2013 (excepting 1997 and 1999–2001. Scheel 2002; Scheel et al. 2007). The seasonally earliest survey was in 2002 (25–28 May) and the latest in 2006 (24–26 June). Octopus dens were recognised by one or more of: the presence of an octopus, a den excavated in a characteristic fashion, or the presence of distinct marks (drills, bites) indicative of octopus predation on prey remains (Dodge and Scheel 1999). Dens were assessed for occupancy by using a green alder branch as a flexible probe, and only occupied dens were counted. Sites were visited by researchers each year at Gibbon’s Anchorage on Green Island (60°16′N, 147°27′W) and a nearby cove, and Ellamar near the village of Tatitlik (60°53′N, 146°42′W) and a nearby site on Busby Island for a total of six surveys in most years (two each at Gibbon’s Anchorage and Ellamar, one each at the nearby sites on Green and Busby Islands). Each was of an ~1000-m2 section of lower intertidal beach surveyed on foot during low water. Actual area surveyed was measured using laser rangefinders and all octopus counts were standardised to count per 1000 m2 (see Scheel 2002 for detailed methods). Where possible without damage to the site or octopus, octopuses were captured from their dens and weighed.
Biomass estimates derived from summer bottom-trawl surveys, Gulf of Alaska
Conners et al. (2012b) reported biomass estimates for octopuses over the Gulf of Alaska on the basis of semi-annual National Marine Fisheries Service (NMFS) bottom-trawl surveys for the period 1984–2011. Although these estimates are based on ‘fisheries-independent data collected by proper design-based sampling’, the sample comes primarily from the western Gulf of Alaska and the central gyre; most samples (>85%) caught no octopuses, a typical survey had n < 100 octopuses over this entire region, and surveys were conducted only every other year (Conners et al. 2012b). These authors reported that the estimates may be the most reliable biomass data available and yet also may have substantially underestimated biomass because of inefficiencies of sampling for octopuses by bottom trawl. Trawl-caught octopuses were small (predominantly <5 kg in weight; 50% of individuals <0.5 kg) owing to gear-size bias. It is important to recognise that these data were biomass estimates not counts; and were derived from pooled octopus data of several species. Although the exact species composition of this catch is unknown, because of their larger size, biomass estimates were dominated by E. dofleini: In 2011, 79% of individuals caught were E. dofleini (Conners et al. 2012b); thus, owing to their large size, by mass, >95% of the sample were E. dofleini. By contrast, data from the PWS and Puget Sound surveys (Sections 2.1.1, 2.1.3, 2.1.4) were counts of individual E. dofleini. I used these estimates from the period 1999–2011 to correspond closely with the period for which data were available from the summer PWS survey and the winter Puget Sound diver survey (see below).
Autumn pot-fishing surveys, Prince William Sound, Alaska
The Alaska Department of Fish and Game (ADFG) has since 1999 conducted an annual spot shrimp (Pandalus platyceros) survey in PWS during October. These surveys were conducted at eight sites per year in northern and western PWS. One of eight locations differed in 1999–2008 from that fished in 2009–2011. The sites were chosen to survey shrimp, not octopus, abundance. A single string of 11 baited kite-style shrimp pots (15 × 41 × 91 cm, covered with black woven fabric) was fished at each of four stations within each site (44 pots per site). Soak times averaged 20–22 h. Incidental to the shrimp surveys, the counts of octopuses caught at each station were recorded, and occasionally octopus weights were also recorded. For purposes of analyses reported here, data were provided by ADFG (K. Goldman, pers. comm.), and I calculated catch per unit effort as number of octopuses per 10 strings fished. On the basis of 2012–2013 data (N. Hollenbeck and D. Scheel, unpubl. data), ~75% (by count) of octopuses captured in this survey were E. dofleini, and 25% a possible undescribed species (Hollenbeck and Scheel 2012; Toussaint et al. 2012).
Winter diver surveys, Seattle Washington
The Puget Sound Octopus Census was started in 2000 as a SCUBA survey by the Seattle Aquarium. The survey has been conducted each year since in coordination with Octopus Week at the Seattle Aquarium. The seasonally earliest survey was in 2011 (14–17 January) and the latest in 2005 (19–21 February). Each year, octopuses were counted by volunteer divers on each dive at sites chosen by the divers over a period of 1–6 days (1 day each in 2000–2001, typically 3 days thereafter, but 6 days in 2012). Divers reported octopus encounters, dive site, number of divers and related information to the Seattle Aquarium. No search area nor dive times were reported for these surveys. Encounters with Octopus rubescens, the only other octopus commonly encountered by divers in Puget Sound, were removed from the count by the Seattle Aquarium on the basis of diver descriptions. For purposes of analyses reported here, data were provided by the Seattle Aquarium (K. Kegel, pers. comm.), and I calculated catch (sightings) per unit effort as octopuses per diver on a given dive. Dive durations were not reported and so count per hour of search could not be used. In practice, the average dive durations of volunteer divers, surveying many of the same sites at the same depths year after year, are not expected to vary greatly among years. Data on the number of divers were not available for 2009, so this year was removed from further analyses. Some divers estimated the size of octopuses, typically as ball- or fruit-sized (such as, for example, a volleyball, an orange). I converted these to centimeter mantle-length estimates by using standard ball or average fruit sizes.
Sea-surface temperature
The pre-survey interval maximising the SST-density correlation
I used the Pacific decadal oscillation index (PDO) to identify the time period over which to consider mean SST correlations with octopus density. The PDO is an index of SST anomalies over the entire extra-tropical North Pacific (Zhang et al. 1997, available at http://jisao.washington.edu/pdo/PDO.latest, accessed 13 March 2012).
I calculated the time-lagged correlation of the monthly mean PDO with octopus density from the summer PWS surveys only. I examined the correlation for each month over the 30 months (2.5 years) preceding the survey. The life expectancy of E. dofleini is estimated at 3–5 years (Hartwick 1983a), but octopuses in the intertidal are juveniles up to 9 kg (Scheel 2002) and, hence, unlikely to have recruited to the substrate from the plankton more than two and a half years pre-survey. Monthly correlations were negative and strongest in October to March of each winter in this period.
I next considered seasonal means of the monthly PDO. The correlation of octopus density with the 30-month winter average PDO (–0.77) was stronger than that with the average PDO of the previous 6 months (–0.41), the survey month (–0.46), 28-month spring average (–0.69), or the 24-month winter average (–0.70). I therefore chose the previous two and half winters as the period over which to examine SST for areas with greatest correlation to June octopus survey densities.
The ocean area maximising the SST-density correlation
I next obtained SST data from the NOAA NCEP re-analysis (Kalnay et al. 1996) and used area-weighted averages across the region, reported as monthly means. From these data, I calculated seasonal means of the monthly means for winter months (October to March) of the past 30 months (2.5 years) previous to each June survey.
I calculated the R2 coefficient for the regression of annual June octopus density averaged across sites with the previous 30-month October–March average monthly SST (hereafter, winter SST), using data from 1995 to 2011. The initial choice of area across which to average winter SST included the waters of Prince William Sound, Cook Inlet and adjacent waters of the Gulf of Alaska (59°N, 215°E to 61°N, 206°EA). The trend of the R2 of this regression with changing area sampled was calculated by iteratively repositioning the north, south, east and west boundaries to expand or contract the search area. Boundary changes that increased or did not change R2 were retained, whereas those reducing R2 were abandoned, until additional changes in boundaries in all directions resulted in decreases rather than increases in the R2. This technique is thus a simple hill climbing algorithm, identifying the geographic region of a local maximum in the correlation within the northern Gulf of Alaska. This exploratory method was necessary because of the lack of understanding of what factors and what areas of the current regime may make fluctuations in octopus populations most predictable.
The final area included in the regression of density against winter SST was fixed at the area where R2 was at a local maximum and subsequent incremental border changes resulted in lower R2. For this region, I reported the regression using survey and temperature data from 1995 (for the PWS summer beach-walk survey data) or 1999 (if available) to 2011. In addition, for surveys with more recent data, I also reported the regression statistics including the two additional years of 2012 and 2013, not included in the hill-climbing algorithm, thus providing a small degree of independence between the identification of the area maximising the SST-density correlation and the calculation of the P-value. I used Fisher’s F-statistic to test whether the regression differed significantly from a null model of zero slope. For PWS data (summer beach-walk survey, autumn shrimp-pot survey) and the summer Gulf of Alaska biomass estimates, I used the geographic area that maximised R2 for the summer beach-walk survey. For Puget Sound, I began with the coastal area including Puget Sound and repeated the hill-climbing algorithm until it reached a local maximum. I report the P-values without family-wise error-rate correction (e.g. Bonferroni) for multiple hypotheses (each incremental shift in geographic area) for several reasons. First, each geographic search does not constitute an independent hypothesis but, instead, is a step in a hill-climbing algorithm and is not independent of adjacent steps. Second, the intent of the hill-climbing algorithm is to suggest mechanistic hypotheses (such as a link between upwelling productivity and octopus density) rather than test them. Instead, the Fisher’s F-test examines the hypothesis that slope of the maximised correlation is significantly different from zero (no correlation). Third, I present the results of multiple steps of the hill-climbing algorithm, include the initial (defined as the area where octopuses were surveyed), the shape of the relation between geographic area and R2 (the hill-climbing landscape) and the final area(s) (see Results below). Finally, in the absence of detailed planktonic surveys across a broad area of the Gulf of Alaska, it is simply not possible to identify an area of water most influential to local octopus density without some exploratory comparison of the correlation with octopus density of oceanographic variables in different areas.
Results
Early summer beach-walk surveys, PWS, Alaska
Enteroctopus dofleini densities ranged from 0 to 3.7 octopuses 1000 m–2 over 93 measured early summer beach-walk surveys comprising a total of over 178 800 m2 in 15 years of the period from 1995 to 2013. Weights were obtained for n = 246 octopuses from beach-walk samples. More than 85% of these animals were <5 kg and just over one-quarter were <1 kg in weight. Densities on measured transects (Fig. 1) were low during the period 1995–2006, followed by a period of rising, higher and more variable averages in 2007–2013.

|
During the first period (1995–2004), with little trend in octopus density, octopus size distribution was even, with >10% of the sample occurring in each size class from <1 to 4 kg. In contrast, during the 2 years from 2005 to 2006, the size distribution was strongly unimodal, with many small octopuses captured and very few large individuals (Fig. 2). Subsequently, during the period 2007–2013, with increasing trends in octopus density, size distribution was again more even in size classes larger than 1 kg, whereas new recruits <1 kg comprised almost 40% of the sample. Thus, the increase in local density appears to have started with high recruitment in 2005–2006, and been maintained during subsequent years through both continued recruitment and survival across size classes.

|
The R2 coefficient for the regression of June octopus density with the previous 30-month winter SST increased in the Gulf of Alaska to the south and east of the Sound, but decreased to the west from the starting region of PWS (Figs 3, 4). This correlation reached a maximum for a rectangle encompassing the eastern Gulf of Alaska from south of Queen Charlotte Islands to south-eastern Alaska around Glacier Bay (hereafter, eastern GOA region). The increase between geographic regions was substantial, with the R2 for the eastern GOA region twice that using PWS SST (Fig. 4, Table 2).

|

|
Octopus densities following the coldest winter periods were more than six times those following warmer periods (minimum average, 0.32 per 1000 m2 in 2004; maximum 2.08 in 2010). The average octopus density was significantly negatively correlated with winter average SST for the eastern GOA region during the study period from 1995 to 2011 (Table 2). This correlation dropped somewhat to R2 = 0.48, (F1,13 = 12, P = 0.004) when the two additional years 2012–2013 were included (Fig. 5). If the data are divided into three time-periods of five surveys each (1995–2003, 2004–2008, 2009–2013), a strong negative correlation is present between SST and octopus density in each of the first two periods (R2 = 0.51 in the first, 0.41 in the second), but is absent during the third (R2 = 0.0), when 30-month average SSTs were at their coldest and least variable (see Fig. 1).

|
Biomass estimates derived from summer bottom-trawl surveys, Gulf of Alaska
The NMFS biomass estimates were derived from summer bottom-trawl surveys (n = 7, because of sampling alternate years only). Octopuses were captured most commonly in these surveys in waters adjacent to Kodiak Island, Alaska. The octopuses were predominantly <2 kg in weight, and 50% were <0.5 kg. The annual octopus biomass estimate was not significantly correlated with winter SST for the eastern GOA region over the period 1999–2011, although the trend was towards a negative relationship (Table 2).
Autumn pot-fishing surveys, PWS, Alaska
The autumn shrimp survey resulted in a by-catch of 106 octopuses over the years 1999–2011, with an annual effort of 28–32 pot strings of 10 pots each. Size data were available for a few of these animals only during the years 2006, 2007 and 2011 (n = 20 octopuses). These octopuses were predominantly (80%) <2 kg and the largest was 4.4 kg. The catch per unit effort of autumn pot-fishing surveys was not significantly correlated with winter SST for the eastern GOA region over the period 1999–2011, although, as for the other surveys, the trend was towards a negative relationship (Table 2).
Winter diver surveys, Seattle Washington
Volunteer survey divers on a total of 871 dives counted a total of 472 octopuses during the survey in the years 2000–2013. The annual average depth of those octopuses for which depth was reported varied from 13 to 19 m (44–62 feet). Size estimates were available for 318 (67%) of these octopuses. Octopuses in this survey were substantially larger than those in the summer or autumn surveys (PWS). In the summer survey, 5-kg octopuses had mantle lengths of 15–25 cm. On the basis of visual estimates by divers, only 32–46% of winter-surveyed octopuses (Puget Sound) were this size or smaller, and 50% had mantle-length estimates over 40 cm.
The R2 coefficient for the regression of annual winter-survey octopus sightings per diver in the Seattle surveys with the previous 30-month winter SST (using the period 2000–2011, excluding 2009 for which effort was not available) was at a local maximum for SST in an area extending 48.6–50°N and 120–135°W. This was due east of the southernmost portion of the area selected to maximise the summer-survey correlation (PWS) and extended further east to include Puget Sound itself (Fig. 3). Similarly to the results the summer surveys, octopus counts per diver following the coldest winter periods were 10 times those following warmer periods (minimum average, 0.13 per diver in Puget Sound in 2007, maximum 1.4 in 2010). The maximum counts occurred in the same year in both of these regions. The Puget Sound correlation again had a negative slope for the years 2000–2011 (Table 2) for both the Puget Sound area alone as well as for the region of local maximum. The correlation was only slightly lower (R2 = 0.58, F1,9 = 15.2, P = 0.002) with the inclusion of the two additional years 2012–2013 (Fig. 5).
Discussion
I examined four datasets reflecting abundance trends of E. dofleini (the giant Pacific octopus) in the north-eastern Pacific (PWS, GOA, and Puget Sound) over a period from the mid- or late-1990s until 2011–2013 (e.g. Fig. 1). Octopus abundance in these areas appears to be influenced in biologically realistic ways by the Alaska Current as it diverges from the North Pacific Current off southern British Columbia and Washington State and turns northward (Fig. 3). In both PWS and Puget Sound, the survey methods were local and the study areas small. Movement in and out of the study area does not necessarily mean a change in population size, and it is reasonable that local changes over time influenced survey results. Survey sites may vary over time in factors affecting octopus habitat selection (Scheel 2002; Scheel and Bisson 2012), for example, in the number of sea otters visiting the areas, local harvest pressure, or kelp cover. Nonetheless, each visual survey measured an index of local octopus abundance. SST variation accounted for more than 50% of variation in these measures, and thus appears to be as important as, or more so than, local factors.
The biological relevance of the correlations of SST with abundances presented here is evident from several factors. First, for all datasets, the direction of the trend was negative. Second, the two datasets from visual surveys (summer PWS beach walks and winter Puget Sound octopus census) were significantly correlated (Table 2; Results), with winter SST accounting for 48–61% of the variance in octopus counts (Fig. 5; Results). Both of these visual surveys were designed specifically to search for and census E. dofleini. The other two datasets, derived from by-catch data targeting other species, showed non-significant negative trends of octopus abundance with winter SST (Table 2). The summer GOA by-catch biomass estimate differed from the others used here in being a biomass estimate, in sampling primarily near Kodiak Island (further west of the other samples, Fig. 3), and in only surveying alternate years, resulting in a lower sample size and resolution. Although the autumn PWS pot survey counted individual octopuses, sampled in PWS, and was conducted annually, like the GOA survey, this sampling did not target octopuses and had the smallest sample size (counted octopuses) of any of the surveys (see Results). These two surveys were size-biased, detecting primarily octopuses of less than 2 kg, whereas the PWS and Puget Sound targeted surveys sampled smaller individuals as well as larger individuals of 3 to >6 kg. In both by-catch surveys, octopus species were not discriminated, although estimates of the proportion of E. dofleini in these samples exceeded 75%. Third, the PWS and Puget Sound correlations were negative with SST in the survey areas themselves (Table 2), before the hill-climbing algorithm to search for an area of locally maximum correlation. Fourth, the effect was large (6–10-fold variation in abundance) and the maximums occurred in the same year in each region.
I identified the area of winter SST used to model octopus abundance by searching for an area of water most correlated with the summer PWS beach-walk survey results. This area search identified biologically relevant areas in several ways also. First, the search identified that higher correlations occurred moving upstream along major GOA currents from the survey area in PWS. Similarly, for Puget Sound, the search was begun in Puget Sound and identified higher correlations upstream relative to the North Pacific Current (Fig. 3). Second, the preliminary search identified winter SST with a time lag up to 2.5 years (30 months), consistent with octopus life history and the likely age range of individuals detected in the summer PWS beach-walk surveys. Third, although the hill-climbing methodology does not constitute multiple independent hypotheses (see Materials and methods), the P-values obtained (Results, PWS P = 0.004, Puget Sound P = 0.002) were still significantly different from the null hypothesis (that the slopes of the correlations were not different than zero) even if a Bonferroni correction for family-wise error higher than a factor of 10 was applied.
The eastern GOA region (Fig. 3) that was strongly correlated with summer PWS (and possibly winter Puget Sound) octopus abundance is home to mesoscale eddies that affect plankton and cephalopod productivity. Mesoscale eddies (Haida and Sitka eddies, Crawford et al. 2005) form during the winter and dominate spring plankton productivity offshore. Copepods, other crustacea and pteropod mollusks, which may be prey of planktonic octopus paralarvae, all occurred at higher abundance within Haida eddies (Mackas et al. 2005) and commercial squid catches were elevated in the high-productivity periphery of a Haida eddy relative to surrounding waters, indicating that cephalopod productivity in particular may be influenced by eddies (Whitney and Robert 2002). Eddy average magnitude is positively correlated with the winter SST of the eastern GOA (Henson and Thomas 2008). Increased eddy activity in the Alaska Current is associated with downwelling wind conditions (Henson and Thomas 2008). In upwelling systems, octopus productivity is negatively correlated with temperature indexes (Table 1), as also reported here for PWS and Puget Sound octopuses. Thus, eddy activity may provide a link between ocean temperature in this region, plankton productivity and cephalopod recruitment.
In Alaska, E. dofleini is caught as by-catch, particularly in Pacific cod pot (trap) fisheries. Until 2011, these by-catch were managed as part of the ‘other species’ complex also including sharks, squids and sculpins (Reuter et al. 2010). Beginning in 2011, octopuses (all species) have been managed as a species group. Management for octopuses is considered data-poor, and that year, the overfishing limit was set as the average of the past year’s catch (Conners et al. 2012b). An unexpectedly high by-catch of octopuses resulted in the closure of the Aleutian Island-Bering Sea Pacific pot fishery for cod. In 2012 and 2013, the overfishing limit was set using the average biomass estimate of the last three surveys. Even though these limits are set using the best available data, the Scientific and Statistical Committee of the North Pacific Fisheries Management Council has called for alternative approaches or a survey for octopuses.
On the basis of life-history considerations, cephalopod abundance is expected to correlate with biologically appropriate environmental variation (e.g. see Pierce et al. 2008; Rodhouse 2010; Higgins et al. 2013). In the present paper, two small-scale targeted visual surveys (in PWS and Puget Sound) showed correlations with SST in eastern GOA waters, as expected on the basis of life-history parameters, accounting for ~50% of the variance in octopus counts over a period of more than a decade. Unfortunately, this strength of correlation did not extend to size-biased by-catch datasets of a smaller sample size in two fisheries targeting other species. Although limited, these findings suggest that visual surveys for E. dofleini may be more predictable than are pot or trawl by-catch surveys, and may be more predictable estimators of variation in octopus populations. Other studies have also found that visual surveys differ substantially from trawl surveys, even when octopuses are targeted in each (Katsanevakis and Verriopoulos 2004). Population variation is expected to be related to environmental variation, and this relationship is often useful for management of squid and octopus populations (see references in Table 1). There has been a call for ‘alternatives and surveys’ to supplement the Tier 6 trophic estimation methods currently being tried for by-catch management (Conners et al. 2012b). Predictive models based on easily available oceanographic variables provide one possibility for such an alternative. The negative correlation found between winter average SST in the eastern GOA and octopus counts is just such a relationship, and as such, has potential as an alternative for by-catch management. Validation of such a method may require continued surveys targeted to visually census octopuses.
Acknowledgements
I thank R. Anderson, K. Kegel, the Seattle Aquarium, the volunteer divers of the Puget Sound Octopus Census, K. Goldman, the Alaska Department of Fish and Game, D. Urban and the National Marine Fisheries Service for their willingness to share data, and Nicholas Bond (NOAA) and those attending the first Seattle Aquarium Giant Pacific Octopus Symposium and Workshop in 2012 for comments on a preliminary version of the ideas presented here. Thanks go to Captain N. Oppen of the R/V Tempest for his professionalism, hard work, dedication to the long-term success of these surveys and for his good humour in the field; and to fellow researchers T. L. S. Vincent, students of the annual summer octopus expeditions 2001–2013 at Alaska Pacific University (APU), Thomas L. and P. J. Vincent, J. P. and L. H. Scheel, and many others for collaboration in aspects of these studies. The manuscript benefited from review by two anonymous reviewers. Long-term financial support for this study was provided through APU from donations by the Pollock Conservation Consortium (2001–2013); with additional support from the Exxon Valdez Oil Spill Trustee Council (1995–1997), West Coast and Polar Regions Underseas Research Center (University of Alaska Fairbanks National Undersea Research Program, NOAA 1998, 2010), National Science Foundation (Grant #0115882 2001–2003), Alaska Space Grant Program NASA (2008–2010), and Alaska Fisheries Development Foundation (2006) to D. Scheel and T. L. S. Vincent. Findings and conclusions presented by the author are his own and do not necessarily reflect the views or positions of the supporting organisations. The research was conducted under Alaska Department of Fish and Game Fish Resource permits to D. Scheel, and complies with the current laws of the United States. The author declares that he has no conflict of interest.
References
Agnew, D. J., Beddington, J. R., and Hill, S. L. (2002). The potential use of environmental information to manage squid stocks. Canadian Journal of Fisheries and Aquatic Sciences 59, 1851–1857.| The potential use of environmental information to manage squid stocks.Crossref | GoogleScholarGoogle Scholar |
Anderson, P. J., and Piatt, J. F. (1999). Community reorganization in the Gulf of Alaska following ocean climate regime shift. Marine Ecology Progress Series 189, 117–123.
| Community reorganization in the Gulf of Alaska following ocean climate regime shift.Crossref | GoogleScholarGoogle Scholar |
Anderson, C. I. H., and Rodhouse, P. G. (2001). Life cycles, oceanography, and variability: ommastrephid squid in variable oceanographic environments. Fisheries Research 54, 133–143.
| Life cycles, oceanography, and variability: ommastrephid squid in variable oceanographic environments.Crossref | GoogleScholarGoogle Scholar |
André, J., Haddon, M., and Pecl, G. T. (2010). Modelling climate-change-induced nonlinear thresholds in cephalopod population dynamics. Global Change Biology 16, 2866–2875.
| Modelling climate-change-induced nonlinear thresholds in cephalopod population dynamics.Crossref | GoogleScholarGoogle Scholar |
Bakun, A., and Csirke, J. (1998). Environmental processes and recruitment variability. In ‘Squid Recruitment Dynamics: the Genus Illex as a Model, the Commerical Illex Species, and Influences on Variability’. (Eds P. G. Rodhouse, E. G. Dawe and R. K. O’Dor.) pp. 105–124. (FAO: Rome.)
Boyle, P., and Boletzky, S.v. (1996). Cephalopod populations: definition and dynamics. Philosophical Transactions: Biological Sciences 351, 985–1002.
| Cephalopod populations: definition and dynamics.Crossref | GoogleScholarGoogle Scholar |
Conners, M. E., Conrath, C., and Brewer, R. (2012a). ‘Field Studies in Support of Stock Assessment for the Giant Pacific Octopus Enteroctopus dofleini.’ (Alaska Fisheries Science Center, North Pacific Fisheries Management Council: Seattle, WA.)
Conners, M. E., Aydin, K., and Conrath, C. (2012b). ‘NPFMC Gulf of Alaska SAFE 22. Assessment of the Octopus Stock Complex in the Gulf of Alaska.’ pp. 647–687. (Alaska Fisheries Science Center, North Pacific Fisheries Management Council: Seattle, WA.)
Cooney, R. T., Allen, J. R., Bishop, M. A., Eslinger, D. L., Kline, T., Norcross, B. L., McRoy, C. P., Milton, J., Olsen, J., Patrick, V., Paul, A. J., Salmon, D., Scheel, D., Thomas, G. L., Vaughan, S. L., and Willette, T. M. (2001). Ecosystem control of pink salmon (Oncorhynchus gorbuscha) and Pacific herring (Clupea pallasi) populations in Prince William Sound, Alaska. Fisheries Oceanography 10, 1–13.
| Ecosystem control of pink salmon (Oncorhynchus gorbuscha) and Pacific herring (Clupea pallasi) populations in Prince William Sound, Alaska.Crossref | GoogleScholarGoogle Scholar |
Crawford, W. R., Brickley, P. J., Peterson, T. D., and Thomas, A. C. (2005). Impact of Haida Eddies on chlorophyll distribution in the eastern Gulf of Alaska. Deep-sea Research. Part II, Topical Studies in Oceanography 52, 975–989.
| Impact of Haida Eddies on chlorophyll distribution in the eastern Gulf of Alaska.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXksVyjsL0%3D&md5=4a0ea5b26a2f08b180c22df16f0956c9CAS |
Dawe, E. G., and Brodziak, J. K. T. (1998). Trophic relationships, ecosystem variability and recruitment. In ‘Squid Recruitment Dynamics: the Genus Illex as a Model, the Commerical Illex Species, and Influences on Variability’. (Eds P. G. Rodhouse, E. G. Dawe and R. K. O’Dor.) pp. 125–156. (FAO: Rome.)
Demicheli, M., Martínez, A., Ortega, L., Scarabino, F., Maytía, S., and Demicheli, A. (2006). Mass stranding of Argonauta nodosa Lightfoot, 1786 (Cephalopoda, Argonautidae) along the Uruguayan coast (southwestern Atlantic). Revista de Biología Marina y Oceanografía 41, 147–153.
Dodge, R., and Scheel, D. (1999). Remains of the prey – recognizing the midden piles of Octopus dofleini (Wulker). The Veliger 42, 260–266.
Garofalo, G., Ceriola, L., Gristina, M., Fiorentino, F., and Pace, R. (2010). Nurseries, spawning grounds and recruitment of Octopus vulgaris in the Strait of Sicily, central Mediterranean Sea. ICES Journal of Marine Science 67, 1363–1371.
Garstang, W. (1900). The plague of octopus on the south coast, and its effect on the crab and lobster fisheries. Journal of the Marine Biological Association of the United Kingdom 6, 260–273.
| The plague of octopus on the south coast, and its effect on the crab and lobster fisheries.Crossref | GoogleScholarGoogle Scholar |
Guerra, Á., González, Á. F., and Rocha, F. (2002). Appearance of the common paper nautilus Argonauta argo related to the increase of the sea surface temperature in the north-eastern Atlantic. Journal of the Marine Biological Association of the United Kingdom 82, 855–858.
| Appearance of the common paper nautilus Argonauta argo related to the increase of the sea surface temperature in the north-eastern Atlantic.Crossref | GoogleScholarGoogle Scholar |
Hartwick, B. (1983a). Octopus dofleini. In ‘Cephalopod Life Cycles. Vol. 1’. (Ed. P. R. Boyle.) pp. 277–291. (Academic Press: London.)
Hartwick, E. B. (1983b). Octopus dofleini. In ‘Cephalopod Life Cycles. Vol. I. Species Accounts’. (Ed. P. R. Boyle.) pp. 277–291. (Academic Press: London.)
Hartwick, E. B., and Barriga, I. (1997). Octopus dofleini: biology and fisheries in Canada. In ‘Proceedings of the Workshop on The Fishery and Market Potential of Octopus in California’, 31 August–8 September 1989, Avalon, CA, USA. (Eds M. A. Lang and F. G. Hochberg.) pp. 45–56. (Smithsonian Institution: Washington, DC.)
Hendrickson, L. (2004). Population biology of northern shortfin squid (Illex illecebrosus) in the Northwest Atlantic Ocean and initial documentation of a spawning area. ICES Journal of Marine Science 61, 252–266.
| Population biology of northern shortfin squid (Illex illecebrosus) in the Northwest Atlantic Ocean and initial documentation of a spawning area.Crossref | GoogleScholarGoogle Scholar |
Henson, S. A., and Thomas, A. C. (2008). A census of oceanic anticyclonic eddies in the Gulf of Alaska. Deep-sea Research. Part I. Oceanographic Research Papers 55, 163–176.
| A census of oceanic anticyclonic eddies in the Gulf of Alaska.Crossref | GoogleScholarGoogle Scholar |
Higgins, K. L., Semmens, J. M., Doubleday, Z. A., and Burridge, C. P. (2013). Life history matters: comparisons of population structuring in sympatric octopus species that differ in the presence of a pelagic larval stage. Marine Ecology Progress Series 486, 203–212.
| Life history matters: comparisons of population structuring in sympatric octopus species that differ in the presence of a pelagic larval stage.Crossref | GoogleScholarGoogle Scholar |
Hollenbeck, N., and Scheel, D. (2012). Is there a previously unknown cryptic species of giant Pacific octopus in Prince William Sound, Alaska? American Malacological Bulletin 43, 7–9.
Jorgensen, E. M. (2009). ‘Field Guide to Squids and Octopods of the Eastern North Pacific and Bering Sea.’ (Alaska Sea Grant College Program: Fairbanks, AK.)
Kalnay, E., Kanamitsu, M., Kistler, R., Collins, W., Deaven, D., Gandin, L., Iredell, M., Saha, S., White, G., Woollen, J., Zhu, Y., Leetmaa, A., Reynolds, R., Chelliah, M., Ebisuzaki, W., Higgins, W., Janowiak, J., Mo, K. C., Ropelewski, C., Wang, J., Jenne, R., and Joseph, D. (1996). The NCEP/NCAR reanalysis 40-year project. Bulletin of the American Meteorological Society 77, 437–471.
| The NCEP/NCAR reanalysis 40-year project.Crossref | GoogleScholarGoogle Scholar |
Katsanevakis, S., and Verriopoulos, G. (2004). Abundance of Octopus vulgaris on soft sediment. Scientia Marina 68, 553–560.
| Abundance of Octopus vulgaris on soft sediment.Crossref | GoogleScholarGoogle Scholar |
Katsanevakis, S., and Verriopoulos, G. (2006). Modelling the effect of temperature on hatching and settlement patterns of meroplanktonic organisms: the case of the octopus. Scientia Marina 70, 699–708.
Kubodera, T. (1991). Distribution and abundance of the early life stages of octopus, Octopus dofleini Wülker, 1910 in the north Pacific. Bulletin of Marine Science 49, 235–243.
Laidig, T. E., Adams, P. B., Baxter, C. H., and Butler, J. L. (1995). Feeding on euphausiids by Octopus rubescens. California Fish and Game 81, 77–79.
Lima, F. D., Leite, T. S., Haimovici, M., Nóbrega, M. F., and Oliveira, J. E. L. (2014). Population structure and reproductive dynamics of Octopus insularis (Cephalopoda: Octopodidae) in a coastal reef environment along northeastern Brazil. Fisheries Research 152, 86–92.
| Population structure and reproductive dynamics of Octopus insularis (Cephalopoda: Octopodidae) in a coastal reef environment along northeastern Brazil.Crossref | GoogleScholarGoogle Scholar |
Mackas, D. L., Tsurumi, M., Galbraith, M. D., and Yelland, D. R. (2005). Zooplankton distribution and dynamics in a North Pacific Eddy of coastal origin: II. Mechanisms of eddy colonization by and retention of offshore species. Deep-sea Research. Part II, Topical Studies in Oceanography 52, 1011–1035.
| Zooplankton distribution and dynamics in a North Pacific Eddy of coastal origin: II. Mechanisms of eddy colonization by and retention of offshore species.Crossref | GoogleScholarGoogle Scholar |
O’Dor, R. K. (1998). Squid life history strategies. In ‘Squid Recruitment Dynamics: the Genus Illex as a Model, the Commerical Illex Species, and Influences on Variability’. (Eds P. G. Rodhouse, E. G. Dawe and R. K. O’Dor.) pp. 233–254. (FAO: Rome.)
Okutani, T., and Kawaguchi, T. (1983). A mass occurrence of Argonauta argo (Cephalopoda: Octopoda) along the coast of Shimane prefecture, Western Japan Sea. Venus 41, 281–290.
Otero, J., Alvarez-Salgado, X. A., Gonzalez, A. F., Miranda, A., Groom, S. B., Cabanas, J. M., Casas, G., Wheatley, B., and Guerra, A. (2008). Bottom up control of common octopus Octopus vulgaris in the Galician upwelling system, northeast Atlantic ocean. Marine Ecology Progress Series 362, 181–192.
| Bottom up control of common octopus Octopus vulgaris in the Galician upwelling system, northeast Atlantic ocean.Crossref | GoogleScholarGoogle Scholar |
Pierce, G. J., Boyle, P. R., Hastie, L. C., and Shanks, A. M. (1994). Distribution and abundance of the fished population of Loligo forbesi in UK waters: analysis of fishery data. Fisheries Research 21, 193–216.
| Distribution and abundance of the fished population of Loligo forbesi in UK waters: analysis of fishery data.Crossref | GoogleScholarGoogle Scholar |
Pierce, G. J., Valvanis, V. D., Guerra, A., Jereb, P., Orsi-Relini, L., Bellido, J. M., Katara, I., Piatkowski, U., Pereira, J., Balguerias, E., Sobrino, I., Lefkaditou, E., Wang, J., Santurtun, M., Boyle, P. R., Hastie, L. C., MacLeod, C. D., Smith, J. M., Viana, M., Gonzalez, A. F., and Zuur, A. F. (2008). A review of cephalopod-environment interactions in European seas. Hydrobiologia 612, 49–70.
| A review of cephalopod-environment interactions in European seas.Crossref | GoogleScholarGoogle Scholar |
Rees, W. J., and Lumby, J. R. (1954). The abundance of octopus in the English channel. Journal of the Marine Biological Association of the United Kingdom 33, 515–536.
| The abundance of octopus in the English channel.Crossref | GoogleScholarGoogle Scholar |
Reuter, R. F., Conners, M. E., Dicosimo, J., Gaichas, S., Ormseth, O., and Tenbrink, T. T. (2010). Managing non-target, data-poor species using catch limits: lessons from the Alaskan groundfish fishery. Fisheries Management and Ecology 17, 323–335.
Robert, M., Faraj, A., McAllister, M. K., and Rivot, E. (2010). Bayesian state-space modelling of the De Lury depletion model: strengths and limitations of the method, and application to the Moroccan octopus fishery. ICES Journal of Marine Science 67, 1272–1290.
| Bayesian state-space modelling of the De Lury depletion model: strengths and limitations of the method, and application to the Moroccan octopus fishery.Crossref | GoogleScholarGoogle Scholar |
Rodhouse, P. G. (2008). Large-scale range expansion and variability. In ‘Ommastrephid squid populations: a review of environmental links’. California Cooperative Oceanic Fisheries Investigation report 49, pp. 83–89. Available at http://www.calcofi.org/ccpublications/ccreports/calcofi-reports-pdf.html [Verified 12 January 2015].
Rodhouse, P. G. (2010). Effects of environmental variability and change on cephalopod populations: an introduction to the CIAC ‘09 symposium special issue. ICES Journal of Marine Science 67, 1311–1313.
Sakurai, Y., Kiyofuji, H., Saitoh, S., Goto, T., and Hiyama, Y. (2000). Changes in inferred spawning areas of Todarodes pacificus (Cephalopoda: Ommastrephidae) due to changing environmental conditions. ICES Journal of Marine Science 57, 24–30.
| Changes in inferred spawning areas of Todarodes pacificus (Cephalopoda: Ommastrephidae) due to changing environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Scheel, D. (2002). Characteristics of habitats used by Enteroctopus dofleini in Prince William Sound and Cook Inlet, Alaska. Marine Ecology 23, 185–206.
| Characteristics of habitats used by Enteroctopus dofleini in Prince William Sound and Cook Inlet, Alaska.Crossref | GoogleScholarGoogle Scholar |
Scheel, D., and Anderson, R. C. (2012). Variability in the diet specialization of Enteroctopus dofleini in the eastern Pacific examined from midden contents. American Malacological Bulletin 30, 267–279.
| Variability in the diet specialization of Enteroctopus dofleini in the eastern Pacific examined from midden contents.Crossref | GoogleScholarGoogle Scholar |
Scheel, D., and Bisson, L. (2012). Movement patterns of giant Pacific octopuses, Enteroctopus dofleini (Wülker, 1910). Journal of Experimental Marine Biology and Ecology 416–417, 21–31.
| Movement patterns of giant Pacific octopuses, Enteroctopus dofleini (Wülker, 1910).Crossref | GoogleScholarGoogle Scholar |
Scheel, D., Lauster, A., and Vincent, T. L. S. (2007). Habitat ecology of Enteroctopus dofleini from middens and live prey surveys in Prince William Sound, AK. In ‘Cephalopods Present and Past: New Insights and Fresh Perspectives’. (Eds N. H. Landman, R. A. Davis and R. H. Mapes.) pp. 434–458. (Springer: Dordrecht, Netherlands.)
Sims, D. W., Genner, M. J., Southward, A. J., and Hawkins, S. J. (2001). Timing of squid migration reflects North Atlantic climate variability. Proceedings. Biological Sciences 268, 2607–2611.
| Timing of squid migration reflects North Atlantic climate variability.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FgsVCqtQ%3D%3D&md5=2367b79b83d61546f6e7c48d06bf7d92CAS |
Sobrino, I., Silva, L., Bellido, J. M., and Ramos, F. (2002). Rainfall, river discharges and sea temperature as factors affecting abundance of two coastal benthic cephalopod species in the Gulf of Cádiz (SW Spain). Bulletin of Marine Science 71, 851–865.
Thiaw, M., Gascuel, D., Thiao, D., Thiaw, O. T., and Jouffre, D. (2011). Analysing environmental and fishing effects on a short-lived species stock: the dynamics of the octopus Octopus vulgaris population in Senegalese waters. African Journal of Marine Science 33, 209–222.
| Analysing environmental and fishing effects on a short-lived species stock: the dynamics of the octopus Octopus vulgaris population in Senegalese waters.Crossref | GoogleScholarGoogle Scholar |
Toussaint, R. K., Scheel, D., Sage, G. K., and Talbot, S. L. (2012). Nuclear and mitochondrial markers reveal evidence for genetically segregated cryptic speciation in giant Pacific octopuses from Prince William Sound, Alaska. Conservation Genetics 13, 1483–1497.
| Nuclear and mitochondrial markers reveal evidence for genetically segregated cryptic speciation in giant Pacific octopuses from Prince William Sound, Alaska.Crossref | GoogleScholarGoogle Scholar |
Vidal, E. A. G., Haimovici, M., and Hackbart, V. C. S. (2010). Distribution of paralarvae and small juvenile cephalopods in relation to primary production in an upwelling area off southern Brazil. ICES Journal of Marine Science 67, 1346–1352.
Vincent, T. L. S., Scheel, D., and Hough, K. R. (1998). Some aspects of diet and foraging behavior of Octopus dofleini (Wülker, 1910) in its northernmost range. Marine Ecology 19, 13–29.
| Some aspects of diet and foraging behavior of Octopus dofleini (Wülker, 1910) in its northernmost range.Crossref | GoogleScholarGoogle Scholar |
Waluda, C. M., Rodhouse, P. G., Podesta, G. P., Trathan, P. N., and Pierce, G. J. (2001). Surface oceanography of the inferred hatching grounds of Illex argentinus (Cephalopoda: Ommastrephidae) and influences on recruitment variability. Marine Biology 139, 671–679.
| Surface oceanography of the inferred hatching grounds of Illex argentinus (Cephalopoda: Ommastrephidae) and influences on recruitment variability.Crossref | GoogleScholarGoogle Scholar |
Whitney, F., and Robert, M. (2002). Structure of Haida Eddies and their transport of nutrient from coastal margins into the NE Pacific Ocean. Journal of Oceanography 58, 715–723.
| Structure of Haida Eddies and their transport of nutrient from coastal margins into the NE Pacific Ocean.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XptV2hsbc%3D&md5=ecb7ce7f89845bf57cca9f798826ebd5CAS |
Zhang, Y., Wallace, J. M., and Battisti, D. S. (1997). ENSO-like interdecadal variability: 1900–93. Journal of Climate 10, 1004–1020.
| ENSO-like interdecadal variability: 1900–93.Crossref | GoogleScholarGoogle Scholar |
A 59°N, 145°W to 61°N, 154°W, expressed in degrees east = 360 – degrees west. This defaulted to NOAA grid boundaries 61.9–58.1°N by 206.3–215.6°E.