The art of otolith chemistry: interpreting patterns by integrating perspectives
Benjamin D. Walther
Department of Life Sciences, Texas A&M University – Corpus Christi, 6300 Ocean Drive, Corpus Christi, TX 78412, USA. Email: benjamin.walther@tamucc.edu
Marine and Freshwater Research 70(12) 1643-1658 https://doi.org/10.1071/MF18270
Submitted: 28 July 2018 Accepted: 1 November 2018 Published: 21 January 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
The ability to obtain high-resolution chemical profiles across otoliths has expanded with technological advancements that prompted an explosion of data from diverse taxa in coastal, marine and freshwater systems worldwide. The questions pursued by most otolith chemists fall broadly into six categories: identifying origins, tracking migration, reconstructing environments, quantifying growth or physiology, validating ages and assessing diets. Advances in instrumentation have widened the periodic table of otolith elements, and two-dimensional mapping has further illuminated spatial heterogeneity across these complex structures. Although environmental drivers of observed elemental signatures in otoliths are often assumed to be paramount, multiple intrinsic and extrinsic factors can disrupt simple relationships between an element and a single environmental parameter. An otolith chemical profile is not a direct photograph of an environment, but rather an impressionistic image filtered through the multifaceted experiences of the fish itself. A ‘signal-to-noise’ approach that assesses the relative magnitudes of variation from intrinsic and extrinsic factors on chemical profiles may be a promising way to resolve the factor of interest against the ‘noise’ of others. A robust appreciation of environmental drivers, physiological regulation and calcification dynamics that affect the ability to effectively interpret otolith chemical patterns is necessary to drive the field forward.
Additional keywords: barium, carbonates, increments, membranes, stable isotopes, strontium.
Introduction
Observers actually know that despite the apparent simplicity of the laws governing their formation, the works of nature are infinitely varied, from the most important to the least, no matter what their species or family … the quarters of an orange, the leaves of a tree, the petals of a flower are never identical; it thus seems that every kind of beauty draws its charm from this diversity. [Pierre-Auguste Renoir in 1884; Nochlin 1966, p. 46]
A robust understanding of migration, life history strategies and growth dynamics is critical for effective management of fishes in marine, coastal and freshwater systems worldwide. The practice of otolithology, or the broad use of otoliths to investigate fish dynamics (sensu Gaemers 1978), has yielded an explosion of data and subsequent insight into the dynamics of fishes, their communities and ecosystems. The field has proliferated in the types of questions that can be asked with these remarkable structures, spurred on by monumental discoveries such as the identification of annual (Reibisch 1899) and daily (Pannella 1971) increments. A particularly fruitful expansion of this field derives from the increasingly detailed and high-throughput chemical analyses of otolith increments. Much of this growth has been tightly coupled to technological advances, such as the development and refinement of laser technology allowing multi-element high-resolution and high-precision measurements that are, importantly, reasonably affordable for a wide array of fish ecologists. The expansion and maturation of the otolith chemistry subfield is evident in the published proceedings of the International Otolith Symposia (IOS) over the years. For instance, in the book published after the Hilton Head, North Carolina, IOS, only 10% (4 of 40) of contributed papers focused on the elemental or isotopic composition of otoliths (Secor et al. 1995), whereas in the two special issues published after the Mallorca, Spain, symposium, 48% of contributed papers incorporated chemical analyses of otoliths or analogous structures such as vertebrae (Morales-Nin and Geffen 2015; Geffen et al. 2016). A review of the titles of contributed talks and posters at the IOS in Keelung, Taiwan, in 2018 showed an estimated 43% of talks and 34% of posters focused on otolith chemistry techniques (see http://isis.cmima.csic.es/aforo/presentations/IOS2018Taiwan.pdf, accessed 22 November 2018). Beyond the official IOS and their proceedings, other special sessions devoted to otoliths and comparable structures have become regular features at marine and aquatic science conferences, with their own associated publications (e.g. Walther et al. 2017; Hunter et al. 2018). Clearly, a growing number of investigators is turning towards chemical tools to unravel key information about fish life histories.
The increasing prominence of otolith chemistry can be seen in the broader body of published literature. To assess the overall publication trends in otolith chemistry, a literature search was conducted in ISI Web of Science (conducted on 3 October 2018 with search terms: otolith AND (chem* OR microchem* OR elem* OR isotop*)) from 1967 through 2017 to quantify annual publications of elemental and isotopic analyses of fish otoliths since the work of Devereux (1967) on oxygen isotope ratios. Results were culled to remove non-fish biomedical human and animal model research, exclude fluorescent marking research and retain only peer-reviewed journal articles (excluding book chapters or conference abstracts), leaving a total of 1505 papers published over five decades (Fig. 1a). Although this search is undoubtedly an undercount, it illustrates the non-linear trajectory and rapid growth in published work in recent decades, driven in large part by advances in instrumentation, experimental validation and high-throughput approaches. The critical role of the periodic symposia can be seen in upticks following IOS years (1993 in Hilton Head, USA; 1998 in Bergen, Norway; 2004 in Townsville, Australia; 2009 in Monterey, USA; and 2014 in Mallorca, Spain), with publications of associated books or special issues, such as this one. Given that publication rates in all subject areas have also grown significantly over the same time period, the annual percentage of otolith chemistry papers relative to total otolith publications as indexed in the Aquatic Sciences and Fisheries Abstracts (ASFA; search term: otolith*) was calculated (Fig. 1b). Although percentages were initially high in the early years due to low numbers of total otolith publications each year before 1990, the proportion increased steadily throughout the 1990s and subsequently stabilised in the past decade. These trends are similar to those observed in prior assessments of annual publication rates (Campana and Thorrold 2001; Secor 2010; Starrs et al. 2016; Tanner et al. 2016). Clearly, the field is robust, with an average of 91 publications annually constituting an average of 19% of all otolith publications since 2013.
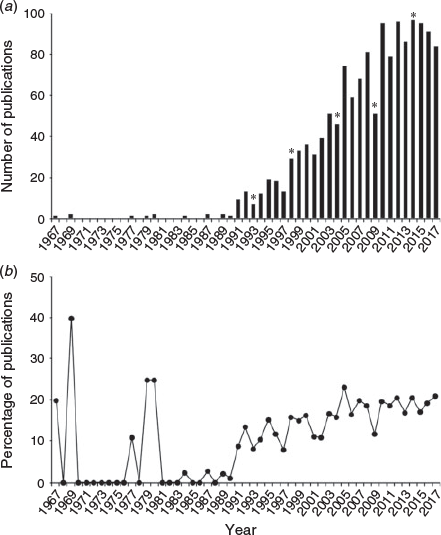
|
Although the applications of otolith chemistry are increasingly diverse, the primary questions investigated can be broadly grouped into six major categories: (1) identifying natal origins and connectivity patterns; (2) assessing individual variability in migration patterns; (3) reconstructing environmental exposure histories (e.g. temperature, dissolved oxygen, pollution); (4) quantifying growth and physiological dynamics (e.g. metabolism, stress, reproduction); (5) validating age estimates; and (6) determining dietary histories.
Investigating any of these questions requires the appropriate selection of chemical proxies that reveal the targeted variable of interest, an extractive or in situ analysis of sufficient material to yield quantifiable measurements of the analyte and appropriate placement and resolution of analyses in relation to increment spacing. A given element may yield information for multiple questions, given the possibility of both extrinsic and intrinsic control on elemental incorporation. Still, otoliths hold incredible promise for revealing comprehensive life history information about the lives of fishes. This paper reviews developments in otolith chemistry research by using a metaphor to structure the discussion of critical frontiers in the field. Specifically, it is argued that an otolith is like a painting. Much like a painting is influenced by multiple ‘filters’ that influence the final image, including the studio, the canvas, the media and the artist, an otolith is influenced by analogous filters, including the environment, the constituent elements and isotopes, the biogenic structure composition and the physiology of the fish itself. Our challenge as otolith chemists is to interpret the observed chemical patterns much like a museum visitor interprets the painting. Before this metaphor is used, the paper begins by briefly considering the historical foundations of this field and the technological advancements that have led to a proliferation of otolith chemistry data that require interpretation.
Historical foundations
The growth of the field prompts us to reflect on the origins of otolith chemistry. Far from springing from a single source of inspiration, otolith chemistry can be viewed as a natural evolution of the broader advances in biogeochemistry across systems and taxa. The case of strontium is emblematic of this evolution. The element was originally discovered in 1790 by Adair Crawford and William Cruickshank and named for the small village of Strontian, Scotland, where unidentified minerals were unearthed in local lead mines (Mellor 1961). Strontium was first isolated in 1808 by electrolysis by the great chemist Sir Humphry DavyA, who also isolated calcium, magnesium and barium (Knight 1998). The potential for strontium to reveal biogeochemical processes and elemental cycling was quickly realised. Forchhammer (1865) first discovered strontium in seawater and living tissues of Fucus algae, indicating the possibility of active biotic uptake. Dieulafait (1877) identified strontium in fossilised and modern brachiopods and suggested that shell composition reflected contemporary ocean chemical composition. Papillon (1870) made one of the first assessments of strontium in living vertebrates and, through experimental diet manipulations, discovered that strontium in pigeon bones was derived at least in part from dietary sources. These early investigations prompted a wide-ranging number of publications quantifying strontium in water, soil and biological tissues throughout the late 19th and early 20th centuries (for a review, see Odum 1950).
The first systematic approach to understanding strontium cycling came with the ground-breaking work of the ecologist Howard T. Odum. A graduate student at Yale, Odum was encouraged to investigate what he called ‘the strontium problem’ by his supervisor G. Evelyn Hutchinson. Hutchinson and Odum recognised that a comprehensive model of strontium cycling was not merely a chemical curiosity, but a window into global ecosystem processes and the biotic and abiotic connections that link diverse systems (Limburg 2004). For his thesis, Odum (1950) analysed 1100 samples of Sr/Ca in water, sediment, rocks and biota to quantitatively understand the magnitudes of elemental reservoirs and transfers among them. He used these measurements to propose large-scale cycling models that addressed weathering, riverine transport, atmospheric deposition and ocean cycling, in addition to uptake and incorporation into living tissues and calcified hard parts (Odum 1950). This remarkable body of work was the basis for a series of papers that addressed specific aspects of cycling dynamics (Odum 1951a, 1951b, 1957a, 1957b). Notably, Odum (1951b) experimentally measured the relationship between water and Physa gastropod shells and calculated a distribution factor, or partition coefficient, to quantify elemental incorporation rates for this species, much like otolith chemists continue to calculate today. Although the effect of ambient water composition on elemental uptake was clear, Odum was keenly aware of the possibility of taxonomic and physiological factors to regulate uptake. He hypothesised that membrane transport capacity, indicated by the thickness of the tissue separating seawater from the calcifying surface, and the complexity of the circulatory system could explain taxonomic differences in strontium uptake from algae to vertebrates (Odum 1957a; Fig. 2). Note that Fig. 2 includes fish otolith measurements, which is the first reported quantitative measurement of otolith strontium I have found in the literature. Odum’s work was foundational for the field of elemental tracer ecology in general, and emphasised many of the same issues otolith chemists grapple with today, including geological controls on water composition, temporal and spatial variability in ambient composition and physiological mediation of elemental uptake dynamics. These questions continue to drive important field-based and experimental work in otolith chemistry.

|
Currently, otolith chemistry as a discipline is diverse in both analytical approaches and the types of questions investigated using these remarkable biogenic structures. Probe-based instrumentation, such as laser ablation inductively coupled plasma mass spectrometry (ICP-MS), the current workhorse of the field, has improved in sensitivity and precision for many analytes. Laser wavelengths of 213 or 193 nm are common in many ICP-MS facilities, and femtosecond lasers hold promise for further improvements in analyses with single- and multiple-collector ICP-MS (Lord et al. 2011; Yang et al. 2011). Concordantly, the improvement in spatial resolution has allowed more targeted analyses of specified life history periods. Although whole otolith dissolutions were common in the 1990s, increasingly precise probe-based methods have allowed targeting of natal cores or post-settlement periods or complete life history transects across all growth increments. Furthermore, two-dimensional spatial mapping of elemental or isotopic distributions are possible with ICP-MS (Woodhead et al. 2007; Wang et al. 2013; McGowan et al. 2014; Petrus et al. 2017) or techniques such as proton-induced energy emission spectroscopy and scanning X-ray fluorescence microscopy (Limburg and Elfman 2017). These mapping approaches yield valuable visualisations of chemical heterogeneity across otoliths, allowing researchers to identify concentric fluctuations in an analyte, such as strontium, as well as radial differences in elemental patterns that would not be evident from a single transect alone (Fig. 3). Although these topographic quantifications of otolith elements have been explored before (e.g. Gauldie et al. 1991; Tzeng et al. 1997), they are becoming more readily available with improvements in instrumentation and mapping software.

|
How do we interpret chemical patterns?
With an expanding scope of analytical possibilities comes a persistent challenge for the otolith chemist. Elemental maps provide unprecedented insight into chemical patterns across structures, but have these advances translated into increased understanding about the life histories of these fishes? Put simply, how do we interpret these maps? A common tool that otolith chemists have used to translate chemical patterns into meaningful life history information is a partition coefficient, or discrimination coefficient, which is simply the ratio of the concentration of element E ([E]) in an otolith to the concentration in water, as follows:

This simple metric is most directly applicable when determining partitioning between an accreting solid and the immediately surrounding fluid. Although used extensively for calcifying organisms and the water they lived in, this metric has long been recognised to be a simplification that omits important intermediary transitions that can have dramatic effects on elemental uptake. If ultimately derived from seawater, elements must pass through multiple membranes and barriers before incorporation into an otolith, with possibility of discrimination or concentration at every step (Campana 1999). These barrier transitions may not be equilibrium processes, and many factors, including temperature, precipitation rate and solution composition, can affect partition coefficients in both abiogenic and biogenic precipitates. For these reasons, Morse and Bender (1990) urged workers to ‘use considerable caution in interpreting data on the composition of natural carbonates’. For fishes, a more explicit decomposition of the participation coefficient was suggested by Walther et al. (2010) to recognise the strong roles that each barrier transition may play in mediating overall uptake:
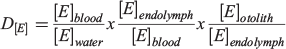
Indeed, this equation itself is insufficient given that elements may derive partially or predominantly from dietary sources. Thus, a companion equation to quantify elemental uptake from the diet would be:
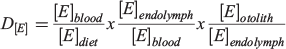
Clearly, a partition coefficient is a convenient shorthand, and the potential for physiological regulation or decoupling poses considerable challenges for attempts to interpret observed chemical patterns across otolith increments. These difficulties have been recognised for some time by otolith chemists, although practitioners must continuously remind themselves of the multifaceted intrinsic and extrinsic dynamics that influence otolith chemistry. The challenge remains how to appreciate these complexities yet continue to move the field of otolith chemistry forward without stagnation. To this end, a qualitative conceptual framework using an artistic metaphor is proposed below that may hold value in approaching the challenge of otolith chemistry interpretation.
A metaphor
Imagine you are at a museum, wandering through galleries and admiring the artwork. You notice a painting on a wall that stops you in your tracks with its beauty and power. You have never seen this image before, but something about its use of colour and form intrigues you. You wonder to yourself: when was this painted? Who was the artist? What were they trying to say? This prompts you to learn everything you can about the artist, their life and times, and the potential messages encoded in the image that so arrested you.
This metaphor of art appreciation has value when attempting to interpret the complex chemical patterns we observe in otoliths. Superficially, the ability to analyse and produce dramatic spatial maps of elemental variability in otoliths represented by shapes and colours has obvious parallels to a painted landscape. But more deeply, this metaphor gives us a framework for appreciating the many interpretive layers that can be brought to bear when attempting to decode an image, either artistic or ichthyological. Consider the 19th century artistic movement of Impressionism, which was practiced by artists including Claude Monet, Pierre-Auguste Renoir and Camille Pissaro, among others. Evolving out of the prior paradigms of Naturalism and Realism, which emphasised faithful and even idealised representations of nature, Impressionism used novel combinations of brushstrokes and colour to not only represent the world around the artists, but also to incorporate the observer’s subjective experience (Thompson 2000). Mood, perspective, experience and the artist’s interpretation of the world around them were integrated into images that communicated more than a simple image of their environment. One of the first paintings to be considered Impressionist and an inspiration for the label itself was the painting Impression, Sunrise (1872) by Claude Monet (Fig. 4). A depiction of rowboats in the port of La Havre, the work uses bold brushstrokes that are visible swathes of paint when viewed up close but meld at distance to illustrate a familiar scene. The painting also preserves a moment in time that captures the particular light, colour and shadows unique to an early sunrise, and evokes the experience of the painter that morning. The art critic Jules-Antoine Castagnary wrote in 1874 that these types of paintings were Impressionist ‘in the sense that they render not the landscape, but the sensation produced by the landscape’ (Rubin 1999). The artist is inserting themselves as a filter through which the landscape is observed, recorded and interpreted. Impressionism explicitly integrates images of the natural world with the subjective experience of the observer and the artist, and these paintings can be analysed and appreciated through multiple lenses. This perspective has metaphorical parallels to the challenges otolith chemists face when interpreting the often complex chemical patterns that are influenced by physiological and environmental factors alike.

|
Critical analysis of paintings can occur at many levels, as can interpretation of otolith chemistry patterns. The first level is that of the studio, or the environment in which the painting was made. Did the artist paint outdoors or inside, from a model or a memory? More broadly, when was this painting made in time, and what were the social and political settings that influenced the artist? The second level is that of the media, or the materials used to create the image. Did the artist use oils or gouaches, a few colours or a wide palette? How did the choice of media inform the image and its representation of the subject? The third level is that of the canvas, or the surface on which the image was painted. Was the canvas primed or bare, made of linen or cotton, or perhaps made of something entirely different, like a brick wall or the inside of a limestone cave? How did the canvas composition influence the choice of media and subject matter, and vice versa? Finally, the fourth level is that of the artist. Who was this person, and what was their motivation? Were they old or young, healthy or sickly, a loner or sociable? How did their life influence the way they saw the world and the way they made paintings? Each of these levels can be thought of as a filter that stands between reality, such as a landscape, and the one that eventually ends up in the final painting. Instead of a faithful snapshot of reality, the painting is filtered through the perspective of the artist and the materials they use to represent their environments.
Thus, a painting holds more information than simply what the landscape actually looked like because it simultaneously provides insight about the artist themselves. This is precisely the same challenge that we face when interpreting chemical patterns in otoliths. The ‘studio’ is the environment in which the fish lives. This includes the geographic and physical setting, as well as the spatial variability in the elements and isotopes that become proxies for the environment. The ‘media’ are the elements themselves. Each element records its own unique set of information and, together, a suite of elements provides the palette that can be observed. The ‘canvas’ is the otolith itself, with its specific organic and inorganic composition that mediates elemental incorporation dynamics. The canvas may change significantly when investigating otolith analogues, such as scales, fin rays or vertebrae, which are increasingly popular alternative structures that have their own growth dynamics and compositional properties. Finally, but most importantly, the fish is the Artist that ultimately integrates these filters and creates an otolith reflecting its individual experience. Internal characteristics such as physiological status, maturity and stress can all act as filters that modify the eventual chemical pattern, much like an artist’s subjective perception influences the paintings they make. For the remainder of this paper, I will use this metaphorical framework to highlight a few specific advances in each of these four areas, and I conclude with a proposed conceptual approach to integrate these perspectives.
The studio
A large proportion of otolith research seeks to identify natal origins or reconstruct movements through distinct habitats. Central to most of these efforts is the assumption that ambient variability in chemical constituents will be reflected in the accreted increments of the life history stage of interest, with uptake derived primarily through a water-derived pathway. For elements or isotope ratios in otoliths that have been validated to strongly or at least partially reflect the ambient dissolved composition, geographic variability in such constituents is an ultimate control on the spatial resolution of this tool (Elsdon et al. 2008). The ‘studio’, therefore, is the chemical background through which a fish travels naturally or experiences during experimental manipulations, and the degree to which the myriad environments a fish inhabits are chemically distinct determines whether such reconstructions from otoliths are even possible.
Considerable progress in geospatial mapping of chemical variability has been essential for many fields that use composition of animal tissues to determine migration pathways. Much of this work has been driven by researchers investigating terrestrial fauna and isotopic markers such as δ18O, δD and δ13C in hard or soft tissues (West et al. 2010; Hobson and Koehler 2015). The creation of high-resolution maps of isotope variability, or isoscapes, requires a combination of field-collected samples and models that interpolate and predict isotope composition for unsampled locations. Animals of unknown origin can then be assayed for the marker of interest and matched to the isoscape to quantitatively estimate putative locations of origin or migration. A principle concern in these efforts is parameterising temporal variability of an isotope marker in a given location (Wunder 2010; Trueman et al. 2012). The accuracy of locational assignments requires temporal variability to be smaller than differences between locations; with large overlap in signature values between locations, the ability to confidently determine which location was the true origin decreases. For isotope ratios that are especially labile and sensitive to climatic conditions, such as δ18O, it is important to remember that these isoscapes are not static but dynamic, and time is a crucial variable (Dutton et al. 2005; Walther and Thorrold 2009). Although much of this isoscape mapping work has been targeted towards terrestrial landscapes, similar tools have been used to construct marine isoscapes, although limited spatial coverage of sampling in some ocean basins remain an issue (McMahon et al. 2013a, 2013b).
A powerful and increasingly popular isotope system for migration studies is 87Sr/86Sr ratios. The utility of this system is due to several attractive features, including strong geological control that leads to significant nested variation at local to continental scales (Banner 2004) and the lack of apparent fractionation during uptake, meaning water values are directly recorded in biogenic structures without physiological decoupling (Beard and Johnson 2000). Because of the dominant influence of geological composition and age on this radiogenic ratio, predictive maps based on rock age and type can be constructed for use to assign natal origins or habitat use histories in fishes (Kennedy et al. 2000, 2002; Hegg et al. 2013). A primary value of these models and maps is their ability to provide at least first-order estimations about whether certain habitats may be chemically distinguishable. Thus, these maps provide guidance on the feasibility of testing hypotheses about migration between specific locations (e.g. Humston et al. 2017). This type of a priori prediction of chemical variability is incredibly valuable given limited budgets and difficulties in sampling logistics that restrict the ability to sample water from all putative habitats before a project begins. These maps, to put it simply, reveal what questions we might ask.
Models that predict large-scale variation in 87Sr/86Sr ratios have become increasingly sophisticated over the past decade. Barnett-Johnson et al. (2008) predicted >90% of the variability in ratios recorded in salmonid otoliths in the western US with a model based on the proportion of the watershed containing granitic bedrock. Subsequently, Bataille and Bowen (2012) developed a model of bedrock lithology for silicates and carbonates as well as age to estimate rock-specific radiogenic decay of 87Rb to 87Sr from parent rock compositions, which were combined with geographic information system (GIS) geology maps to estimate 87Sr/86Sr variation across the contiguous US. This model then incorporated weathering coefficients for specific rock types combined with hydrological flow measurements to estimate local catchment compositions for specific regions. The flux-weighted models that incorporated bedrock type, age and weathering had a high degree of accuracy (~70%) in predicting observed 87Sr/86Sr ratios in specific regions. This model was further improved by Bataille et al. (2014) through refinements of model components including siliciclastic sedimentary rock submodel improvements to account for grain recycling, and an updated weathering model for Alaska that incorporated permafrost and glacial processes that can affect Sr flux at higher latitudes. These models point to the need to incorporate both general isotope systematics as well as regional hydrodynamic processes to more accurately estimate the dissolved 87Sr/86Sr present in streams and rivers that fish inhabit, thereby allowing highly accurate estimation of natal origins and migration patterns of freshwater and diadromous fishes (Brennan and Schindler 2017).
For fish that transit salinity gradients, a central concern is understanding how targeted elements vary across an estuary. The theory and practice of hydrological mixing dynamics are of paramount concern to discern whether movements between salinity regimes are even detectable. Although the pertinent concepts about mixing have been discussed previously (e.g. Kraus and Secor 2004; Gillanders 2005; Milton and Chenery 2005; Walther and Limburg 2012), they are worth revisiting in the context of understanding the ‘studio’ in which otoliths are formed. First, when considering differences between fresh and marine chemical signatures, the chemical compositions of both water masses on either end of a mixing dynamic, or endmembers, must be known. For example, the larger the difference between fresh and marine Sr concentrations, the greater the likelihood of detecting a movement between those habitats. For an element like Sr, where marine concentrations are relatively homogeneous and constant on ecological time scales compared to fresh waters (de Villiers 1999), it is the freshwater endmember that is of most concern for otolith chemists. The strong geological control on freshwater Sr composition therefore requires one to inspect a geological map of the catchment to investigate the bedrock composition of the drainages. Given significant variation in Sr composition among lithologies, some systems, such as those dominated by marine carbonates, may have very similar or nearly identical compositions to that of marine waters (Brown and Severin 2009). With limited differences in the selected chemical composition between fresh and marine endmembers, there is little scope for detecting movement in those systems. This ultimate environmental control on the utility of chemical tracking for a particular system thus requires the researcher to carefully consider the regional geological setting before selecting otolith chemistry as a viable tool.
Beyond considering the endmembers themselves, the mixing dynamics across the salinity gradient are also of great interest. A dissolved element is considered conservative if it exhibits linear mixing between two water masses, such as fresh and marine endmembers. Non-conservative mixing is indicated by positive or negative curvature in the relationship between the element and salinity, which results when there are internal kinetics that result in net addition or removal of the constituent at an intermediate salinity (Ward and Montague 1996). Note that a linear relationship with salinity does not mean that there is an absence of kinetics for the constituent in the estuary, only that there is no net kinetic addition or removal at mid-salinities. A relevant example of this is dissolved Ba, which exhibits net addition at low salinities where riverine particles containing adsorbed Ba first hit the salt wedge and Ba ions are desorbed and released into the water column (Coffey et al. 1997). This low salinity peak in Ba is observed in most estuaries worldwide, with the magnitude of the peak depending on the particulate load and geological composition of the catchment (Sinclair and McCulloch 2004).
However, the ability to determine whether an element is conservative or non-conservative based on linear or non-linear behaviour requires that specific set of assumptions are met. If one or more of those assumptions are violated, non-linear relationships between the element and salinity may be observed even if the element itself is still behaving conservatively. Critical assumptions include: (1) there are only two endmembers (violated if there are multiple streams or even groundwater contributions with distinct chemical compositions all contributing to the estuary); (2) that salinity itself is conservative (violated if strong evaporative processes cause alterations in salinity that are not due to mixing); and (3) that the endmember compositions are temporally stable with respect to the residence time of the estuary (violated if short-term flood or drought regimes alter upstream weathering of unique lithologies, for example). Thus, the observation of non-linear behaviour can, on the one hand, indicate non-conservative processes, but may indicate on the other hand that one or more assumptions have been violated. Why is this of concern for otolith chemists? The shape and scope of the mixing curves for these tracer elements are again the ultimate control dictating whether movements are discernible. The hydrodynamics and elemental kinetics of the system are useful to identify whether movement between specific salinity regimes is resolvable, and they may also indicate important ecological considerations, such as the contribution of multiple catchments (and therefore potential habitats for migration) or the dominance of evaporative processes that could induce salinity stress for stationary fishes (Barnett-Johnson et al. 2008; Gillanders and Munro 2012; Hegg et al. 2015; Mohan and Walther 2015). Careful consideration of the environmental processes and their controls on constituent behaviour is key to the successful use and interpretation of otolith chemistry patterns as a movement tracking tool.
The media
Turning to the media, or the chemical constituents that may capture some aspect of the life of a fish, we must identify the palette of elements and isotopes available for observation. A small handful of elements has dominated the literature, in part because certain elements are readily incorporated into the aragonitic crystal lattice, they may pass through Ca channels and substitute directly for Ca, thereby more effectively representing the ambient water composition, and because of their relative abundance in otoliths, rendering them reliably assayed with common instrumentation. Thus, the alkaline earth metals isolated by Sir Humphry Davy, such as Sr and Ba, are of great utility. In addition, the isotopic ratios of C and O are readily quantified in any carbonate structure and useful given the great deal of work on fractionation and isotope systematics in other aragonitic skeletons, such as those of scleractinian corals. The other key to a useful ‘colour’ is that the element or isotope ratio varies environmentally with a gradient such as salinity or responds to another variable of interest like temperature or dissolved oxygen (Radtke 1989; Thorrold et al. 1997; Elsdon and Gillanders 2002; Limburg et al. 2015). These required characteristics naturally winnow to the spectrum of potentially useful elements down to a handful, which are typically sufficient for many questions and systems.
However, with advances in instrumental sensitivities and precisions and expanding experimental validations to understand uptake dynamics of other elements, our palette may expand. Less frequently investigated elements such as Fe, Pb, Cu, Zn and S (bulk and isotope ratios) are detectable in otoliths, although analytical challenges for quantifying their concentrations persist (Spencer et al. 2000; Limburg and Elfman 2010; Daverat et al. 2012; Di Franco et al. 2014; Hüssy et al. 2016; Doubleday et al. 2018). The degree to which some of these elements reflect ambient water compositions or are highly regulated by physiological discrimination is poorly known (but see Geffen et al. (1998) and Milton and Chenery (2001b), among others). Experimental validations of these alternative elements are rare, due in part to the difficulty in rearing fish in carefully controlled elemental environments with minimal contamination of trace metals such as Fe and Pb during rearing or analysis (Arslan and Secor 2008; Selleslagh et al. 2016). Yet, we must encourage new otolith chemists to devise ways to experimentally manipulate water and diets of these alternative elements in an attempt to widen the available palette beyond the now-traditional elements.
A key feature of an otolith is that it is essentially a mixed-media artwork. Rather than simply an abiotic accretion, the structure is biogenic and created with a complex protein lattice that forms the scaffolding on which the carbonate crystals accrete (Murayama et al. 2005; Miller et al. 2006; Weigele et al. 2016). The nature and composition of the organic fraction of an otolith is still being revealed, posing both challenges and opportunities for interpretations of chemical patterns. The degree to which proteins bind and alter elemental availability for transport across membranes or incorporation into the crystal structure itself contributes greatly to the ability of an element to accurately reflect ambient water compositions (Payan et al. 1999, 2004a, 2004b; Borelli et al. 2001, 2003a, 2003b). A careful consideration of protein–element interactions at all stages along the relevant physiological pathways is important. For example, Thomas et al. (2017) found that although the dissolved fraction of Ca, Sr and Ba in endolymph is large, a significant proportion of those same elements is protein bound and thus potentially unavailable for incorporation into the otolith. Within the otolith itself, the assumption that elements substitute for Ca in the carbonate crystal may not be uniformly accurate, and the protein lattice itself may facilitate the uptake of some elements (McFadden et al. 2016). Izzo et al. (2016) found that the fraction of protein-bound elements within otoliths varied, with minimal proportions of Ba bound to proteins but larger proportions of protein-bound Mn and Cu. These element-specific protein interactions contribute, in part, to their respective partition coefficients and illustrate the complexities of attempting to interpret elemental patterns in these mixed-media structures.
In addition to affecting uptake dynamics of traditional elements, the protein matrix itself can be assayed for vital information about the life of a fish. Although low in total concentration, the proteins offer the potential for insight into dietary dynamics of fishes that cannot be probed effectively with elements such as Sr and Ba. The primary limitation for assaying the organic fraction has been purifying sufficient material for analysis. However, new methods for isolating and quantifying proteins show great promise. For example, Lueders-Dumont et al. (2018) found that otolith-bound δ15N distinguished between farmed and wild salmonids, and Sirot et al. (2017) used otolith δ15N and δ13C together to investigate the diets of both modern and archived otoliths. Beyond just bulk analysis, the ability to quantify compound-specific amino acid stable isotope ratios is a frontier with much potential. The advantage of this compound-specific approach is the use of certain amino acids that undergo minimal trophic fractionation (‘essential amino acids’) to estimate baseline carbon fixation sources and other highly fractionated amino acids to estimate trophic position (McClelland and Montoya 2002; Larsen et al. 2013; Nielsen et al. 2015). The ground-breaking work by McMahon et al. (2011a, 2011b, 2016) showed that this approach was possible in otoliths, and δ13C values of amino acids effectively distinguished fish who fed on mangrove or seagrass food webs. More recently, Vane et al. (2018) used δ15N of ‘trophic’ and ‘source’ amino acids to estimate ontogenetic trophic shifts of mobile fishes. This compound-specific approach is likely to become more frequently used as methods and instrumentation become more accessible. Truly, the organic fraction of otoliths is a fruitful area of study not only to increase our understanding of elemental incorporation dynamics, but also to open the door to additional questions about dietary and metabolic histories contained in the proteins themselves.
The canvas
The canvas refers to the structure on which the chemical patterns are created. As described above, an otolith is composed primarily of calcium carbonate but with a small but significant fraction of organic matter, thereby influencing the choice of elemental and isotopic proxies that can be investigated. The properties of otoliths, including their acellular structure and lack of metabolic reworking, are fundamental to our ability to interpret patterns as time-stamped reflections of a particular period in the life of a fish (Campana and Thorrold 2001). Although the primary form of most otoliths is aragonite, other crystal forms may be present in particular species, such as vaterite and calcite, which have important implications for the relative abundance and incorporation dynamics of particular elements (Tzeng et al. 2007; Pracheil et al. 2017). Thus, the properties of an otolith are paramount for dictating how and which chemical proxies are incorporated and effectively interpreted. Parallel with the growth in the field of otolith chemistry has been the rapid development of alternative and complementary chemical records in other structures, such as scales, fin spines and rays, eye lenses and vertebrae. These ‘otolith analogues’ have their own set of challenges and opportunities, largely dictated by the relative proportion of calcified and uncalcified material, the degree of metabolic stability and the periodicity of increment formation (for a review, see Tzadik et al. 2017). Workers investigating these alternative structures tend to come from a background of either soft tissue stable isotope research or otolith chemistry, with concomitant focus on one or the other set of proxies in these structures, although work using combinations of proxies may become more frequent.
Scales offer the potential for paired organic isotope assays with Sr/Ca and Ba/Ca measurements with less limitations of sample size as encountered with otoliths (Woodcock and Walther 2014; Seeley et al. 2015). However, their overlain bipartite structure renders the interpretation of interior increments difficult for many species (Hutchinson and Trueman 2006; Trueman and Moore 2007), resorption may occur and the periodicity of scale increment formation may be irregular or decoupled from that in otoliths for some species (Campana 2001; Abecasis et al. 2008; Upton et al. 2012). Furthermore, workers must be cautious to exclude regenerated scales if they wish to obtain a more complete life history record (Seeley et al. 2017), and scales only begin formation at squamation, which means they are typically useless for determining larval origins. Fin spines and rays offer similar potential for assays of organic stable isotope ratios, although with comparable uncertainties about increment periodicity, metabolic reworking and record completeness. A primary reason to use structures such as fin spines or scales is the non-lethality of sampling, which may be required for work with imperilled species or catch-and-release fisheries.
Other structures that are sampled lethally include eye lenses and vertebrae. Vertebrae have been targeted by those investigating migrations and the life history of sharks, where otoliths are not available for analysis (Raoult et al. 2016; Smith et al. 2016; McMillan et al. 2017). One intriguing and exciting reason to investigate vertebrae in these organisms is the presence of material accreted before birth, potentially providing information about in utero growth, and therefore maternal diets and movements (Carlisle et al. 2015). Although, as with the other structures, the potential for metabolic instability and lack of experimental validation of uptake and turnover for many of these long-lived and large species is a continued impediment (but see Werry et al. 2011; Smith et al. 2013). Eye lens composition is an emerging technique that uses separated laminae for individual analyses of stable isotopes or elemental composition (Wallace et al. 2014; Quaeck-Davies et al. 2018). For all these structures, continued investigation into transport, incorporation and stability of chemical signatures is key. One potential value-added way to rapidly advance this field is for researchers who conduct experiments on otolith chemical uptake dynamics to retain as many tissues as possible (vertebrae, eye lenses, scales etc.) from the fish at the conclusion of the experiment. Although costly to analyse multiple structures, I encourage researchers to reach out to one another to attempt to coordinate analyses among multiple laboratories to maximise outputs. Importantly, this multilaboratory effort could allow comparisons of patterns in the same element across multiple structures, which is sorely needed in this field.
Finally, for otolith chemists attempting to understand elemental uptake dynamics into these carbonate structures, we must stay familiar with the advances in other biogenic carbonate research, including work on scleractinian corals, foraminifera and sclerosponges. Certainly, the taxonomic and physiological constraints are quite different in these disparate groups, but theoretical and methodological advances in coral sclerochronology, for example, could provide a framework for modelling and experimental approaches to understanding comparable processes in fishes. Researchers in those fields are engaged in similar attempts to understand how physiological processes are regulating elemental movement across membranes to the calcification surface and to what extent those transport dynamics may influence a proxy such as the Sr/Ca ratio and temperature relationship. Current debate revolves around the extent to which Rayleigh fractionation in partially closed calcification systems is also mediated by Ca2+-ATPase activity, which itself responds to environmental conditions such as temperature, pH, aragonite saturation state and elemental concentrations (Gaetani et al. 2011; Thien et al. 2014; DeCarlo et al. 2015; Tanaka et al. 2015; Giri et al. 2018). Combined models that incorporate direct environmental and vital effects on elemental incorporation have been developed (e.g. DeCarlo et al. 2016), which could be usefully adapted and modified for fish physiologies and provide a framework for future investigation and experimentation. Thus, keeping pace with advances in analogous fields that are investigating alternative ‘canvases’ could allow otolith chemists to make great strides in understanding the interplay between biogenic and abiogenic factors without starting from scratch.
The artist
Finally, we come to the ultimate integrator of extrinsic and intrinsic drivers and the creator of the otolith itself: the fish. As the artist responsible for the patterns we observe in these structures, the ‘perspective’ of each fish must be considered when interpreting chemical signatures recorded in otoliths. Although it is objectively obvious that the physiology and experience of the fish is important, it is all too easy to think of an otolith as a simple recorder of the environment with no filtering of the ambient signal. A survey of the otolith chemistry literature will find numerous statements to the effect that ‘elements in water are recorded in otoliths’ as a justification for using the approach to study movement dynamics. This language is imprecise in a few ways. First, it implies passive and direct incorporation of elements without any physiological modification (either concentration or discrimination) during the various pathways from water to otolith. Second, it assumes water is the only source of otolith elements, which may be incorrect for some constituents. Third, it collectively lumps the uptake of all elements into a single monolithic group, implying that transport dynamics are similar for any element found in an otolith. Although some elements do reflect ambient water composition, as modified by physiology, temperature and other factors, other elements (e.g. Mg) have not yet been found to accurately reflect water composition and may more closely reflect metabolism (Woodcock et al. 2012; Limburg et al. 2018). This language is of course a shorthand and could be forgiven because not every publication on otolith chemistry requires a full exposition of the complexities of uptake in the Introduction of a paper. But we must be cautious to not let literary shorthand devolve into faulty assumptions. Students and new practitioners of otolith chemistry must be especially careful to remind themselves of these assumptions and do their best to use accurate phraseology.
As mentioned throughout this paper, the myriad intrinsic factors that can potentially regulate or even decouple otolith elemental composition from that in the environment are complex and, in some cases, poorly understood. Metaphorically, the fish as an artist creates its artwork that is both constrained and enabled by its health, condition, behaviour and environment. Taxonomic groups with divergent physiologies could be viewed as analogous to artistic movements with recognisable styles. Impressionists, Surrealists and Modernists produce identifiably different artworks, much like Istiophoridae, Salmonidae and Megalopidae produce distinct otoliths. Although these issues have been recognised throughout the history of this field, much work remains to be done to disentangle the relative effects of these processes. A focus on ionic transport of elements across membranes is critical (Hirose et al. 2003). Many of the central elements in our palette, such as Sr and Ba, are assumed to mimic the behaviour of Ca and move through Ca ion channels. If these ions do follow such transport pathways, then calcium homeostasis and the hormonal and other pertinent regulatory processes are of critical concern for understanding physiological mediation of ion behaviour. As is well known in the physiological literature, maintenance of homeostasis will be affected by a wide variety of physiological and life history perturbations, including stress, circadian rhythms, vitellogenesis, reproduction, osmoregulation and metamorphosis (Marshall and Grosell 2006). Sex and reproductive status are potentially quite influential, particularly for marine fish, which live in more chemically homogeneous environments (Sturrock et al. 2014, 2015), but these parameters are not always recorded in wild-collected fish samples (exceptions include Edmonds et al. 1991; Campana et al. 1994; Gauldie 1996; Secor and Piccoli 1996; Milton and Chenery 2001a; Ashford et al. 2006; Brenkman et al. 2007; Han and Tzeng 2007; Jónsdóttir et al. 2007; Arkhipkin et al. 2009; Albuquerque et al. 2012). Of particular relevance to those tracking diadromous movements is the altered ion regulation requirements for fish inhabiting fresh or marine environments where they are hyperosmotic or hypo-osmotic respectively. Elemental partition coefficients are known to vary with salinity (Elsdon and Gillanders 2003; Gillanders and Munro 2012; Panfili et al. 2015), and ion regulation may be a partial explanation for this phenomenon.
Together, the importance of ion transport processes points to a continued need for experimentation that explicitly integrates physiological and environmental perspectives to understand uptake. Here, otolith chemists may find useful collaboration with physiologists already heavily engaged in ion transport dynamics, particularly because that field has grappled with the effects of aquatic acidification on stress, homeostasis, ion transport and carbonate accretion responses (Pörtner 2008; Heuer and Grosell 2014; Esbaugh 2018). Complementary approaches to quantifying environmental experiences, such as archival tagging technologies, can also be usefully coupled with otolith chemistry analyses to disentangle extrinsic forcing on observed chemical patterns (Darnaude et al. 2014; Darnaude and Hunter 2018). Further, meta-analyses of the existing wide range of experimental evaluations of factors influencing elemental uptake in otoliths will be critical for highlighting gaps where future efforts can be focused (Izzo et al. 2018). Much as a painting is created by an artist with an idiosyncratic set of experiences and perspectives, an otolith does not form spontaneously in water, but rather is created by a fish with its own physiological dynamics and experiential history that mediate and influence the observed patterns incorporated into the structure itself.
Signal-to-noise approach
The continued challenge for practitioners of otolith chemistry is to bear these multifarious and interactive extrinsic and intrinsic factors in mind when attempting to interpret a chemical pattern for a specific question. How to reconcile these issues while still deriving meaningful information from these structures? One potential approach, at least conceptually, borrows terminology from the field of signal processing. Specifically, we may consider an otolith chemical pattern to be similar to an acoustic waveform or captured image, with the target signal of interest embedded with additional information, or noise, that obscures its resolution. Of central interest, then, is the signal-to-noise ratio (SNR), which dictates whether our signal has a chance of being resolved from the ‘background’ of other dynamic factors.
Consider the case of attempting to identify a fish movement across a salinity gradient with otolith chemistry (Fig. 5). The expectation is that a tracer such as Sr/Ca varies substantially across the salinity gradient with the freshwater endmember identifiably lower than the marine endmember (Fig. 5a). However, as discussed above, this assumption must be validated and a catchment with a limestone-dominated bedrock may have elevated freshwater Sr/Ca and therefore a minimal difference between endmembers (Fig. 5b). These two scenarios impose very different constraints on the ability to detect a movement from fresh to marine waters, particularly given other factors that include additional variation on the final otolith pattern (depicted here as random noise, but which could also manifest as systematic or non-random alterations). If the magnitude of these alternative influences is moderate, then a movement across an environmental backdrop of a large difference between endmembers should still be resolvable. In that scenario, the SNR is high (Fig. 5c). However, with reduced endmember difference, the noise may be equivalent in magnitude to the signal, the SNR is low and the movement is more difficult to detect (Fig. 5d). Of course, the categorisation of particular factors as signal or noise depends on the question being asked. An investigator may identify a physiological transition, such as reproductive status, as the desired signal, in which case chemical variability induced by movements across environmental gradients would be the noise for that study.
In order to implement this type of SNR approach, the relative magnitudes of pertinent factors must be known at least to a first-order approximation. If magnitudes of the effects of intrinsic and non-target extrinsic factors are known, then the magnitude of the required chemical gradient to impart detectable signals of movement could be estimated. This estimation may be possible for some taxonomic groups given current experimental results. Species-specific or stock-specific differences in uptake dynamics may challenge our ability to extrapolate these results to species where experimental work is limited (Clarke et al. 2011; Barnes and Gillanders 2013; Chang and Geffen 2013). However, multi-elemental comparisons among proxies that have varying degrees of physiological control is a promising way to identify the relative effects of intrinsic and extrinsic factors (Grammer et al. 2017). This SNR framework could be useful in pointing towards gaps in experimental knowledge and guide new practitioners towards essential preliminary steps when evaluating whether otolith chemistry will be a viable tool.
Conclusion
Returning to the artistic metaphor, we must consider the museum patron who enjoys and analyses the art in front of them. This patron is the scientist attempting to understand something about the life and times of fishes. Much like the art historians that now use advanced image processing and even elemental analyses to establish provenance and creation of important artworks (Marin et al. 2015; Cucci et al. 2016), otolith chemistry tools have grown in sophistication and accessibility as instrumentation has advanced. Yet, technological advances do not eliminate the possibility that the observer is misled when interpreting a pattern. Observer bias is an ever-present issue in biological studies (Holman et al. 2015; Kardish et al. 2015), and otolith chemists must caution themselves about the possibility that they are biased towards observing particular types of patterns, or they may simply not recognise patterns that are present. Scientists are the final filter through which the data are passed before an interpretation is produced.
Despite these many challenges inherent to the interpretation of otolith chemistry patterns, the field has grown dramatically over recent decades and afforded great insight into the life histories, population dynamics and habitat requirements of a diverse range of species. An otolith is truly an integrated portrait of the life of a fish, including information about the environment, its physiology and growth patterns in one singular structure. Rather than turning a blind eye to these complicated issues, our field has flourished by embracing this complexity and applying innovative experimentation and adoption of technological improvements to disentangle these dynamics. By reminding ourselves that an otolith reflects not just the external environment, but also the singular point of view of each fish, we can continue to interpret these complicated patterns. We use otoliths to appreciate not just the landscape imprinted in the image, but also the perspective of the artist. As visitors to these aquatic galleries, we are truly lucky to have such rich and rewarding images to contemplate.
Conflicts of interest
The author declares that he has no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
Sincere thanks to Chia-Hui Wang and the International Otolith Symposium (IOS) Organizing Committee for inviting the author to present a keynote talk at the Sixth IOS held 15–20 April 2018 in Keelung, Taiwan. The author thanks several important mentors who have guided and supported him in this field over the years: Simon Thorrold, Bronwyn Gillanders, Michael Kingsford and Karin Limburg. Thanks also to David Secor and one anonymous reviewer who provided insightful comments that greatly improved the manuscript. This paper is dedicated to the author’s grandmother, Dwaine Walther, who taught him that a love of science and a love of art are compatible.
References
Abecasis, D., Bentes, L., Coelho, R., Correia, C., Lino, P. G., Monteiro, P., Gonçalves, J. M. S., Ribeiro, J., and Erzini, K. (2008). Ageing seabreams: a comparative study between scales and otoliths. Fisheries Research 89, 37–48.| Ageing seabreams: a comparative study between scales and otoliths.Crossref | GoogleScholarGoogle Scholar |
Albuquerque, C., Miekeley, N., Muelbert, J., Walther, B., and Jaureguizar, A. (2012). Estuarine dependency in a marine fish evaluated with otolith chemistry. Marine Biology 159, 2229–2239.
| Estuarine dependency in a marine fish evaluated with otolith chemistry.Crossref | GoogleScholarGoogle Scholar |
Arkhipkin, A. I., Schuchert, P. C., and Danyushevsky, L. (2009). Otolith chemistry reveals fine population structure and close affinity to the Pacific and Atlantic oceanic spawning grounds in the migratory southern blue whiting (Micromesistius australis australis). Fisheries Research 96, 188–194.
| Otolith chemistry reveals fine population structure and close affinity to the Pacific and Atlantic oceanic spawning grounds in the migratory southern blue whiting (Micromesistius australis australis).Crossref | GoogleScholarGoogle Scholar |
Arslan, Z., and Secor, D. H. (2008). High resolution micromill sampling for analysis of fish otoliths by ICP-MS: effects of sampling and specimen preparation on trace element fingerprints. Marine Environmental Research 66, 364–371.
| High resolution micromill sampling for analysis of fish otoliths by ICP-MS: effects of sampling and specimen preparation on trace element fingerprints.Crossref | GoogleScholarGoogle Scholar | 18640714PubMed |
Ashford, J. R., Arkhipkin, A. I., and Jones, C. M. (2006). Can the chemistry of otolith nuclei determine population structure of Patagonian toothfish Dissostichus eleginoides? Journal of Fish Biology 69, 708–721.
| Can the chemistry of otolith nuclei determine population structure of Patagonian toothfish Dissostichus eleginoides?Crossref | GoogleScholarGoogle Scholar |
Banner, J. L. (2004). Radiogenic isotopes: systematics and applications to earth surface processes and chemical stratigraphy. Earth-Science Reviews 65, 141–194.
| Radiogenic isotopes: systematics and applications to earth surface processes and chemical stratigraphy.Crossref | GoogleScholarGoogle Scholar |
Barnes, T. C., and Gillanders, B. M. (2013). Combined effects of extrinsic and intrinsic factors on otolith chemistry: implications for environmental reconstructions. Canadian Journal of Fisheries and Aquatic Sciences 70, 1159–1166.
| Combined effects of extrinsic and intrinsic factors on otolith chemistry: implications for environmental reconstructions.Crossref | GoogleScholarGoogle Scholar |
Barnett-Johnson, R., Pearson, T. E., and Ramos, F. C. (2008). Tracking natal origins of salmon using isotopes, otoliths, and landscape geology. Limnology and Oceanography 53, 1633–1642.
| Tracking natal origins of salmon using isotopes, otoliths, and landscape geology.Crossref | GoogleScholarGoogle Scholar |
Bataille, C. P., and Bowen, G. J. (2012). Mapping 87Sr/86Sr variations in bedrock and water for large scale provenance studies. Chemical Geology 304–305, 39–52.
| Mapping 87Sr/86Sr variations in bedrock and water for large scale provenance studies.Crossref | GoogleScholarGoogle Scholar |
Bataille, C. P., Brennan, S. R., Hartmann, J., Moosdorf, N., Wooller, M. J., and Bowen, G. J. (2014). A geostatistical framework for predicting variations in strontium concentrations and isotope ratios in Alaskan rivers. Chemical Geology 389, 1–15.
| A geostatistical framework for predicting variations in strontium concentrations and isotope ratios in Alaskan rivers.Crossref | GoogleScholarGoogle Scholar |
Beard, B. L., and Johnson, C. M. (2000). Strontium isotope composition of skeletal material can determine the birth place and geographic mobility of humans and animals. Journal of Forensic Sciences 45, 1049–1061.
| Strontium isotope composition of skeletal material can determine the birth place and geographic mobility of humans and animals.Crossref | GoogleScholarGoogle Scholar | 11005180PubMed |
Borelli, G., Mayer-Gostan, N., De Pontual, H., Boeuf, G., and Payan, P. (2001). Biochemical relationships between endolymph and otolith matrix in the trout (Oncorhynchus mykiss) and turbot (Psetta maxima). Calcified Tissue International 69, 356–364.
| Biochemical relationships between endolymph and otolith matrix in the trout (Oncorhynchus mykiss) and turbot (Psetta maxima).Crossref | GoogleScholarGoogle Scholar | 11800233PubMed |
Borelli, G., Guibbohni, M. E., Mayer-Gostan, N., Priouzeau, F., De Pontual, H., Allemand, D., Puverel, S., Tambutte, E., and Payan, P. (2003a). Daily variations of endolymph composition: relationship with the otolith calcification process in trout. The Journal of Experimental Biology 206, 2685–2692.
| Daily variations of endolymph composition: relationship with the otolith calcification process in trout.Crossref | GoogleScholarGoogle Scholar | 12819274PubMed |
Borelli, G., Mayer-Gostan, N., Merle, P. L., De Pontual, H., Boeuf, G., Allemand, D., and Payan, P. (2003b). Composition of biomineral organic matrices with special emphasis on turbot (Psetta maxima) otolith and endolymph. Calcified Tissue International 72, 717–725.
| Composition of biomineral organic matrices with special emphasis on turbot (Psetta maxima) otolith and endolymph.Crossref | GoogleScholarGoogle Scholar | 14563001PubMed |
Brenkman, S. J., Corbett, S. C., and Volk, E. C. (2007). Use of otolith chemistry and radiotelemetry to determine age-specific migratory patterns of anadromous bull trout in the Hoh River, Washington. Transactions of the American Fisheries Society 136, 1–11.
| Use of otolith chemistry and radiotelemetry to determine age-specific migratory patterns of anadromous bull trout in the Hoh River, Washington.Crossref | GoogleScholarGoogle Scholar |
Brennan, S. R., and Schindler, D. E. (2017). Linking otolith microchemistry and dendritic isoscapes to map heterogeneous production of fish across river basins. Ecological Applications 27, 363–377.
| Linking otolith microchemistry and dendritic isoscapes to map heterogeneous production of fish across river basins.Crossref | GoogleScholarGoogle Scholar | 27875020PubMed |
Brown, R. J., and Severin, K. P. (2009). Otolith chemistry analyses indicate that water Sr : Ca is the primary factor influencing otolith Sr : Ca for freshwater and diadromous fish but not for marine fish. Canadian Journal of Fisheries and Aquatic Sciences 66, 1790–1808.
| Otolith chemistry analyses indicate that water Sr : Ca is the primary factor influencing otolith Sr : Ca for freshwater and diadromous fish but not for marine fish.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E. (1999). Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Marine Ecology Progress Series 188, 263–297.
| Chemistry and composition of fish otoliths: pathways, mechanisms and applications.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197–242.
| Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., and Thorrold, S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Sciences 58, 30–38.
| Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations?Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Fowler, A. J., and Jones, C. M. (1994). Otolith elemental fingerprinting for stock identification of Atlantic cod (Gadus morhua) using laser ablation ICPMS. Canadian Journal of Fisheries and Aquatic Sciences 51, 1942–1950.
| Otolith elemental fingerprinting for stock identification of Atlantic cod (Gadus morhua) using laser ablation ICPMS.Crossref | GoogleScholarGoogle Scholar |
Carlisle, A. B., Goldman, K. J., Litvin, S. Y., Madigan, D. J., Bigman, J. S., Swithenbank, A. M., Kline, T. C., and Block, B. A. (2015). Stable isotope analysis of vertebrae reveals ontogenetic changes in habitat in an endothermic pelagic shark. Proceedings of the Royal Society of London – B. Biological Sciences 282, –20411446.
| Stable isotope analysis of vertebrae reveals ontogenetic changes in habitat in an endothermic pelagic shark.Crossref | GoogleScholarGoogle Scholar |
Chang, M.-Y., and Geffen, A. J. (2013). Taxonomic and geographic influences on fish otolith microchemistry. Fish and Fisheries 14, 458–492.
| Taxonomic and geographic influences on fish otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Clarke, L. M., Thorrold, S. R., and Conover, D. O. (2011). Population differences in otolith chemistry have a genetic basis in Menidia menidia. Canadian Journal of Fisheries and Aquatic Sciences 68, 105–114.
| Population differences in otolith chemistry have a genetic basis in Menidia menidia.Crossref | GoogleScholarGoogle Scholar |
Coffey, M., Dehairs, F., Collette, O., Luther, G., Church, T., and Jickells, T. (1997). The behaviour of dissolved barium in estuaries. Estuarine, Coastal and Shelf Science 45, 113–121.
| The behaviour of dissolved barium in estuaries.Crossref | GoogleScholarGoogle Scholar |
Cucci, C., Delaney, J. K., and Picollo, M. (2016). Reflectance hyperspectral imaging for investigation of works of art: Old Master paintings and illuminated manuscripts. Accounts of Chemical Research 49, 2070–2079.
| Reflectance hyperspectral imaging for investigation of works of art: Old Master paintings and illuminated manuscripts.Crossref | GoogleScholarGoogle Scholar | 27677864PubMed |
Darnaude, A. M., and Hunter, E. (2018). Validation of otolith δ18O values as effective natural tags for shelf-scale geolocation of migrating fish. Marine Ecology Progress Series 598, 167–185.
| Validation of otolith δ18O values as effective natural tags for shelf-scale geolocation of migrating fish.Crossref | GoogleScholarGoogle Scholar |
Darnaude, A. M., Sturrock, A., Trueman, C. N., Mouillot, D., EIMF Campana, S. E., and Hunter, E. (2014). Listening in on the past: what can otolith δ18O values really tell us about the environmental history of fishes? PLoS One 9, e108539.
| Listening in on the past: what can otolith δ18O values really tell us about the environmental history of fishes?Crossref | GoogleScholarGoogle Scholar | 25279667PubMed |
Daverat, F., Lanceleur, L., Pecheyran, C., Eon, M., Dublon, J., Pierre, M., Schafer, J., Baudrimont, M., and Renault, S. (2012). Accumulation of Mn, Co, Zn, Rb, Cd, Sn, Ba, Sr, and Pb in the otoliths and tissues of eel (Anguilla anguilla) following long-term exposure in an estuarine environment. The Science of the Total Environment 437, 323–330.
| Accumulation of Mn, Co, Zn, Rb, Cd, Sn, Ba, Sr, and Pb in the otoliths and tissues of eel (Anguilla anguilla) following long-term exposure in an estuarine environment.Crossref | GoogleScholarGoogle Scholar | 22954653PubMed |
de Villiers, S. (1999). Seawater strontium and Sr/Ca variability in the Atlantic and Pacific oceans. Earth and Planetary Science Letters 171, 623–634.
| Seawater strontium and Sr/Ca variability in the Atlantic and Pacific oceans.Crossref | GoogleScholarGoogle Scholar |
DeCarlo, T. M., Gaetani, G. A., Holcomb, M., and Cohen, A. L. (2015). Experimental determination of factors controlling U/Ca of aragonite precipitated from seawater: implications for interpreting coral skeleton. Geochimica et Cosmochimica Acta 162, 151–165.
| Experimental determination of factors controlling U/Ca of aragonite precipitated from seawater: implications for interpreting coral skeleton.Crossref | GoogleScholarGoogle Scholar |
DeCarlo, T. M., Gaetani, G. A., Cohen, A. L., Foster, G. L., Alpert, A. E., and Stewart, J. A. (2016). Coral Sr-U thermometry. Paleoceanography 31, 626–638.
| Coral Sr-U thermometry.Crossref | GoogleScholarGoogle Scholar |
Devereux, I. (1967). Temperature measurements from oxygen isotope ratios of fish otoliths. Science 155, 1684–1685.
| Temperature measurements from oxygen isotope ratios of fish otoliths.Crossref | GoogleScholarGoogle Scholar | 6020293PubMed |
Di Franco, A., Bulleri, F., Pennetta, A., De Benedetto, G., Clarke, K. R., and Guidetti, P. (2014). Within-otolith variability in chemical fingerprints: implications for sampling designs and possible environmental interpretation. PLoS One 9, e101701.
| Within-otolith variability in chemical fingerprints: implications for sampling designs and possible environmental interpretation.Crossref | GoogleScholarGoogle Scholar | 25000202PubMed |
Dieulafait, L. (1877). La strontiane, sa diffusion dans la nature minérale et dans la nature vivante, à l’époque actuelle et dans la série des temps géologiques. Conséquences relatives aux eaux minérales salifères. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 84, 1303–1305.
Doubleday, Z. A., Cliff, J., Izzo, C., and Gillanders, B. M. (2018). Untapping the potential of sulfur isotope analysis in biominerals. Marine Ecology Progress Series 598, 159–166.
| Untapping the potential of sulfur isotope analysis in biominerals.Crossref | GoogleScholarGoogle Scholar |
Dutton, A., Wilkinson, B. H., Welker, J. M., Bowen, G. J., and Lohmann, K. C. (2005). Spatial distribution and seasonal variation in 18O/16O of modern precipitation and river water across the conterminous USA. Hydrological Processes 19, 4121–4146.
| Spatial distribution and seasonal variation in 18O/16O of modern precipitation and river water across the conterminous USA.Crossref | GoogleScholarGoogle Scholar |
Edmonds, J., Caputi, N., and Morita, M. (1991). Stock discrimination by trace-element analysis of otoliths of orange roughy (Hoplostethus atlanticus), a deep-water marine teleost. Marine and Freshwater Research 42, 383–389.
| Stock discrimination by trace-element analysis of otoliths of orange roughy (Hoplostethus atlanticus), a deep-water marine teleost.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., and Gillanders, B. M. (2002). Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Canadian Journal of Fisheries and Aquatic Sciences 59, 1796–1808.
| Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., and Gillanders, B. M. (2003). Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri. Marine Ecology Progress Series 260, 263–272.
| Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., Wells, B. K., Campana, S. E., Gillanders, B. M., Jones, C. M., Limburg, K. E., Secor, D. H., Thorrold, S. R., and Walther, B. D. (2008). Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology – an Annual Review 46, 297–330.
| Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences.Crossref | GoogleScholarGoogle Scholar |
Esbaugh, A. J. (2018). Physiological implications of ocean acidification for marine fish: emerging patterns and new insights. Journal of Comparative Physiology – B. Biochemical, Systemic, and Environmental Physiology 188, 1–13.
| Physiological implications of ocean acidification for marine fish: emerging patterns and new insights.Crossref | GoogleScholarGoogle Scholar | 28547292PubMed |
Forchhammer, G. (1865). IV. On the composition of sea-water in the different parts of the ocean. Philosophical Transactions of the Royal Society of London 155, 203–262.
| IV. On the composition of sea-water in the different parts of the ocean.Crossref | GoogleScholarGoogle Scholar |
Gaemers, P. A. M. (1978). Welcome and introduction to the first meeting of fish specialists within the framework of I.G.C.P. Project 124. Mededelingen van de Werkgroep voor Tertiaire en Kwartaire Geologie 15, 125–128.
Gaetani, G. A., Cohen, A. L., Wang, Z., and Crusius, J. (2011). Rayleigh-based, multi-element coral thermometry: a biomineralization approach to developing climate proxies. Geochimica et Cosmochimica Acta 75, 1920–1932.
| Rayleigh-based, multi-element coral thermometry: a biomineralization approach to developing climate proxies.Crossref | GoogleScholarGoogle Scholar |
Gauldie, R. W. (1996). Effects of temperature and vaterite replacement on the chemistry of metal ions in the otoliths of Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences 53, 2015–2026.
| Effects of temperature and vaterite replacement on the chemistry of metal ions in the otoliths of Oncorhynchus tshawytscha.Crossref | GoogleScholarGoogle Scholar |
Gauldie, R. W., Coote, G., Mulligan, K. P., West, I. F., and Merrett, N. R. (1991). Otoliths of deep water fishes: structure, chemistry and chemically coded life histories. Comparative Biochemistry and Physiology – A. Physiology 100, 1–31.
| Otoliths of deep water fishes: structure, chemistry and chemically coded life histories.Crossref | GoogleScholarGoogle Scholar |
Geffen, A. J., Pearce, N. J. G., and Perkins, W. T. (1998). Metal concentrations in fish otoliths in relation to body composition after laboratory exposure to mercury and lead. Marine Ecology Progress Series 165, 235–245.
| Metal concentrations in fish otoliths in relation to body composition after laboratory exposure to mercury and lead.Crossref | GoogleScholarGoogle Scholar |
Geffen, A. J., Morales-Nin, B., and Gillanders, B. M. (2016). Fish otoliths as indicators in ecosystem based management: results of the 5th International Otolith Symposium (IOS2014). Marine and Freshwater Research 67, i–iv.
| Fish otoliths as indicators in ecosystem based management: results of the 5th International Otolith Symposium (IOS2014).Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M. (2005). Otolith chemistry to determine movements of diadromous and freshwater fish. Aquatic Living Resources 18, 291–300.
| Otolith chemistry to determine movements of diadromous and freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Gillanders, B. M., and Munro, A. R. (2012). Hypersaline waters pose new challenges for reconstructing environmental histories of fish based on otolith chemistry. Limnology and Oceanography 57, 1136–1148.
| Hypersaline waters pose new challenges for reconstructing environmental histories of fish based on otolith chemistry.Crossref | GoogleScholarGoogle Scholar |
Giri, S. J., Swart, P. K., and Devlin, Q. B. (2018). The effect of changing seawater Ca and Mg concentrations upon the distribution coefficients of Mg and Sr in the skeletons of the scleractinian coral Pocillopora damicornis. Geochimica et Cosmochimica Acta 222, 535–549.
| The effect of changing seawater Ca and Mg concentrations upon the distribution coefficients of Mg and Sr in the skeletons of the scleractinian coral Pocillopora damicornis.Crossref | GoogleScholarGoogle Scholar |
Grammer, G. L., Morrongiello, J. R., Izzo, C., Hawthorne, P. J., Middleton, J. F., and Gillanders, B. M. (2017). Coupling biogeochemical tracers with fish growth reveals physiological and environmental controls on otolith chemistry. Ecological Monographs 87, 487–507.
| Coupling biogeochemical tracers with fish growth reveals physiological and environmental controls on otolith chemistry.Crossref | GoogleScholarGoogle Scholar |
Han, Y. S., and Tzeng, W. N. (2007). Sex-dependent habitat use by the Japanese eel Anguilla japonica in Taiwan. Marine Ecology Progress Series 338, 193–198.
| Sex-dependent habitat use by the Japanese eel Anguilla japonica in Taiwan.Crossref | GoogleScholarGoogle Scholar |
Hegg, J. C., Kennedy, B. P., and Fremier, A. K. (2013). Predicting strontium isotope variation and fish location with bedrock geology: understanding the effects of geologic heterogeneity. Chemical Geology 360–361, 89–98.
| Predicting strontium isotope variation and fish location with bedrock geology: understanding the effects of geologic heterogeneity.Crossref | GoogleScholarGoogle Scholar |
Hegg, J. C., Giarrizzo, T., and Kennedy, B. P. (2015). Diverse early life-history strategies in migratory Amazonian catfish: implications for conservation and management. PLoS One 10, e0129697.
| Diverse early life-history strategies in migratory Amazonian catfish: implications for conservation and management.Crossref | GoogleScholarGoogle Scholar | 26153984PubMed |
Heuer, R. M., and Grosell, M. (2014). Physiological impacts of elevated carbon dioxide and ocean acidification on fish. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology 307, R1061–R1084.
| Physiological impacts of elevated carbon dioxide and ocean acidification on fish.Crossref | GoogleScholarGoogle Scholar | 25163920PubMed |
Hirose, S., Kaneko, T., Naito, N., and Takei, Y. (2003). Molecular biology of major components of chloride cells. Comparative Biochemistry and Physiology – B. Biochemistry & Molecular Biology 136, 593–620.
| Molecular biology of major components of chloride cells.Crossref | GoogleScholarGoogle Scholar |
Hobson, K. A., and Koehler, G. (2015). On the use of stable oxygen isotope (δ18O) measurements for tracking avian movements in North America. Ecology and Evolution 5, 799–806.
| On the use of stable oxygen isotope (δ18O) measurements for tracking avian movements in North America.Crossref | GoogleScholarGoogle Scholar | 25691999PubMed |
Holman, L., Head, M. L., Lanfear, R., and Jennions, M. D. (2015). Evidence of experimental bias in the life sciences: why we need blind data recording. PLoS Biology 13, e1002190.
| Evidence of experimental bias in the life sciences: why we need blind data recording.Crossref | GoogleScholarGoogle Scholar | 26154287PubMed |
Humston, R., Doss, S. S., Wass, C., Hollenbeck, C., Thorrold, S. R., Smith, S., and Bataille, C. P. (2017). Isotope geochemistry reveals ontogeny of dispersal and exchange between main-river and tributary habitats in smallmouth bass Micropterus dolomieu. Journal of Fish Biology 90, 528–548.
| Isotope geochemistry reveals ontogeny of dispersal and exchange between main-river and tributary habitats in smallmouth bass Micropterus dolomieu.Crossref | GoogleScholarGoogle Scholar | 27615608PubMed |
Hunter, E., Laptikhovsky, V. V., and Hollyman, P. R. (2018). Innovative use of sclerochronology in marine resource management. Marine Ecology Progress Series 598, 155–158.
| Innovative use of sclerochronology in marine resource management.Crossref | GoogleScholarGoogle Scholar |
Hüssy, K., Gröger, J., Heidemann, F., Hinrichsen, H. H., and Marohn, L. (2016). Slave to the rhythm: seasonal signals in otolith microchemistry reveal age of eastern Baltic cod (Gadus morhua). ICES Journal of Marine Science 73, 1019–1032.
| Slave to the rhythm: seasonal signals in otolith microchemistry reveal age of eastern Baltic cod (Gadus morhua).Crossref | GoogleScholarGoogle Scholar |
Hutchinson, J. J., and Trueman, C. N. (2006). Stable isotope analyses of collagen in fish scales: limitations set by scale architecture. Journal of Fish Biology 69, 1874–1880.
| Stable isotope analyses of collagen in fish scales: limitations set by scale architecture.Crossref | GoogleScholarGoogle Scholar |
Izzo, C., Doubleday, Z. A., and Gillanders, B. M. (2016). Where do elements bind within the otoliths of fish? Marine and Freshwater Research 67, 1072–1076.
| Where do elements bind within the otoliths of fish?Crossref | GoogleScholarGoogle Scholar |
Izzo, C., Reis‐Santos, P., and Gillanders, B. M. (2018). Otolith chemistry does not just reflect environmental conditions: a meta‐analytic evaluation. Fish and Fisheries 19, 441–454.
| Otolith chemistry does not just reflect environmental conditions: a meta‐analytic evaluation.Crossref | GoogleScholarGoogle Scholar |
Jónsdóttir, I. G., Marteinsdottir, G., and Campana, S. E. (2007). Contribution of different spawning components to the mixed stock fishery for cod in Icelandic waters. ICES Journal of Marine Science 64, 1749–1759.
| Contribution of different spawning components to the mixed stock fishery for cod in Icelandic waters.Crossref | GoogleScholarGoogle Scholar |
Kardish, M. R., Mueller, U. G., Amador-Vargas, S., Dietrich, E. I., Ma, R., Barrett, B., and Fang, C.-C. (2015). Blind trust in unblinded observation in ecology, evolution, and behavior. Frontiers in Ecology and Evolution 3, 51.
| Blind trust in unblinded observation in ecology, evolution, and behavior.Crossref | GoogleScholarGoogle Scholar |
Kennedy, B. P., Blum, J. D., Folt, C. L., and Nislow, K. H. (2000). Using natural strontium isotopic signatures as fish markers: methodology and application. Canadian Journal of Fisheries and Aquatic Sciences 57, 2280–2292.
| Using natural strontium isotopic signatures as fish markers: methodology and application.Crossref | GoogleScholarGoogle Scholar |
Kennedy, B. P., Klaue, A., Blum, J. D., Folt, C. L., and Nislow, K. H. (2002). Reconstructing the lives of fish using Sr isotopes in otoliths. Canadian Journal of Fisheries and Aquatic Sciences 59, 925–929.
| Reconstructing the lives of fish using Sr isotopes in otoliths.Crossref | GoogleScholarGoogle Scholar |
Keys, T. E. (1956). The ‘Salmonia’ of Sir Humphry Davy. Bulletin of the Medical Library Association 44, 431–442.
| 13364552PubMed |
Knight, D. M. (1998). ‘Humphry Davy: Science and Power.’ (Cambridge University Press: Cambridge, UK.)
Kraus, R. T., and Secor, D. H. (2004). Incorporation of strontium into otoliths of an estuarine fish. Journal of Experimental Marine Biology and Ecology 302, 85–106.
| Incorporation of strontium into otoliths of an estuarine fish.Crossref | GoogleScholarGoogle Scholar |
Larsen, T., Ventura, M., Andersen, N., O’Brien, D. M., Piatkowski, U., and McCarthy, M. D. (2013). Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting. PLoS One 8, e73441.
| Tracing carbon sources through aquatic and terrestrial food webs using amino acid stable isotope fingerprinting.Crossref | GoogleScholarGoogle Scholar | 24069196PubMed |
Limburg, K. E. (2004). The biogeochemistry of strontium: a review of H. T. Odum’s contributions. Ecological Modelling 178, 31–33.
| The biogeochemistry of strontium: a review of H. T. Odum’s contributions.Crossref | GoogleScholarGoogle Scholar |
Limburg, K. E., and Elfman, M. (2010). Patterns and magnitude of Zn : Ca in otoliths support the recent phylogenetic typology of Salmoniformes and their sister groups. Canadian Journal of Fisheries and Aquatic Sciences 67, 597–604.
| Patterns and magnitude of Zn : Ca in otoliths support the recent phylogenetic typology of Salmoniformes and their sister groups.Crossref | GoogleScholarGoogle Scholar |
Limburg, K. E., and Elfman, M. (2017). Insights from two-dimensional mapping of otolith chemistry. Journal of Fish Biology 90, 480–491.
| Insights from two-dimensional mapping of otolith chemistry.Crossref | GoogleScholarGoogle Scholar | 27312827PubMed |
Limburg, K. E., Walther, B. D., Lu, Z. L., Jackman, G., Mohan, J., Walther, Y., Nissling, A., Weber, P. K., and Schmitt, A. K. (2015). In search of the dead zone: use of otoliths for tracking fish exposure to hypoxia. Journal of Marine Systems 141, 167–178.
| In search of the dead zone: use of otoliths for tracking fish exposure to hypoxia.Crossref | GoogleScholarGoogle Scholar |
Limburg, K. E., Wuenschel, M. J., Hüssy, K., Heimbrand, Y., and Samson, M. (2018). Making the otolith magnesium chemical calendar-clock tick: plausible mechanism and empirical evidence. Reviews in Fisheries Science & Aquaculture 26, 479–493.
| Making the otolith magnesium chemical calendar-clock tick: plausible mechanism and empirical evidence.Crossref | GoogleScholarGoogle Scholar |
Lord, C., Tabouret, H., Claverie, F., Pécheyran, C., and Keith, P. (2011). Femtosecond laser ablation ICP‐MS measurement of otolith Sr : Ca and Ba : Ca composition reveal differential use of freshwater habitats for three amphidromous Sicyopterus (Teleostei: Gobioidei: Sicydiinae) species. Journal of Fish Biology 79, 1304–1321.
| Femtosecond laser ablation ICP‐MS measurement of otolith Sr : Ca and Ba : Ca composition reveal differential use of freshwater habitats for three amphidromous Sicyopterus (Teleostei: Gobioidei: Sicydiinae) species.Crossref | GoogleScholarGoogle Scholar | 22026607PubMed |
Lueders-Dumont, J. A., Wang, X. T., Jensen, O. P., Sigman, D. M., and Ward, B. B. (2018). Nitrogen isotopic analysis of carbonate-bound organic matter in modern and fossil fish otoliths. Geochimica et Cosmochimica Acta 224, 200–222.
| Nitrogen isotopic analysis of carbonate-bound organic matter in modern and fossil fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Marin, E., Padro, A., Miquel, A., and Garcia, J. F. (2015). Characterization of paintings by laser ablation-inductively coupled plasma–mass spectrometry. Analytical Letters 48, 167–179.
| Characterization of paintings by laser ablation-inductively coupled plasma–mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
Marshall, W. S., and Grosell, M. (2006). Ion transport, osmoregulation, and acid–base balance. In ‘The Physiology of Fishes’. (Eds D. H. Evans and J. B. Claiborne.) pp. 177–230. (CRC Press: Boca Raton, FL, USA.)
McClelland, J. W., and Montoya, J. P. (2002). Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83, 2173–2180.
| Trophic relationships and the nitrogen isotopic composition of amino acids in plankton.Crossref | GoogleScholarGoogle Scholar |
McFadden, A., Wade, B., Izzo, C., Gillanders, B. M., Lenehan, C. E., and Pring, A. (2016). Quantitative electron microprobe mapping of otoliths suggests elemental incorporation is affected by organic matrices: implications for the interpretation of otolith chemistry. Marine and Freshwater Research 67, 889–898.
| Quantitative electron microprobe mapping of otoliths suggests elemental incorporation is affected by organic matrices: implications for the interpretation of otolith chemistry.Crossref | GoogleScholarGoogle Scholar |
McGowan, N., Fowler, A. M., Parkinson, K., Bishop, D. P., Ganio, K., Doble, P. A., Booth, D. J., and Hare, D. J. (2014). Beyond the transect: an alternative microchemical imaging method for fine scale analysis of trace elements in fish otoliths during early life. The Science of the Total Environment 494–495, 177–186.
| Beyond the transect: an alternative microchemical imaging method for fine scale analysis of trace elements in fish otoliths during early life.Crossref | GoogleScholarGoogle Scholar | 25046609PubMed |
McMahon, K., Berumen, M., Mateo, I., Elsdon, T., and Thorrold, S. (2011a). Carbon isotopes in otolith amino acids identify residency of juvenile snapper (Family: Lutjanidae) in coastal nurseries. Coral Reefs 30, 1135–1145.
| Carbon isotopes in otolith amino acids identify residency of juvenile snapper (Family: Lutjanidae) in coastal nurseries.Crossref | GoogleScholarGoogle Scholar |
McMahon, K. W., Fogel, M. L., Johnson, B. J., Houghton, L. A., and Thorrold, S. R. (2011b). A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids. Canadian Journal of Fisheries and Aquatic Sciences 68, 1330–1340.
| A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids.Crossref | GoogleScholarGoogle Scholar |
McMahon, K. W., Hamady, L. L., and Thorrold, S. R. (2013a). Ocean ecogeochemistry: a review. In ‘Oceanography and Marine Biology: an Annual Review’. (Eds R. N. Hughes, D. Hughes, and I. P. Smith.) Vol. 51, pp. 327–374. (CRC Press: Boca Raton, FL, USA.)
McMahon, K. W., Hamady, L. L., and Thorrold, S. R. (2013b). A review of ecogeochemistry approaches to estimating movements of marine animals. Limnology and Oceanography 58, 697–714.
| A review of ecogeochemistry approaches to estimating movements of marine animals.Crossref | GoogleScholarGoogle Scholar |
McMahon, K. W., Thorrold, S. R., Houghton, L. A., and Berumen, M. L. (2016). Tracing carbon flow through coral reef food webs using a compound-specific stable isotope approach. Oecologia 180, 809–821.
| Tracing carbon flow through coral reef food webs using a compound-specific stable isotope approach.Crossref | GoogleScholarGoogle Scholar | 26590916PubMed |
McMillan, M., Izzo, C., Wade, B., and Gillanders, B. M. (2017). Elements and elasmobranchs: Hypotheses, assumptions and limitations of elemental analysis. Journal of Fish Biology 90, 559–594.
| Elements and elasmobranchs: Hypotheses, assumptions and limitations of elemental analysis.Crossref | GoogleScholarGoogle Scholar | 27859234PubMed |
Mellor, J. W. (1961). The alkaline earths: the history of calcium, strontium and barium. In ‘A Comprehensive Treatise on Inorganic and Theoretical Chemistry’. Vol. III, pp. 619–622. (Wiley: New York, NY, USA.)
Miller, M. B., Clough, A. M., Batson, J. N., and Vachet, R. W. (2006). Transition metal binding to cod otolith proteins. Journal of Experimental Marine Biology and Ecology 329, 135–143.
| Transition metal binding to cod otolith proteins.Crossref | GoogleScholarGoogle Scholar |
Milton, D. A., and Chenery, S. R. (2001a). Can otolith chemistry detect the population structure of the shad hilsa Tenualosa ilisha? Comparison with the results of genetic and morphological studies. Marine Ecology Progress Series 222, 239–251.
| Can otolith chemistry detect the population structure of the shad hilsa Tenualosa ilisha? Comparison with the results of genetic and morphological studies.Crossref | GoogleScholarGoogle Scholar |
Milton, D. A., and Chenery, S. R. (2001b). Sources of uptake of trace metals in otoliths of juvenile barramundi (Lates calcarifer). Journal of Experimental Marine Biology and Ecology 264, 47–65.
| Sources of uptake of trace metals in otoliths of juvenile barramundi (Lates calcarifer).Crossref | GoogleScholarGoogle Scholar |
Milton, D. A., and Chenery, S. R. (2005). Movement patterns of barramundi Lates calcarifer, inferred from 87Sr/86S and Sr/Ca ratios in otoliths, indicate non-participation in spawning. Marine Ecology Progress Series 301, 279–291.
| Movement patterns of barramundi Lates calcarifer, inferred from 87Sr/86S and Sr/Ca ratios in otoliths, indicate non-participation in spawning.Crossref | GoogleScholarGoogle Scholar |
Mohan, J. A., and Walther, B. D. (2015). Spatiotemporal variation of trace elements and stable isotopes in subtropical estuaries: II. Regional, local, and seasonal salinity–element relationships. Estuaries and Coasts 38, 769–781.
| Spatiotemporal variation of trace elements and stable isotopes in subtropical estuaries: II. Regional, local, and seasonal salinity–element relationships.Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B., and Geffen, A. J. (2015). The use of calcified tissues as tools to support management: the view from the 5th International Otolith Symposium. ICES Journal of Marine Science 72, 2073–2078.
| The use of calcified tissues as tools to support management: the view from the 5th International Otolith Symposium.Crossref | GoogleScholarGoogle Scholar |
Morse, J. W., and Bender, M. L. (1990). Partition coefficients in calcite – examination of factors influencing the validity of experimental results and their application to natural systems. Chemical Geology 82, 265–277.
| Partition coefficients in calcite – examination of factors influencing the validity of experimental results and their application to natural systems.Crossref | GoogleScholarGoogle Scholar |
Murayama, E., Herbomel, P., Kawakami, A., Takeda, H., and Nagasawa, H. (2005). Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae. Mechanisms of Development 122, 791–803.
| Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae.Crossref | GoogleScholarGoogle Scholar | 15905077PubMed |
Nielsen, J. M., Popp, B. N., and Winder, M. (2015). Meta-analysis of amino acid stable nitrogen isotope ratios for estimating trophic position in marine organisms. Oecologia 178, 631–642.
| Meta-analysis of amino acid stable nitrogen isotope ratios for estimating trophic position in marine organisms.Crossref | GoogleScholarGoogle Scholar | 25843809PubMed |
Nochlin, L. (1966). ‘Impressionism and Post-Impressionism 1874–1904: Sources and Documents.’ (Prentice-Hall: Englewood Cliffs, NJ, USA.)
Odum, H. T. (1950). The biogeochemistry of strontium: with discussion on the ecological integration of elements. Ph.D. Thesis, Yale University, New Haven, CT, USA.
Odum, H. T. (1951a). Notes on the strontium content of sea water, celestite Radiolaria, and strontianite snail shells. Science 114, 211–213.
| Notes on the strontium content of sea water, celestite Radiolaria, and strontianite snail shells.Crossref | GoogleScholarGoogle Scholar | 14866198PubMed |
Odum, H. T. (1951b). The stability of the world strontium cycle. Science 114, 407–411.
| The stability of the world strontium cycle.Crossref | GoogleScholarGoogle Scholar | 14892741PubMed |
Odum, H. T. (1957a). Biogeochemical deposition of strontium. Publications of the Institute of Marine Science 4, 38–114.
Odum, H. T. (1957b). Strontium in natural waters. Publications of the Institute of Marine Science 4, 22–37.
Panfili, J., Darnaude, A. M., Vigliola, L., Jacquart, A., Labonne, M., and Gilles, S. (2015). Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths. Journal of Experimental Marine Biology and Ecology 467, 65–70.
| Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths.Crossref | GoogleScholarGoogle Scholar |
Pannella, G. (1971). Fish otoliths: daily growth layers and periodical patterns. Science 173, 1124–1127.
| Fish otoliths: daily growth layers and periodical patterns.Crossref | GoogleScholarGoogle Scholar |
Papillon, F. (1870). Recherches expérimentales sur les modifications de la composition immédiate des os. Comptes Rendus Hebdomadaires des Séances de l’Académie des Sciences 71, 372–374.
Payan, P., Edeyer, A., De Pontual, H., Borelli, G., Boeuf, G., and Mayer-Gostan, N. (1999). Chemical composition of saccular endolymph and otolith in fish inner ear: lack of spatial uniformity. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 277, R123–R131.
| Chemical composition of saccular endolymph and otolith in fish inner ear: lack of spatial uniformity.Crossref | GoogleScholarGoogle Scholar |
Payan, P., De Pontual, H., Boeuf, G., and Mayer-Gostan, N. (2004a). Endolymph chemistry and otolith growth in fish. Comptes Rendus. Palévol 3, 535–547.
| Endolymph chemistry and otolith growth in fish.Crossref | GoogleScholarGoogle Scholar |
Payan, P., De Pontual, H., Edeyer, A., Borelli, G., Boeuf, G., and Mayer-Gostan, N. (2004b). Effects of stress on plasma homeostasis, endolymph chemistry, and check formation during otolith growth in rainbow trout (Oncorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Sciences 61, 1247–1255.
| Effects of stress on plasma homeostasis, endolymph chemistry, and check formation during otolith growth in rainbow trout (Oncorhynchus mykiss).Crossref | GoogleScholarGoogle Scholar |
Petrus, J. A., Chew, D. M., Leybourne, M. I., and Kamber, B. S. (2017). A new approach to laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) using the flexible map interrogation tool ‘Monocle’. Chemical Geology 463, 76–93.
| A new approach to laser ablation–inductively coupled plasma–mass spectrometry (LA-ICP-MS) using the flexible map interrogation tool ‘Monocle’.Crossref | GoogleScholarGoogle Scholar |
Pörtner, H. O. (2008). Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Marine Ecology Progress Series 373, 203–217.
| Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view.Crossref | GoogleScholarGoogle Scholar |
Pracheil, B. M., Chakoumakos, B. C., Feygenson, M., Whitledge, G. W., Koenigs, R. P., and Bruch, R. M. (2017). Sturgeon and paddlefish (Acipenseridae) sagittal otoliths are composed of the calcium carbonate polymorphs vaterite and calcite. Journal of Fish Biology 90, 549–558.
| Sturgeon and paddlefish (Acipenseridae) sagittal otoliths are composed of the calcium carbonate polymorphs vaterite and calcite.Crossref | GoogleScholarGoogle Scholar | 27461067PubMed |
Quaeck-Davies, K., Bendall, V. A., MacKenzie, K. M., Hetherington, S., Newton, J., and Trueman, C. N. (2018). Teleost and elasmobranch eye lenses as a target for life-history stable isotope analyses. PeerJ 6, e4883.
| Teleost and elasmobranch eye lenses as a target for life-history stable isotope analyses.Crossref | GoogleScholarGoogle Scholar | 29888128PubMed |
Radtke, R. L. (1989). Strontium–calcium concentration ratios in fish otoliths as environmental indicators. Comparative Biochemistry and Physiology – A. Comparative Physiology 92, 189–193.
| Strontium–calcium concentration ratios in fish otoliths as environmental indicators.Crossref | GoogleScholarGoogle Scholar |
Raoult, V., Peddemors, V. M., Zahra, D., Howell, N., Howard, D. L., de Jonge, M. D., and Williamson, J. E. (2016). Strontium mineralization of shark vertebrae. Scientific Reports 6, 29698.
| Strontium mineralization of shark vertebrae.Crossref | GoogleScholarGoogle Scholar | 27424768PubMed |
Reibisch, J. (1899). Über die Eizahl bei Pleuronectes platessa und die Altersbestimmung dieser Form aus den Otolithen. Wissenschaftliche Meeresuntersuchungen von der Kommission zur wissenschaftlichen Untersuchug und der Biologischen Anstalt auf Helgoland 4, 233–248.
Rubin, J. H. (1999). ‘Impressionism.’ (Phaidon Press: London, UK.)
Secor, D. H. (2010). Is otolith science transformative? New views on fish migration. Environmental Biology of Fishes 89, 209–220.
| Is otolith science transformative? New views on fish migration.Crossref | GoogleScholarGoogle Scholar |
Secor, D. H., and Piccoli, P. M. (1996). Age- and sex-dependent migrations of striped bass in the Hudson River as determined by chemical microanalysis of otoliths. Estuaries 19, 778–793.
| Age- and sex-dependent migrations of striped bass in the Hudson River as determined by chemical microanalysis of otoliths.Crossref | GoogleScholarGoogle Scholar |
Secor, D. H., Dean, J. M., and Campana, S. E. (Eds) (1995). ‘Recent Developments in Fish Otolith Research.’ (University of South Carolina Press: Columbia, SC, USA.)
Seeley, M., Miller, N., and Walther, B. (2015). High resolution profiles of elements in Atlantic tarpon (Megalops atlanticus) scales obtained via cross-sectioning and laser ablation ICP-MS: a literature survey and novel approach for scale analyses. Environmental Biology of Fishes 98, 2223–2238.
| High resolution profiles of elements in Atlantic tarpon (Megalops atlanticus) scales obtained via cross-sectioning and laser ablation ICP-MS: a literature survey and novel approach for scale analyses.Crossref | GoogleScholarGoogle Scholar |
Seeley, M. E., Logan, W. K., and Walther, B. D. (2017). Consistency of elemental and isotope-ratio patterns across multiple scales from individual fish. Journal of Fish Biology 91, 928–946.
| Consistency of elemental and isotope-ratio patterns across multiple scales from individual fish.Crossref | GoogleScholarGoogle Scholar | 28776676PubMed |
Selleslagh, J., Echard, A., Pecheyran, C., Baudrimont, M., Lobry, J., and Daverat, F. (2016). Can analysis of Platichthys flesus otoliths provide relevant data on historical metal pollution in estuaries? Experimental and in situ approaches. The Science of the Total Environment 557–558, 20–30.
| Can analysis of Platichthys flesus otoliths provide relevant data on historical metal pollution in estuaries? Experimental and in situ approaches.Crossref | GoogleScholarGoogle Scholar | 26994790PubMed |
Sinclair, D. J., and McCulloch, M. T. (2004). Corals record low mobile barium concentrations in the Burdekin River during the 1974 flood: evidence for limited Ba supply to rivers? Palaeogeography, Palaeoclimatology, Palaeoecology 214, 155–174.
| Corals record low mobile barium concentrations in the Burdekin River during the 1974 flood: evidence for limited Ba supply to rivers?Crossref | GoogleScholarGoogle Scholar |
Sirot, C., Grønkjær, P., Pedersen, J. B., Panfili, J., Zetina-Rejon, M., Tripp-Valdez, A., Ramos-Miranda, J., Flores-Hernandez, D., Sosa-Lopez, A., and Darnaude, A. M. (2017). Using otolith organic matter to detect diet shifts in Bardiella chrysoura, during a period of environmental changes. Marine Ecology Progress Series 575, 137–152.
| Using otolith organic matter to detect diet shifts in Bardiella chrysoura, during a period of environmental changes.Crossref | GoogleScholarGoogle Scholar |
Smith, W. D., Miller, J. A., and Heppell, S. S. (2013). Elemental markers in elasmobranchs: effects of environmental history and growth on vertebral chemistry. PLoS One 8, e62423.
| Elemental markers in elasmobranchs: effects of environmental history and growth on vertebral chemistry.Crossref | GoogleScholarGoogle Scholar | 24098320PubMed |
Smith, W. D., Miller, J. A., Márquez-Farías, J. F., and Heppell, S. S. (2016). Elemental signatures reveal the geographic origins of a highly migratory shark: prospects for measuring population connectivity. Marine Ecology Progress Series 556, 173–193.
| Elemental signatures reveal the geographic origins of a highly migratory shark: prospects for measuring population connectivity.Crossref | GoogleScholarGoogle Scholar |
Spencer, K., Shafer, D. J., Gauldie, R. W., and DeCarlo, E. H. (2000). Stable lead isotope ratios from distinct anthropogenic sources in fish otoliths: a potential nursery ground stock marker. Comparative Biochemistry and Physiology – A. Molecular & Integrative Physiology 127, 273–284.
| Stable lead isotope ratios from distinct anthropogenic sources in fish otoliths: a potential nursery ground stock marker.Crossref | GoogleScholarGoogle Scholar |
Starrs, D., Ebner, B. C., and Fulton, C. J. (2016). All in the ears: unlocking the early life history biology and spatial ecology of fishes. Biological Reviews of the Cambridge Philosophical Society 91, 86–105.
| All in the ears: unlocking the early life history biology and spatial ecology of fishes.Crossref | GoogleScholarGoogle Scholar | 25424431PubMed |
Sturrock, A., Trueman, C., Milton, J., Waring, C., Cooper, M., and Hunter, E. (2014). Physiological influences can outweigh environmental signals in otolith microchemistry research. Marine Ecology Progress Series 500, 245–264.
| Physiological influences can outweigh environmental signals in otolith microchemistry research.Crossref | GoogleScholarGoogle Scholar |
Sturrock, A. M., Hunter, E., Milton, J. A., EIMF Johnson, R. C., Waring, C. P., and Trueman, C. N. (2015). Quantifying physiological influences on otolith microchemistry. Methods in Ecology and Evolution 6, 806–816.
| Quantifying physiological influences on otolith microchemistry.Crossref | GoogleScholarGoogle Scholar |
Tanaka, K., Holcomb, M., Takahashi, A., Kurihara, H., Asami, R., Shinjo, R., Sowa, K., Rankenburg, K., Watanabe, T., and McCulloch, M. (2015). Response of Acropora digitifera to ocean acidification: constraints from δ11B, Sr, Mg, and Ba compositions of aragonitic skeletons cultured under variable seawater pH. Coral Reefs 34, 1139–1149.
| Response of Acropora digitifera to ocean acidification: constraints from δ11B, Sr, Mg, and Ba compositions of aragonitic skeletons cultured under variable seawater pH.Crossref | GoogleScholarGoogle Scholar |
Tanner, S. E., Reis-Santos, P., and Cabral, H. N. (2016). Otolith chemistry in stock delineation: a brief overview, current challenges and future prospects. Fisheries Research 173, 206–213.
| Otolith chemistry in stock delineation: a brief overview, current challenges and future prospects.Crossref | GoogleScholarGoogle Scholar |
Thien, B. M. J., Kulik, D. A., and Curti, E. (2014). A unified approach to model uptake kinetics of trace elements in complex aqueous – solid solution systems. Applied Geochemistry 41, 135–150.
| A unified approach to model uptake kinetics of trace elements in complex aqueous – solid solution systems.Crossref | GoogleScholarGoogle Scholar |
Thomas, O. R. B., Ganio, K., Roberts, B. R., and Swearer, S. E. (2017). Trace element–protein interactions in endolymph from the inner ear of fish: implications for environmental reconstructions using fish otolith chemistry. Metallomics 9, 239–249.
| Trace element–protein interactions in endolymph from the inner ear of fish: implications for environmental reconstructions using fish otolith chemistry.Crossref | GoogleScholarGoogle Scholar |
Thompson, B. (2000). ‘Impressionism: Origin, Practice, Reception.’ (Thames & Hudson: London, UK.)
Thorrold, S. R., Campana, S. E., Jones, C. M., and Swart, P. K. (1997). Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish. Geochimica et Cosmochimica Acta 61, 2909–2919.
| Factors determining δ13C and δ18O fractionation in aragonitic otoliths of marine fish.Crossref | GoogleScholarGoogle Scholar |
Trueman, C. N., and Moore, A. (2007). Use of the stable isotope composition of fish scales for monitoring aquatic ecosystems. In ‘Stable Isotopes as Indicators of Ecological Change’. (Eds T. E. Dawson and R. T. W. Siegwolf.) pp. 145–161. (Academic Press: Burlington, VT, USA.)
Trueman, C. N., MacKenzie, K. M., and Palmer, M. R. (2012). Identifying migrations in marine fishes through stable-isotope analysis. Journal of Fish Biology 81, 826–847.
| Identifying migrations in marine fishes through stable-isotope analysis.Crossref | GoogleScholarGoogle Scholar | 22803737PubMed |
Tzadik, O. E., Curtis, J. S., Granneman, J. E., Kurth, B. N., Pusack, T. J., Wallace, A. A., Hollander, D. J., Peebles, E. B., and Stallings, C. D. (2017). Chemical archives in fishes beyond otoliths: a review on the use of other body parts as chronological recorders of microchemical constituents for expanding interpretations of environmental, ecological, and life-history changes. Limnology and Oceanography, Methods 15, 238–263.
| Chemical archives in fishes beyond otoliths: a review on the use of other body parts as chronological recorders of microchemical constituents for expanding interpretations of environmental, ecological, and life-history changes.Crossref | GoogleScholarGoogle Scholar |
Tzeng, W. N., Severin, K. P., and Wickström, H. (1997). Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla. Marine Ecology Progress Series 149, 73–81.
| Use of otolith microchemistry to investigate the environmental history of European eel Anguilla anguilla.Crossref | GoogleScholarGoogle Scholar |
Tzeng, W., Chang, C., Wang, C., Shiao, J., Iizuka, Y., Yang, Y., You, C., and Lozys, L. (2007). Misidentification of the migratory history of anguillid eels by Sr/Ca ratios of vaterite otoliths. Marine Ecology Progress Series 348, 285–295.
| Misidentification of the migratory history of anguillid eels by Sr/Ca ratios of vaterite otoliths.Crossref | GoogleScholarGoogle Scholar |
Upton, S. A., Walther, B. D., Thorrold, S. R., and Olney, J. E. (2012). Use of a natural isotopic signature in otoliths to evaluate scale-based age determination for American shad. Marine and Coastal Fisheries 4, 346–357.
| Use of a natural isotopic signature in otoliths to evaluate scale-based age determination for American shad.Crossref | GoogleScholarGoogle Scholar |
Vane, K., Wallsgrove, N. J., Ekau, W., and Popp, B. N. (2018). Reconstructing lifetime nitrogen baselines and trophic position of Cynoscion acoupa from δ15N values of amino acids in otoliths. Marine Ecology Progress Series 597, 1–11.
| Reconstructing lifetime nitrogen baselines and trophic position of Cynoscion acoupa from δ15N values of amino acids in otoliths.Crossref | GoogleScholarGoogle Scholar |
Wallace, A. A., Hollander, D. J., and Peebles, E. B. (2014). Stable isotopes in fish eye lenses as potential recorders of trophic and geographic history. PLoS One 9, e108935.
| Stable isotopes in fish eye lenses as potential recorders of trophic and geographic history.Crossref | GoogleScholarGoogle Scholar | 25279946PubMed |
Walther, B. D., and Limburg, K. E. (2012). The use of otolith chemistry to characterize diadromous migrations. Journal of Fish Biology 81, 796–825.
| The use of otolith chemistry to characterize diadromous migrations.Crossref | GoogleScholarGoogle Scholar | 22803736PubMed |
Walther, B. D., and Thorrold, S. R. (2009). Inter-annual variability in isotope and elemental ratios recorded in otoliths of an anadromous fish. Journal of Geochemical Exploration 102, 181–186.
| Inter-annual variability in isotope and elemental ratios recorded in otoliths of an anadromous fish.Crossref | GoogleScholarGoogle Scholar |
Walther, B., Kingsford, M., O’Callaghan, M., and McCulloch, M. (2010). Interactive effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish. Environmental Biology of Fishes 89, 441–451.
| Interactive effects of ontogeny, food ration and temperature on elemental incorporation in otoliths of a coral reef fish.Crossref | GoogleScholarGoogle Scholar |
Walther, B. D., Limburg, K. E., Jones, C. M., and Schaffler, J. J. (2017). Frontiers in otolith chemistry: insights, advances and applications. Journal of Fish Biology 90, 473–479.
| Frontiers in otolith chemistry: insights, advances and applications.Crossref | GoogleScholarGoogle Scholar | 28220478PubMed |
Wang, H. A. O., Grolimund, D., Giesen, C., Borca, C. N., Shaw-Stewart, J. R. H., Bodenmiller, B., and Günther, D. (2013). Fast chemical imaging at high spatial resolution by laser ablation inductively coupled plasma–mass spectrometry. Analytical Chemistry 85, 10107–10116.
| Fast chemical imaging at high spatial resolution by laser ablation inductively coupled plasma–mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
Ward, G. H., and Montague, C. L. (1996). Estuaries. In ‘Handbook of Water Resources Engineering’. (Ed. L. Mays.) pp. 12.1–12.114. (McGraw-Hill: New York, NY, USA.)
Weigele, J., Franz-Odendaal, T. A., and Hilbig, R. (2016). Not all inner ears are the same: otolith matrix proteins in the inner ear of sub-adult cichlid fish, Oreochromis mossambicus, reveal insights into the biomineralization process. The Anatomical Record 299, 234–245.
| Not all inner ears are the same: otolith matrix proteins in the inner ear of sub-adult cichlid fish, Oreochromis mossambicus, reveal insights into the biomineralization process.Crossref | GoogleScholarGoogle Scholar | 26559654PubMed |
Werry, J. M., Lee, S. Y., Otway, N. M., Hu, Y., and Sumpton, W. (2011). A multi-faceted approach for quantifying the estuarine–nearshore transition in the life cycle of the bull shark, Carcharhinus leucas. Marine and Freshwater Research 62, 1421–1431.
| A multi-faceted approach for quantifying the estuarine–nearshore transition in the life cycle of the bull shark, Carcharhinus leucas.Crossref | GoogleScholarGoogle Scholar |
West, J. B., Bowen, G. J., Dawson, T. E., and Tu, K. P. (Eds) (2010). ‘Isoscapes: Understanding Movement, Pattern, and Process on Earth through Isotope Mapping.’ (Springer: Dordrecht, Netherlands.)
Woodcock, S. H., and Walther, B. D. (2014). Trace elements and stable isotopes in Atlantic tarpon scales reveal movements across estuarine gradients. Fisheries Research 153, 9–17.
| Trace elements and stable isotopes in Atlantic tarpon scales reveal movements across estuarine gradients.Crossref | GoogleScholarGoogle Scholar |
Woodcock, S. H., Munro, A. R., Crook, D. A., and Gillanders, B. M. (2012). Incorporation of magnesium into fish otoliths: determining contribution from water and diet. Geochimica et Cosmochimica Acta 94, 12–21.
| Incorporation of magnesium into fish otoliths: determining contribution from water and diet.Crossref | GoogleScholarGoogle Scholar |
Woodhead, J. D., Hellstrom, J., Hergt, J. M., Greig, A., and Maas, R. (2007). Isotopic and elemental imaging of geological materials by laser ablation inductively coupled plasma‐mass spectrometry. Geostandards and Geoanalytical Research 31, 331–343.
Wunder, M. B. (2010). Using isoscapes to model probability surfaces for determining geographic origins. In ‘Isoscapes: Understanding Movement, Pattern, and Process on Earth through Isotope Mapping.’ (Eds J. B. West, G. J. Bowen, T. E. Dawson, and K. P. Tu.) pp. 251–270. (Springer: Dordrecht, Netherlands.)
Yang, Z. P., Fryer, B. J., Longerich, H. P., Gagnon, J. E., and Samson, I. M. (2011). 785 nm femtosecond laser ablation for improved precision and reduction of interferences in Sr isotope analyses using MC-ICP-MS. Journal of Analytical Atomic Spectrometry 26, 341–351.
| 785 nm femtosecond laser ablation for improved precision and reduction of interferences in Sr isotope analyses using MC-ICP-MS.Crossref | GoogleScholarGoogle Scholar |
A H. Davy was a renowned chemist and polymath whose life and work deserves attention by the fish ecologist. In addition to the isolation of important alkaline earth metals, he discovered the anaesthetic properties of nitrous oxide and served as President of the Royal Society in London. He was also an amateur poet, friend of Samuel Taylor Coleridge and an avid outdoorsman who was passionate about fishing his entire life. One of his final published works was entitled Salmonia, which was a treatise on the practice of fly-fishing and the natural history of salmonids (Keys 1956). He would be surely delighted to learn how his chemical and ichthyological passions have collided in the field of otolith chemistry.



