Using stable-isotope analysis and acoustic telemetry data to infer broad-scale migration patterns of Port Jackson sharks (Heterodontus portusjacksoni)
N. C. Bass A * , N. E. Hussey B and C. Brown
A * , N. E. Hussey B and C. Brown  A
A
A Department of Biological Sciences, Macquarie University, North Ryde, NSW 2109, Australia.
B Department of Integrative Biology, University of Windsor, 401 Sunset Avenue, Windsor, ON, N9B 3P4, Canada.
Marine and Freshwater Research 74(4) 387-397 https://doi.org/10.1071/MF22180
Submitted: 7 September 2022 Accepted: 22 December 2022 Published: 3 February 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
Context: Understanding migratory species’ habitat selection is complicated by variation in movement strategies. Stable-isotope analysis provides a powerful tool to investigate such variation.
Aims: We used acoustic telemetry and stable-isotope analysis to better understand the movement strategies of Port Jackson sharks.
Methods: We compared the δ13C and δ15N values of fin tissue from acoustically tracked individuals that undertook three distinct movement strategies. Hierarchical cluster analysis was then used to cluster movement strategies of a larger sample of sharks on the basis of δ13C and δ15N values.
Key results: Tracked individuals that remained in Jervis Bay were enriched in 13C, compared with those that migrated south after the breeding season. Individuals were assigned to six clusters and δ13C and δ15N values indicated that migrating males and females may utilise different geographical areas or niches during the non-breeding season.
Conclusions: By using stable isotope analysis and acoustic telemetry, we identified distinct groups of Port Jackson sharks with similar broad-scale movement strategies.
Implications: These variable movement strategies may lead to different reproductive fitness advantages on an individual and population level, having implications for the broader ecosystem, given the important role mesopredators play in southern reef marine ecosystems.
Keywords: acoustic tracking, differential migration, dispersal, ecology, elasmobranchs, isotopes, marine, movement, stable isotopes.
Introduction
The life histories of species that undertake broadscale migrations are influenced by the complex interaction between the abiotic and biotic factors at breeding grounds, non-breeding sites and during migration. The non-breeding phase of the reproductive cycle plays an important role in influencing population dynamics through its relative effect on individual survival, condition and future reproductive success (e.g. Saino et al. 2004; Leyrer et al. 2013; Goodenough et al. 2017). Several physiological, behavioural and life-history-related traits that influence breeding phenology and investment are shaped by the selective pressures operating while individuals are at non-breeding sites. For example, Goodenough et al. (2017) found that pied flycatchers (Ficedula hypoleuca) that overwintered in more mesic habitats with moderate moisture levels (identified by lower δ13C values) had higher levels of breeding success in terms of clutch size, number of young to hatch and number of young to fledge. Studying among-individual variation in non-breeding site selection and its effects on reproductive fitness is essential for a comprehensive understanding of the movement ecology of a species at the population level.
Discerning the non-breeding habitats or locations of marine species that exhibit broadscale migrations can be particularly challenging, given their cryptic nature, vast scales of movement and the medium in which they live. Although electronic tagging, such as acoustic telemetry, archival tags and satellite telemetry can provide detailed insights into animal movement behaviours (Hussey et al. 2015), equipment costs can be prohibitive, they can often be expensive to deploy and maintain, are not applicable to all species or study areas and are often limited by sample size (Sequeira et al. 2019). Further, for acoustic telemetry to generate useful data, tagged individuals must utilise geographical areas and habitats that are monitored by acoustic receivers. Alternatively, methods that allow the retrospective tracking of individuals through the analyses of minor invasive tissue sampling, such as stable isotopes (e.g. Madigan et al. 2018a) and fatty acids (e.g. Cárdenas-Palomo et al. 2018), provide a more economically viable approach that allows investigation of behaviour of large numbers of individuals that may be more reflective of overall population dynamics (Chapman et al. 2015; Madigan et al. 2018b). Analysis of these naturally occurring biochemical markers in tissues can be used to answer questions pertaining to broad-scale movement and migration patterns of individuals (Rubenstein and Hobson 2004; Hussey et al. 2012), discerning residency areas such as overwintering habitat for migratory bird species (Steenweg et al. 2017) and can show population-level variation in past movements (Madigan et al. 2014, 2021).
The application of bulk stable-isotope analysis to investigate movement is based on the premise that variations in biogeochemical processes in spatially distinct areas, i.e. those that have isotopically different baseline δ13C values (or in specific cases δ15N values; Madigan et al. 2021), are reflected in the tissues of consumers that forage on prey resident in those areas (DeNiro and Epstein 1978, 1981). Isotopic turnover rates differ among tissue types; consequently, tissue selection or the use of multiple tissues is an important consideration for stable-isotope studies of movement–foraging ecology. For example, muscle and cartilage from fin tissue of elasmobranchs has a relatively long estimated isotopic turnover rate and can be used to examine long-term integrated movement patterns, whereas plasma has a relatively shorter isotopic turnover rate, which can be used to investigate seasonal movement patterns (MacNeil et al. 2006; Logan and Lutcavage 2010; Matich et al. 2011; Hussey et al. 2012; Kim et al. 2012). Further, given that only a small tissue sample is needed, it is a minor-invasive and powerful tool to study the movements of cryptic and vulnerable species (Hammerschlag and Sulikowski 2011; Hussey et al. 2012).
Ecological studies from a wide range of taxa typically use the conservative fractionation of carbon isotopes (i.e. δ13C; ∼1‰) to examine where an individual forages (e.g. Couturier et al. 2013) because 13C can vary between benthic and pelagic habitats (France 1995) and is influenced by marine, freshwater and terrestrial inputs (Fry and Sherr 1989), whereas the marked fractionation of nitrogen isotopes (i.e. δ15N; 3.4‰) is typically used to estimate trophic position and to examine food-web structure (Hobson 1999; Hussey et al. 2012, 2014; but see Madigan et al. 2018b for system-specific applications of δ15N). In the marine environment, stable-isotope analysis has been successfully employed to reconstruct the movement ecology of seabirds (e.g. Votier et al. 2010), fishes (e.g. Matley et al. 2017), mammals (e.g. Aurioles et al. 2006) and elasmobranchs (e.g. Abrantes and Barnett 2011; Hussey et al. 2011a; French et al. 2018). Specifically, variation in δ13C and δ15N values of shark tissues has allowed inference on broad-scale movement patterns (Hussey et al. 2011a), movements of individuals among distinct habitats (Abrantes and Barnett 2011; Hussey et al. 2011a), as well as sexual (Abrantes and Barnett 2011; French et al. 2018) or ontogenetic segregation (Nielsen et al. 2019; Di Lorenzo et al. 2020). However, the majority of these studies have focused on large, apex-predator species, identifying a need for more focused effort to understand smaller, benthic elasmobranch species (Simpfendorfer and Heupel 2012). These smaller, benthic species, which often occupy mesopredator roles in marine ecosystems, play an important role in linking lower and upper trophic levels as both predators and prey (Ritchie and Johnson 2009). Studying the movement ecology of these species can have important implications not only at the species level, but also for the communities in which they live (Ritchie and Johnson 2009).
Port Jackson shark (Heterodontus portusjacksoni) is a benthic shark species that acts as a tertiary consumer in temperate ecosystems confined to the southern coast of the Australian continent (Powter et al. 2010). Given the assumed important ecological role of adult Port Jackson sharks, as well as their high abundance and high levels of site fidelity, coupled with broad-scale migrations along the Australian coastline (Bass et al. 2017), they represent a useful model species to develop a greater understanding of the role of mesopredators in linking spatially distinct temperate marine ecosystems. Stomach content and fatty acid analyses have been used to quantify the diets of Port Jackson sharks throughout their distribution (Powter et al. 2010; Sommerville et al. 2011; Beckmann 2014), showing significant differences in foraging among regions and by size class. Sommerville et al. (2011) and Powter et al. (2010) noted an ontogenetic shift in the diet of Port Jackson sharks, which Powter et al. (2010) attributed to a shift in ecological role from secondary consumers as juveniles and subadults to tertiary consumers as adults. There have been no in situ studies using stable-isotope analysis to examine the foraging behaviour or movements of Port Jackson sharks. Combining acoustic-telemetry tracking of individuals (Speed et al. 2012; Matich and Heithaus 2014) with stable-isotope analysis of tagged and non-tagged individuals by using analytical approaches such as cluster analysis (Hobson 1999) has the potential to further our understanding of the movement ecology of mesopredatory elasmobranch species.
Here, we used stable-isotope analysis of fin tissue of a large sample of Port Jackson sharks in conjunction with an extensive acoustic telemetry array to monitor the large-scale movements of a subset of individuals to infer the non-breeding habitat for this species. Specifically, we aimed to (1) determine whether variation in stable-isotope values (δ13C and δ15N) among tracked individuals was correlated with known discrete movement strategies derived through long-term acoustic-telemetry tracking, and then (2) define clusters within a larger sample size of individuals on the basis of only δ13C and δ15N values to examine size-based and sex-specific variation in non-breeding movements at the population level. These data advance our understanding of Port Jackson shark movement ecology in temperate ecosystems, showing complex movement behaviours similar to those of larger-bodied species.
Materials and methods
Capture and tagging procedures
Port Jackson sharks were opportunistically hand-caught on snorkel between July and September 2012 at Dent Rock (n = 19) and Plantation Point (n = 109), two subtidal rocky reef habitats located in Jervis Bay, Australia. All Port Jackson sharks were measured (total length, cm), sexed based on the presence or absence of claspers, a fin clip was taken from the trailing edge of the first dorsal fin for stable-isotope and genetic analyses and individuals were tagged with passive integrated transponder (PIT) tags to allow for individual identification. Fin tissue was used for this study to allow for a longer-term assessment of movement and foraging behaviour (Matich et al. 2011) and was obtained using a standardised sampling location to minimise composition variability among samples and to allow for a more accurate comparison among individuals (Hussey et al. 2011b). A subset of the sampled individuals (n = 13) were also fitted with V16-x acoustic transmitters (Innovasea, Halifax, NS, Canada; nominal delay 90 s; expected battery life 7.5 years; frequency 69 kHz). Within Jervis Bay, New South Wales Department of Primary Industries installed an acoustic receiver array (n = 27 receivers; Innovasea VR2-W receivers) to monitor the movements and residency of a number of fish species on rocky reefs within the bay and across the bay entrance. The receivers were estimated to have a detection range of ∼250 m (50% probability of detection; Swadling et al. 2020). Additional receivers were deployed around the Australian coastline (n = 112 between Jervis Bay and Tasmania), as a part of the Integrated Marine Observing System (IMOS) animal-tracking database, a national collaborative research initiative of various universities, government and private research institutions. Details on residency, local movements and migration patterns of Port Jackson sharks based on these data have previously been described by Bass et al. 2017, 2021a, 2021b). The migration data from Bass et al. (2017) are used here to validate the application of isotopes to understand population-level movement behaviour.
All tagging and sampling procedures were conducted in accordance with a New South Wales fisheries permit (P08/0010-3.1) and an Animal Research Authority permit (2012/009) granted by the Macquarie University Animal Ethics Committee.
Tissue collection and processing
Tissue samples for stable-isotope analysis were taken from the trailing edge of the first dorsal fin of each individual and stored at −20°C on returning to the laboratory. Tissue samples were then freeze-dried and transported to the Great Lakes Institute for Environmental Research, University of Windsor, Canada, for stable-isotope analysis. Fin tissue samples were first homogenised and ground into a fine powder and then lipids were extracted to remove their potential bias on δ13C values (Post et al. 2007). In brief, lipid extraction involved agitating samples in a 2:1 chloroform–methanol solution for 24 h, decanting of the solution at the end of this period and drying the resulting tissue pellet for 48 h in a fume cupboard to remove any remaining solvent following standard procedures (Hussey et al. 2012). Urea, a tissue solute known to influence δ15N values in elasmobranchs, was not extracted given expected low concentrations in fin tissue and previous experimental work demonstrating minimal effect of this processing step on derived fin isotope values (Shipley et al. 2017). Processed tissues sampled were weighed into tin capsules (between 400 and 600 μg) and carbon and nitrogen stable-isotope ratios were analysed using a continuous flow isotope-ratio mass spectrometer (Finnigan MAT Deltaplus, Thermo Finnigan, San Jose, CA, USA) interfaced with an elemental analyser (Costech 4010). Stable isotope abundances are expressed in delta notation (δ) because the ratio of a sample to recognised standards in parts per thousand (‰), using the following equation:
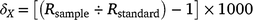
where X is 13C or 15N, Rsample is the corresponding ratio of 13C:12C or 15N:14N and Rstandard represents the isotopic ratios of the respective standards (Pee Dee Belemnite carbonate for δ13C and atmospheric nitrogen for δ15N). The analytical precision for δ15N was <0.20‰ and for δ13C it was <0.12‰ on the basis of more than 100 analyses of NIST standard 8414 and tilapia muscle. Instrument accuracy, on the basis of the analysis of certified NIST standards 8542 and 8547, was 0.08‰ for δ15N and 0.02‰ for δ13C.
Data analysis
Statistical analyses were conducted in RStudio (ver. 1.0.136, RStudio PBC, Boston, MA, USA, see https://rstudio.com/) using the packages ‘stats’ (ver. 4.3.0, see https://stat.ethz.ch/R-manual/R-devel/library/stats/html/00Index.html), ‘Rfit’ (ver. 0.24.2, see https://CRAN.R-project.org/package=Rfit; Kloke and McKean 2012), ‘dplyr’ (ver. 1.0.10, see https://CRAN.R-project.org/package=dplyr), ‘factoextra’ (ver. 1.0.7, see https://CRAN.R-project.org/package=factoextra) and ‘ggplot2’ (ver. 3.4.0, see https://CRAN.R-project.org/package=ggplot2; Wickham 2016).
Detection trends of acoustically tagged individuals based on previous work (see Bass et al. 2017 for further details) showed that the majority of Port Jackson sharks departed Jervis Bay after the breeding season and exhibited southward migrations, with individuals being detected up to 645 km south of Jervis Bay (Fig. 1). However, some individuals were not detected outside of the breeding season and at least two individuals remained in Jervis Bay year-round. For the current study, these tagged individuals were categorised into the following three movement strategies on the basis of their behaviour observed in Bass et al. (2017): those detected within Jervis Bay throughout the year (i.e. resident; n = 2), individuals detected by the IMOS Narooma Line (i.e. southern migrators; n = 6) and those not detected outside of the breeding season (i.e. unknown movements after present in Jervis Bay for the breeding season; n = 5). Owing to the sparse distribution of acoustic receivers along the southern part of the eastern coast of Australia, it is possible that individuals that were not detected outside of the breeding season may have moved southward to or past the IMOS Narooma Line. A Kruskall–Wallis test was used to examine whether variation in δ13C and δ15N values correlated with these three known discrete movement strategies. A Dunn test was used to examine pairwise differences between δ13C values and migration strategy, with P-values adjusted using the Benjamini–Hochberg method.
|
|
Prior to assessing population-level movements beyond the breeding season on the basis of isotope values, we first examined whether tagging location (n = 2; Dent Rock and Plantation Point) influenced overall δ15N and δ13C values of all individuals sampled (n = 128) by using a Kruskall–Wallis test. Given that δ13C and δ15N values did not differ between tagging locations (δ13C: KW = 1.774, d.f. = 2, P = 0.412; δ15N: KW = 2.324, d.f. = 2, P = 0.313), all individuals were combined into a single data set. Because of a non-normal distribution of the data, Kruskall–Wallis test was used to examine potential sex-based effects on δ15N and δ13C values separately. Rank-based estimation regression was then used to identify any sized-based effects on δ15N and δ13C fin-tissue values. Given sexual dimorphism, whereby female Port Jackson sharks are larger than males, we mean-centred the total length data for each sex independently (ranging from zero for the smallest individual to one for the largest individual) prior to undertaking the regression analysis.
To examine size-based and sex-specific variation in non-breeding movements at the population level, K-means clustering was then used to assign individuals into groups on the basis of similarity of their δ13C and δ15N values as a proxy for different movement strategies. The ‘fviz_nbclust()’ function in the ‘factoextra’ package in R was used to determine the optimal number of clusters by using the silhouette method. Following this, an ANOVA was used to test whether δ13C or δ15N values differed among derived clusters. Given our low sample size of acoustically tagged individuals (n = 13) relative to our total sample size (n = 128), a Fisher’s exact test was used to compare the movement strategies of individuals to their cluster assignment, to validate the clusters and to allow assignment of preliminary movement strategies to the derived clusters for the larger non-tagged population sample size.
The derived clusters based on the larger population size were then re-examined to determine whether demographic factors (sex and body size) differed (1) among clusters and (2) among individuals within each cluster, indicative of differential foraging or movement habits. For Point 1, a chi-squared test of association was used to examine whether there was a significant level of association between sex and cluster assignment and a Kruskal–Wallis test was employed to determine whether the mean-centred total lengths (TLs) of individuals differed among derived clusters. To determine whether males and females within each cluster differed in their δ13C and δ15N values (i.e. for Point 2), Kruskal–Wallis tests were used. For size effects, linear regression was used to examine the influence of mean-centred TL on δ13C and δ15N values within each cluster.
Results
General results
In total, 99 male and 29 female Port Jackson sharks were captured, sampled and PIT-tagged at Dent Rock and Plantation Point. Females ranged in body size from 107 cm to 132 cm TL and males from 74 cm to 115 cm TL. There was considerable variation in both δ13C values (mean ± s.e. = −15.4 ± 0.1; range = −12.4 to −20.3) and δ15N (mean ± s.e. = 14.0 ± 0.1; range = 11.9–17.0) of fin tissue among individuals. Although there was no significant difference between the δ13C values of all sampled males and females (KW = 1.6258, d.f. = 1, P = 0.202; Fig. 2), males exhibited higher δ15N values than did females (KW = 4.460, d.f. = 1, P = 0.035; Fig. 2). Additionally, there was no significant relationship between mean-centred TL and δ13C (t = 1.030, P = 0.3051) or δ15N (t = 0.411, P = 0.682) for all sampled sharks.
Determining whether known movement strategies correlate with discrete stable-isotope values
When considering the three movement strategies of Port Jackson sharks derived from acoustic-telemetry tracking, namely, continuously detected in Jervis Bay throughout the year (n = 2), detected at Narooma (200 km south of Jervis Bay) outside of the breeding season (n = 6) and not detected outside of the breeding season (n = 5), there was a significant difference between δ13C values of fin tissue of tagged individuals (KW = 7.640, d.f. = 2, P = 0.022; Fig. 3a). Pairwise comparisons showed that tagged individuals detected in Jervis Bay year-round had significantly lower δ13C values than did those detected at Narooma during the non-breeding season (z = 2.621, P = 0.026), but were not different from tagged individuals that were only seasonally detected in Jervis Bay (i.e. not detected during the migration period; z = 1.710, P = 0.131). The δ13C values of tagged individuals that were only seasonally detected in Jervis Bay and those of tagged individuals that were detected at Narooma were similar (z = −1.320, P = 0.187, Fig. 3a). The δ15N values of tagged individuals across the three different movement strategies did not differ (KW = 0.976, d.f. = 2, P = 0.614, Fig. 3b). Accepting the small sample size, the standard error of isotope values for individuals detected within Jervis Bay throughout the year was lower than that of the two other groups. This suggests that further research may be needed to investigate whether individuals from the Narooma group and the group that were not detected outside of the breeding season fed over a broader range of habitats and geographical areas than did the two individuals that remained in Jervis Bay.
Cluster analysis to assign the population-level sample to movement strategies on the basis of stable-isotope data alone
Cluster analysis on δ13C and δ15N values for the larger population sample size of non-tagged Port Jackson sharks combined with tagged animals found six distinct clusters with a silhouette measure of cohesion and separation of 0.7 (Fig. 4). An ANOVA showed significant differences in fin-tissue δ13C values among clusters (F = 225.2, d.f. = 5, 122, P < 0.001), with Tukey’s post hoc tests identifying all clusters being different, with the exception of Clusters 3 and 5 (diff = 0.315, P = 0.052). There were also significant differences in δ15N values among clusters (F = 41.42, d.f. = 5, 122, P < 0.001), but Tukey’s HSD post hoc tests found that the majority (Clusters 1, 2, 3 and 4) were similar (P > 0.05). A significant association between movement strategy defined from acoustic-telemetry data and cluster assignment based on stable-isotope data was not apparent (Fisher’s exact test; P = 0.180), likely being a result of our small sample size. Nevertheless, Cluster 2, which was the most enriched in 13C, contained both acoustically tagged individuals that were detected in Jervis Bay year-round and no acoustically tagged individuals that were detected at Narooma.
Effect of demographic factors among and within assigned clusters
There was a significant association between sex and cluster assignment (X2 = 16.498, d.f. = 5, P-value = 0.006), but this was most likely to be a result of male bias in sampling within the study population (male: n = 99; female: n = 29). When comparing δ13C and δ15N values of males and females within clusters, no significant differences were observed, with the exception of Cluster 1 (P > 0.05 for all tests). Males in Cluster 1 (n = 14) were significantly enriched in 13C compared with females (n = 8; KW = 6.044, d.f. = 1, P = 0.014). There were no significant differences between the mean-centred TL of individuals among clusters (KW = 10.328, d.f. = 5, P = 0.066). When considering within-cluster variation, only individuals in Cluster 6 showed a significant positive relationship between mean-centred TL and δ13C (F = 8.039, d.f. = 1, 31, P = 0.008). There was no significant relationship between mean-centred TL and δ15N values among individuals within any of the clusters (P > 0.05).
Discussion
The combined application of acoustic telemetry and stable-isotope analysis of fin tissue of Port Jackson sharks provides evidence for potential differences in broad-scale movement patterns of male and female Port Jackson sharks outside of the breeding season. Individuals showed considerable variation in δ13C and δ15N within fin tissue and isotopic variation among acoustically tagged sharks was broadly associated with known movement behaviours. Individuals from our larger non-tagged population sample size were assigned to six clusters on the basis of δ13C and δ15N values, with some clusters matching known movements of acoustically tagged individuals. These clusters suggest that individuals of a mesopredator shark species show marked variation in movement strategies (denoted by δ13C) and potentially foraging behaviour (denoted by δ15N) while not at their breeding grounds. Furthermore, males and females differed in δ15N values, suggesting potential sexual segregation or niche specialisation with respect to geographical area, habitat selection or foraging behaviour beyond the breeding season. While accepting limitations related to sample size (number of individuals tracked and male bias in our population sample) and using fin tissues for stable-isotope analysis (variable composition of fin tissue and unknown fin-tissue incorporation rates for Port Jackson sharks), these data demonstrated the power of combined isotope and telemetry data for cost-effective inference of the movement dynamics of a mesopredator shark. Moreover, differential movement behaviours observed at the population level indicated that managing smaller-bodied shark species is potentially more complex than was previously thought (Shaw 2020).
Variation in δ13C and δ15N values among the assigned clusters suggests that Port Jackson sharks within each cluster feed in different habitats or geographical zones beyond the breeding season. For example, the observed variation in δ13C values among clusters could indicate that groups of Port Jackson sharks feed over a latitudinal or inshore–offshore gradient. Given the distribution of acoustically tagged individuals within clusters and accepting our sample size of tagged individuals, it is possible to infer the migration behaviour of several clusters. Clusters 1, 3, 4 and 6 all contained individuals that were detected at Narooma and those that were not detected outside of the breeding season, indicating a southward migration. Because of the sparse spatial distribution of receivers along the New South Wales coast, it is possible that undetected individuals still migrated south using deeper waters, bypassing the receivers at Narooma (Bass et al. 2017). However, variation among these clusters (i.e. the degree of 13C depletion) could indicate that individuals migrate varying latitudinal distances to habitats along this stretch of coastline (Bass et al. 2017) or that they may preferentially migrate to different inshore or offshore environments (Hobson 1999). Several studies have suggested that intraspecific variation in δ13C and δ15N values of shark tissue may be indicative of intraspecific variation in movement or foraging behaviour (Abrantes and Barnett 2011; Borrell et al. 2011; Marcus et al. 2019). For example, Marcus et al. (2019) suggested that high variability in the δ15N values of whale sharks sampled on Ningaloo reef was indicative of different foraging patterns among individuals characterised by movements between Ningaloo reef and along the Western Australian coastline. Additionally, Li et al. (2016) found that eight species of pelagic shark that are commonly caught in the north-eastern central Pacific Ocean exhibited a reasonably wide range of δ15N and δ13C values, which the authors suggested indicates that each species may forage in distinct habitats or feed on more than one trophic level. Similarly, variation in the δ15N values between clusters of Port Jackson sharks, namely individuals in Cluster 5 and Cluster 6, may indicate feeding on prey at different trophic levels or in habitats that are enriched in 15N during the non-breeding season.
One potential driver for the observed variation in δ13C and δ15N among Port Jackson sharks may relate to partial migration to and from Jervis Bay during the non-breeding season. Individuals within Cluster 2 (n = 14) were less depleted in 13C than were those in all other clusters, indicating that these individuals were feeding in more northerly or inshore waters. Further, two individuals in Cluster 2 were acoustically tagged and were detected within Jervis Bay throughout the year, suggesting that the rest of the individuals within this cluster may have also remained in Jervis Bay throughout the non-breeding season. Anecdotal observations of large aggregations of Port Jackson sharks on deep rocky reefs just outside of Jervis Bay (Paul Daniels, pers. comm.) provides further evidence that not all Port Jackson sharks exhibit long-distance migratory behaviours outside of the breeding season. McLaughlin and O’Gower (1971) also speculated that some individuals may move to deeper waters offshore, whereas others migrate in a southward direction. Partial migration has been identified in a few shark species (Chapman et al. 2012; Papastamatiou et al. 2013) and adds further complexity to understanding the impact of mesopredators in temperate marine ecosystems.
Male and female Port Jackson sharks showed differences in δ13C and δ15N values, suggesting a degree of sexual segregation or niche specialisation with respect to foraging behaviour outside of the breeding season. Previous research at breeding aggregation sites has indicated that males and females show different habitat preferences (Powter and Gladstone 2008, 2009). The association between sex and cluster provides further support of sexual segregation of Port Jackson sharks outside of the breeding season. For example, the tissues of individuals in Cluster 4, which contained a reasonably high proportion of females (female:male = 3:1), were significantly more depleted in 13C, indicating that these individuals were feeding in more southerly or offshore waters. In agreement, Christie (2015) noted female-only aggregations in the Point Cooke Marine Sanctuary (∼30 km south of Melbourne). Female Port Jackson sharks may, similarly to other elasmobranch species (Carrier et al. 1994; Sims et al. 2001; Wearmouth and Sims 2008), utilise different geographical areas from males to avoid harassment, to prepare for the energetically expensive processes of reproduction, egg production and laying or because of their considerably larger size than that of males. Alternatively, Powter et al. (2010) suggested that dietary variation among individual Port Jackson sharks may be a result of variable dentition and structure or morphology of the head and jaw rather than of competition for resources, which could explain the highly variable δ13C and δ15N observed. Understanding the mechanism of sexual segregation has important biological and ecological implications for the reproductive fitness of individuals within populations, as well as broader implications for understanding the movement ecology (i.e. space use) of shark and ray species (Wearmouth and Sims 2008).
There is a growing body of evidence that elasmobranchs of the same sex and age class exhibit intraspecific variation in residency, movement and habitat use within a season or on an annual cycle (Vaudo et al. 2014; Bass et al. 2021a; Espinoza et al. 2021). Intraspecific variation in migration behaviour in other taxa, in particular overwintering or non-breeding locations of birds (Saino et al. 2004; Leyrer et al. 2013; Goodenough et al. 2017; Steenweg et al. 2017), can influence the fitness and reproductive success of individuals, with consequences for their survival and resilience. Further, for species that are susceptible to anthropogenic disturbance, variability in movement ecology may have important implications for their effective management and their adaptability to disturbances. To further explore individual variation in the movement ecology and foraging behaviour of elasmobranchs by using stable-isotope analysis, future studies should sample multiple tissue types (e.g. plasma, red blood cells and muscle tissues) with known incorporation rates (i.e. established through captive-feeding experiments) from the same individual and establish baseline isotope values within ecosystems (i.e. endpoints) throughout the year (Hussey et al. 2012; Matich and Heithaus 2014; Munroe et al. 2015). The compilation of ecosystem baseline isotope values at multiple locations within Jervis Bay and along the eastern coast of Australia combined with shark isotope data would allow the use of isotopic clocks to further investigate population-level movement behaviour (Madigan et al. 2021; Shipley et al. 2021).
Conclusions
The current study represents the first application of stable-isotope analyses to examine population-level variation in the movement patterns of Port Jackson sharks beyond the breeding season and provides evidence for differences in movement strategies among individuals and potentially between sexes. Combined tracking data from acoustic telemetry provided a degree of validation for variation observed in stable-isotope data (e.g. Madigan et al. 2021) and provided further detail into the inter-individual variability observed in this and other studies (Doherty et al. 2010; Matich and Heithaus 2014; Harrison et al. 2017). Moreover, combining the two methodologies provided a reasonable approach to scale up sample sizes, to address limitations of some methods (e.g. sparse acoustic receiver coverage) and to optimise data collected from each individual to better understand the prevalence and complexities of variable movement strategies (Matley et al. 2022). The seasonal immigration of Port Jackson sharks into Jervis Bay from a diverse range of habitats and geographical areas may represent an important influx of nutrients into the ecosystem through egestion (e.g. Williams et al. 2018) or through the deposition of eggs (e.g. Bouchard and Bjorndal 2000; Gende et al. 2002; Fritz and Whiles 2018). Elucidating the behaviour of sharks at these non-breeding locations and the fitness advantages of differential migration strategies has important ecological and management implications for the seasonal communities in which they migrate to (Heithaus et al. 2008) and highlights the important role that coastal and benthic sharks play in connecting distant and diverse ecosystems on both an individual and species level.
Data availability
Acoustic telemetry data were sourced from Australia’s Integrated Marine Observing System (IMOS, http://www.imos.org.au), which is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS). The data can be accessed from the IMOS Animal Acoustic Telemetry Database (animaltracking.aodn.org.au) or upon request from the lead author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research was funded by Sea World Research and Rescue Foundation Inc. (SWR/5/2012, the Australian Research Council (LP140100319), Taronga Conservation Society Australia and the Department of Biological Sciences, Macquarie University.
Acknowledgements
The authors thank Tristan Guttridge, Joanna Day, Sue Newson and various volunteers for assistance with acoustic tagging and obtaining isotope samples in the field.
References
Abrantes, KG, and Barnett, A (2011). Intrapopulation variations in diet and habitat use in a marine apex predator, the broadnose sevengill shark Notorynchus cepedianus. Marine Ecology Progress Series 442, 133–148.| Intrapopulation variations in diet and habitat use in a marine apex predator, the broadnose sevengill shark Notorynchus cepedianus.Crossref | GoogleScholarGoogle Scholar |
Aurioles, D, Koch, PL, and Le Boeuf, BJ (2006). Differences in foraging location of Mexican and California elephant seals: evidence from stable isotopes in pups. Marine Mammal Science 22, 326–338.
| Differences in foraging location of Mexican and California elephant seals: evidence from stable isotopes in pups.Crossref | GoogleScholarGoogle Scholar |
Bass, NC, Mourier, J, Knott, NA, Day, J, Guttridge, T, and Brown, C (2017). Long-term migration patterns and bisexual philopatry in a benthic shark species. Marine and Freshwater Research 68, 1414–1421.
| Long-term migration patterns and bisexual philopatry in a benthic shark species.Crossref | GoogleScholarGoogle Scholar |
Bass, NC, Day, J, Guttridge, TL, Knott, NA, and Brown, C (2021a). Intraspecific variation in diel patterns of rocky reef use suggests temporal partitioning in Port Jackson sharks. Marine and Freshwater Research 72, 1445–1456.
| Intraspecific variation in diel patterns of rocky reef use suggests temporal partitioning in Port Jackson sharks.Crossref | GoogleScholarGoogle Scholar |
Bass, NC, Day, J, Guttridge, TL, Mourier, J, Knott, NA, Vila Pouca, C, and Brown, C (2021b). Residency and movement patterns of adult Port Jackson sharks (Heterodontus portusjacksoni) at a breeding aggregation site. Journal of Fish Biology 99, 1455–1466.
| Residency and movement patterns of adult Port Jackson sharks (Heterodontus portusjacksoni) at a breeding aggregation site.Crossref | GoogleScholarGoogle Scholar |
Beckmann CL (2014) Fatty acid profiles of a benthic chondrichthyan: captive feeding trials and ecological applications. PhD thesis, Flinders University, Adelaide, SA, Australia.
Borrell, A, Aguilar, A, Gazo, M, Kumarran, RP, and Cardona, L (2011). Stable isotope profiles in whale shark (Rhincodon typus) suggest segregation and dissimilarities in the diet depending on sex and size. Environmental Biology of Fishes 92, 559–567.
| Stable isotope profiles in whale shark (Rhincodon typus) suggest segregation and dissimilarities in the diet depending on sex and size.Crossref | GoogleScholarGoogle Scholar |
Bouchard, SS, and Bjorndal, KA (2000). Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems. Ecology 81, 2305–2313.
| Sea turtles as biological transporters of nutrients and energy from marine to terrestrial ecosystems.Crossref | GoogleScholarGoogle Scholar |
Carrier, JC, Pratt, HL, and Martin, LK (1994). Group reproductive behaviors in free-living nurse sharks, Ginglymostoma cirratum. Copeia 1994, 646–656.
| Group reproductive behaviors in free-living nurse sharks, Ginglymostoma cirratum.Crossref | GoogleScholarGoogle Scholar |
Cárdenas-Palomo, N, Noreña-Barroso, E, Herrera-Silveira, J, Galván-Magaña, F, and Hacohen-Domené, A (2018). Feeding habits of the whale shark (Rhincodon typus) inferred by fatty acid profiles in the northern Mexican Caribbean. Environmental Biology of Fishes 101, 1599–1612.
| Feeding habits of the whale shark (Rhincodon typus) inferred by fatty acid profiles in the northern Mexican Caribbean.Crossref | GoogleScholarGoogle Scholar |
Chapman, BB, Skov, C, Hulthén, K, Brodersen, J, Nilsson, PA, Hansson, L-A, and Brönmark, C (2012). Partial migration in fishes: definitions, methodologies and taxonomic distribution. Journal of Fish Biology 81, 479–499.
| Partial migration in fishes: definitions, methodologies and taxonomic distribution.Crossref | GoogleScholarGoogle Scholar |
Chapman, DD, Feldheim, KA, Papastamatiou, YP, and Hueter, RE (2015). There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Annual Review of Marine Science 7, 547–570.
| There and back again: a review of residency and return migrations in sharks, with implications for population structure and management.Crossref | GoogleScholarGoogle Scholar |
Christie, A (2015). Account of a mass aggregation of Port Jackson sharks Heterodontus portusjacksoni at Point Cooke Marine Sanctuary, Victoria, Australia. The Victorian Naturalist 132, 108–117.
Couturier, LIE, Rohner, CA, Richardson, AJ, Marshall, AD, Jaine, FRA, Bennett, MB, Townsend, KA, Weeks, SJ, and Nichols, PD (2013). Stable isotope and signature fatty acid analyses suggest reef manta rays feed on demersal zooplankton. PLoS ONE 8, e77152.
| Stable isotope and signature fatty acid analyses suggest reef manta rays feed on demersal zooplankton.Crossref | GoogleScholarGoogle Scholar |
DeNiro, MJ, and Epstein, S (1978). Influence of diet on the distribution of carbon isotopes in animals. Geochimica et Cosmochimica Acta 42, 495–506.
| Influence of diet on the distribution of carbon isotopes in animals.Crossref | GoogleScholarGoogle Scholar |
DeNiro, MJ, and Epstein, S (1981). Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta 45, 341–351.
| Influence of diet on the distribution of nitrogen isotopes in animals.Crossref | GoogleScholarGoogle Scholar |
Di Lorenzo, M, Vizzini, S, Signa, G, Andolina, C, Boscolo Palo, G, Gristina, M, Mazzoldi, C, and Colloca, F (2020). Ontogenetic trophic segregation between two threatened smooth-hound sharks in the Central Mediterranean Sea. Scientific Reports 10, 11011.
| Ontogenetic trophic segregation between two threatened smooth-hound sharks in the Central Mediterranean Sea.Crossref | GoogleScholarGoogle Scholar |
Doherty, CA, Curry, RA, and Munkittrick, KR (2010). Spatial and temporal movements of white sucker: implications for use as a sentinel species. Transactions of the American Fisheries Society 139, 1818–1827.
| Spatial and temporal movements of white sucker: implications for use as a sentinel species.Crossref | GoogleScholarGoogle Scholar |
Espinoza, M, Lédée, EJI, Smoothey, AF, Heupel, MR, Peddemors, VM, Tobin, AJ, and Simpfendorfer, CA (2021). Intra-specific variation in movement and habitat connectivity of a mobile predator revealed by acoustic telemetry and network analyses. Marine Biology 168, 80.
| Intra-specific variation in movement and habitat connectivity of a mobile predator revealed by acoustic telemetry and network analyses.Crossref | GoogleScholarGoogle Scholar |
France, RL (1995). Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Marine Ecology Progress Series 124, 307–312.
| Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications.Crossref | GoogleScholarGoogle Scholar |
French, GCA, Rizzuto, S, Stürup, M, Inger, R, Barker, S, van Wyk, JH, Towner, AV, and Hughes, WOH (2018). Sex, size and isotopes: cryptic trophic ecology of an apex predator, the white shark Carcharodon carcharias. Marine Biology 165, 102.
| Sex, size and isotopes: cryptic trophic ecology of an apex predator, the white shark Carcharodon carcharias.Crossref | GoogleScholarGoogle Scholar |
Fritz, KA, and Whiles, MR (2018). Amphibian-mediated nutrient fluxes across aquatic–terrestrial boundaries of temporary wetlands. Freshwater Biology 63, 1250–1259.
| Amphibian-mediated nutrient fluxes across aquatic–terrestrial boundaries of temporary wetlands.Crossref | GoogleScholarGoogle Scholar |
Fry B, Sherr EB (1989) δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. In ‘Stable isotopes in ecological research’. (Eds PW Rundell, JR Ehleringer, KA Nagy) pp. 196–229. (Springer: New York, NY, USA)
Gende, SM, Edwards, RT, Willson, MF, and Wipfli, MS (2002). Pacific salmon in aquatic and terrestrial ecosystems: Pacific salmon subsidize freshwater and terrestrial ecosystems through several pathways, which generates unique management and conservation issues but also provides valuable research opportunities. BioScience 52, 917–928.
| Pacific salmon in aquatic and terrestrial ecosystems: Pacific salmon subsidize freshwater and terrestrial ecosystems through several pathways, which generates unique management and conservation issues but also provides valuable research opportunities.Crossref | GoogleScholarGoogle Scholar |
Goodenough, AE, Coker, DG, Wood, MJ, and Rogers, SL (2017). Overwintering habitat links to summer reproductive success: intercontinental carry-over effects in a declining migratory bird revealed using stable isotope analysis. Bird Study 64, 433–444.
| Overwintering habitat links to summer reproductive success: intercontinental carry-over effects in a declining migratory bird revealed using stable isotope analysis.Crossref | GoogleScholarGoogle Scholar |
Hammerschlag, N, and Sulikowski, J (2011). Killing for conservation: the need for alternatives to lethal sampling of apex predatory sharks. Endangered Species Research 14, 135–140.
| Killing for conservation: the need for alternatives to lethal sampling of apex predatory sharks.Crossref | GoogleScholarGoogle Scholar |
Harrison, PM, Gutowsky, LFG, Martins, EG, Ward, TD, Patterson, DA, Cooke, SJ, and Power, M (2017). Individual isotopic specializations predict subsequent inter-individual variation in movement in a freshwater fish. Ecology 98, 608–615.
| Individual isotopic specializations predict subsequent inter-individual variation in movement in a freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Heithaus, MR, Frid, A, Wirsing, AJ, and Worm, B (2008). Predicting ecological consequences of marine top predator declines. Trends in Ecology & Evolution 23, 202–210.
| Predicting ecological consequences of marine top predator declines.Crossref | GoogleScholarGoogle Scholar |
Hobson, KA (1999). Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120, 314–326.
| Tracing origins and migration of wildlife using stable isotopes: a review.Crossref | GoogleScholarGoogle Scholar |
Hussey, NE, Dudley, SFJ, McCarthy, ID, Cliff, G, and Fisk, AT (2011a). Stable isotope profiles of large marine predators: viable indicators of trophic position, diet, and movement in sharks? Canadian Journal of Fisheries and Aquatic Sciences 68, 2029–2045.
| Stable isotope profiles of large marine predators: viable indicators of trophic position, diet, and movement in sharks?Crossref | GoogleScholarGoogle Scholar |
Hussey, NE, Chapman, DD, Donnelly, E, Abercrombie, DL, and Fisk, AT (2011b). Fin-icky samples: an assessment of shark fin as a source material for stable isotope analysis. Limnology and Oceanography: Methods 9, 524–532.
| Fin-icky samples: an assessment of shark fin as a source material for stable isotope analysis.Crossref | GoogleScholarGoogle Scholar |
Hussey, NE, MacNeil, MA, Olin, JA, McMeans, BC, Kinney, MJ, Chapman, DD, and Fisk, AT (2012). Stable isotopes and elasmobranchs: tissue types, methods, applications and assumptions. Journal of Fish Biology 80, 1449–1484.
| Stable isotopes and elasmobranchs: tissue types, methods, applications and assumptions.Crossref | GoogleScholarGoogle Scholar |
Hussey, NE, MacNeil, MA, McMeans, BC, Olin, JA, Dudley, SF, Cliff, G, Wintner, SP, Fennessy, ST, and Fisk, AT (2014). Rescaling the trophic structure of marine food webs. Ecology Letters 17, 239–250.
| Rescaling the trophic structure of marine food webs.Crossref | GoogleScholarGoogle Scholar |
Hussey, NE, Kessel, ST, Aarestrup, K, Cooke, SJ, Cowley, PD, Fisk, AT, Harcourt, RG, Holland, KN, Iverson, SJ, Kocik, JF, and Mills Flemming, JE (2015). Aquatic animal telemetry: a panoramic window into the underwater world. Science 348, 1255642.
| Aquatic animal telemetry: a panoramic window into the underwater world.Crossref | GoogleScholarGoogle Scholar |
Kim, SL, del Rio, CM, Casper, D, and Koch, PL (2012). Isotopic incorporation rates for shark tissues from a long-term captive feeding study. Journal of Experimental Biology 215, 2495–2500.
| Isotopic incorporation rates for shark tissues from a long-term captive feeding study.Crossref | GoogleScholarGoogle Scholar |
Kloke, JD, and McKean, JW (2012). Rfit: rank-based estimation for linear models. The R Journal 4, 57–64.
Leyrer, J, Lok, T, Brugge, M, Spaans, B, Sandercock, BK, and Piersma, T (2013). Mortality within the annual cycle: seasonal survival patterns in Afro-Siberian Red Knots Calidris canutus canutus. Journal of Ornithology 154, 933–943.
| Mortality within the annual cycle: seasonal survival patterns in Afro-Siberian Red Knots Calidris canutus canutus.Crossref | GoogleScholarGoogle Scholar |
Li, Y, Zhang, Y, and Dai, X (2016). Trophic interactions among pelagic sharks and large predatory teleosts in the northeast central Pacific. Journal of Experimental Marine Biology and Ecology 483, 97–103.
| Trophic interactions among pelagic sharks and large predatory teleosts in the northeast central Pacific.Crossref | GoogleScholarGoogle Scholar |
Logan, JM, and Lutcavage, ME (2010). Stable isotope dynamics in elasmobranch fishes. Hydrobiologia 644, 231–244.
| Stable isotope dynamics in elasmobranch fishes.Crossref | GoogleScholarGoogle Scholar |
MacNeil, MA, Drouillard, KG, and Fisk, AT (2006). Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Canadian Journal of Fisheries and Aquatic Sciences 63, 345–353.
| Variable uptake and elimination of stable nitrogen isotopes between tissues in fish.Crossref | GoogleScholarGoogle Scholar |
Madigan, DJ, Baumann, Z, Carlisle, AB, Hoen, DK, Popp, BN, Dewar, H, Snodgrass, OE, Block, BA, and Fisher, NS (2014). Reconstructing transoceanic migration patterns of Pacific bluefin tuna using a chemical tracer toolbox. Ecology 95, 1674–1683.
| Reconstructing transoceanic migration patterns of Pacific bluefin tuna using a chemical tracer toolbox.Crossref | GoogleScholarGoogle Scholar |
Madigan, DJ, Baumann, Z, Carlisle, AB, Snodgrass, O, Dewar, H, and Fisher, NS (2018a). Isotopic insights into migration patterns of Pacific bluefin tuna in the eastern Pacific Ocean. Canadian Journal of Fisheries and Aquatic Sciences 75, 260–270.
| Isotopic insights into migration patterns of Pacific bluefin tuna in the eastern Pacific Ocean.Crossref | GoogleScholarGoogle Scholar |
Madigan, DJ, Snodgrass, OE, and Fisher, NS (2018b). From migrants to mossbacks: tracer- and tag-inferred habitat shifts in the California yellowtail Seriola dorsalis. Marine Ecology Progress Series 597, 221–230.
| From migrants to mossbacks: tracer- and tag-inferred habitat shifts in the California yellowtail Seriola dorsalis.Crossref | GoogleScholarGoogle Scholar |
Madigan DJ, Shipley ON, Hussey NE (2021) Applying isotopic clocks to identify prior migration patterns and critical habitats in mobile marine predators. In ‘Conservation physiology: integrating physiology into animal conservation and management’. (Eds CL Madliger, SJ Cooke, CE Franklin, OP Love) pp. 69–86. (Oxford University Press: Oxford, UK)
Marcus, L, Virtue, P, Nichols, PD, Ferreira, LC, Pethybridge, H, and Meekan, MG (2019). Stable isotope analysis of dermis and the foraging behavior of whale sharks at Ningaloo Reef, Western Australia. Frontiers in Marine Science 6, 546.
| Stable isotope analysis of dermis and the foraging behavior of whale sharks at Ningaloo Reef, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Matich, P, and Heithaus, MR (2014). Multi-tissue stable isotope analysis and acoustic telemetry reveal seasonal variability in the trophic interactions of juvenile bull sharks in a coastal estuary. Journal of Animal Ecology 83, 199–213.
| Multi-tissue stable isotope analysis and acoustic telemetry reveal seasonal variability in the trophic interactions of juvenile bull sharks in a coastal estuary.Crossref | GoogleScholarGoogle Scholar |
Matich, P, Heithaus, MR, and Layman, CA (2011). Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. Journal of Animal Ecology 80, 294–305.
| Contrasting patterns of individual specialization and trophic coupling in two marine apex predators.Crossref | GoogleScholarGoogle Scholar |
Matley, JK, Heupel, MR, Fisk, AT, Simpfendorfer, CA, and Tobin, AJ (2017). Measuring niche overlap between co-occurring Plectropomus spp. using acoustic telemetry and stable isotopes. Marine and Freshwater Research 68, 1468–1478.
| Measuring niche overlap between co-occurring Plectropomus spp. using acoustic telemetry and stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Matley, JK, Klinard, NV, Larocque, SM, McLean, MF, Brownscombe, JW, Raby, GD, Nguyen, VM, and Barbosa Martins, AP (2022). Making the most of aquatic animal tracking: a review of complementary methods to bolster acoustic telemetry. Reviews in Fish Biology and Fisheries , .
| Making the most of aquatic animal tracking: a review of complementary methods to bolster acoustic telemetry.Crossref | GoogleScholarGoogle Scholar |
McLaughlin, RH, and O’Gower, AK (1971). Life history and underwater studies of a heterodont shark. Ecological Monographs 41, 271–289.
| Life history and underwater studies of a heterodont shark.Crossref | GoogleScholarGoogle Scholar |
Munroe, SEM, Heupel, MR, Fisk, AT, Logan, M, and Simpfendorfer, CA (2015). Regional movement patterns of a small-bodied shark revealed by stable-isotope analysis. Journal of Fish Biology 86, 1567–1586.
| Regional movement patterns of a small-bodied shark revealed by stable-isotope analysis.Crossref | GoogleScholarGoogle Scholar |
Nielsen, J, Christiansen, JS, Grønkjær, P, Bushnell, P, Steffensen, JF, Kiilerich, HO, Præbel, K, and Hedeholm, R (2019). Greenland shark (Somniosus microcephalus) stomach contents and stable isotope values reveal an ontogenetic dietary shift. Frontiers in Marine Science 6, 125.
| Greenland shark (Somniosus microcephalus) stomach contents and stable isotope values reveal an ontogenetic dietary shift.Crossref | GoogleScholarGoogle Scholar |
Papastamatiou, YP, Meyer, CG, Carvalho, F, Dale, JJ, Hutchinson, MR, and Holland, KN (2013). Telemetry and random-walk models reveal complex patterns of partial migration in a large marine predator. Ecology 94, 2595–2606.
| Telemetry and random-walk models reveal complex patterns of partial migration in a large marine predator.Crossref | GoogleScholarGoogle Scholar |
Post, DM, Layman, CA, Arrington, DA, Takimoto, G, Quattrochi, J, and Montana, CG (2007). Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189.
| Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses.Crossref | GoogleScholarGoogle Scholar |
Powter, DM, and Gladstone, W (2008). Habitat preferences of Port Jackson sharks, Heterodontus portusjacksoni, in the coastal waters of eastern Australia. Proceedings of the Linnean Society of New South Wales 129, 151–165.
Powter, DM, and Gladstone, W (2009). Habitat-mediated use of space by juvenile and mating adult Port Jackson sharks, Heterodontus portusjacksoni, in eastern Australia. Pacific Science 63, 1–14.
| Habitat-mediated use of space by juvenile and mating adult Port Jackson sharks, Heterodontus portusjacksoni, in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Powter, DM, Gladstone, W, and Platell, M (2010). The influence of sex and maturity on the diet, mouth morphology and dentition of the Port Jackson shark, Heterodontus portusjacksoni. Marine and Freshwater Research 61, 74–85.
| The influence of sex and maturity on the diet, mouth morphology and dentition of the Port Jackson shark, Heterodontus portusjacksoni.Crossref | GoogleScholarGoogle Scholar |
Ritchie, EG, and Johnson, CN (2009). Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters 12, 982–998.
| Predator interactions, mesopredator release and biodiversity conservation.Crossref | GoogleScholarGoogle Scholar |
Rubenstein, DR, and Hobson, KA (2004). From birds to butterflies: animal movement patterns and stable isotopes. Trends in Ecology & Evolution 19, 256–263.
| From birds to butterflies: animal movement patterns and stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Saino, N, Szép, T, Ambrosini, R, Romano, M, and Møller, AP (2004). Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proceedings of the Royal Society of London – B. Biological Sciences 271, 681–686.
| Ecological conditions during winter affect sexual selection and breeding in a migratory bird.Crossref | GoogleScholarGoogle Scholar |
Sequeira, AMM, Heupel, MR, Lea, M-A, Eguíluz, VM, Duarte, CM, Meekan, MG, Thums, M, Calich, HJ, Carmichael, RH, Costa, DP, Ferreira, LC, Fernandéz-Gracia, J, Harcourt, R, Harrison, A-L, Jonsen, I, McMahon, CR, Sims, DW, Wilson, RP, and Hays, GC (2019). The importance of sample size in marine megafauna tagging studies. Ecological Applications 29, e01947.
| The importance of sample size in marine megafauna tagging studies.Crossref | GoogleScholarGoogle Scholar |
Shaw, AK (2020). Causes and consequences of individual variation in animal movement. Movement Ecology 8, 12.
| Causes and consequences of individual variation in animal movement.Crossref | GoogleScholarGoogle Scholar |
Shipley, ON, Murchie, KJ, Frisk, MG, Brooks, EJ, O’Shea, OR, and Power, M (2017). Low lipid and urea effects and inter-tissue comparisons of stable isotope signatures in three nearshore elasmobranchs. Marine Ecology Progress Series 579, 233–238.
| Low lipid and urea effects and inter-tissue comparisons of stable isotope signatures in three nearshore elasmobranchs.Crossref | GoogleScholarGoogle Scholar |
Shipley, ON, Newton, AL, Frisk, MG, Henkes, GA, LaBelle, JS, Camhi, MD, Hyatt, MW, Walters, H, and Olin, JA (2021). Telemetry-validated nitrogen stable isotope clocks identify ocean-to-estuarine habitat shifts in mobile organisms. Methods in Ecology and Evolution 12, 897–908.
| Telemetry-validated nitrogen stable isotope clocks identify ocean-to-estuarine habitat shifts in mobile organisms.Crossref | GoogleScholarGoogle Scholar |
Simpfendorfer CA, Heupel MR (2012) Assessing habitat use and movement. In ‘Biology of sharks and their relatives’. (Eds JC Carrier, CA Simpfendorfer, MR Heithaus, KE Yopak) pp. 579–601. (CRC Press: Boca Raton, FL, USA)
Sims, D, Nash, J, and Morritt, D (2001). Movements and activity of male and female dogfish in a tidal sea lough: alternative behavioural strategies and apparent sexual segregation. Marine Biology 139, 1165–1175.
| Movements and activity of male and female dogfish in a tidal sea lough: alternative behavioural strategies and apparent sexual segregation.Crossref | GoogleScholarGoogle Scholar |
Sommerville, E, Platell, ME, White, WT, Jones, AA, and Potter, IC (2011). Partitioning of food resources by four abundant, co-occurring elasmobranch species: relationships between diet and both body size and season. Marine and Freshwater Research 62, 54–65.
| Partitioning of food resources by four abundant, co-occurring elasmobranch species: relationships between diet and both body size and season.Crossref | GoogleScholarGoogle Scholar |
Speed, CW, Meekan, MG, Field, IC, McMahon, CR, Abrantes, K, and Bradshaw, CJA (2012). Trophic ecology of reef sharks determined using stable isotopes and telemetry. Coral Reefs 31, 357–367.
| Trophic ecology of reef sharks determined using stable isotopes and telemetry.Crossref | GoogleScholarGoogle Scholar |
Steenweg, RJ, Crossin, GT, Kyser, TK, Merkel, FR, Gilchrist, HG, Hennin, HL, Robertson, GJ, Provencher, JF, Mills Flemming, J, and Love, OP (2017). Stable isotopes can be used to infer the overwintering locations of prebreeding marine birds in the Canadian Arctic. Ecology and Evolution 7, 8742–8752.
| Stable isotopes can be used to infer the overwintering locations of prebreeding marine birds in the Canadian Arctic.Crossref | GoogleScholarGoogle Scholar |
Swadling, DS, Knott, NA, Rees, MJ, Pederson, H, Adams, KR, Taylor, MD, and Davis, AR (2020). Seagrass canopies and the performance of acoustic telemetry: implications for the interpretation of fish movements. Animal Biotelemetry 8, 8.
| Seagrass canopies and the performance of acoustic telemetry: implications for the interpretation of fish movements.Crossref | GoogleScholarGoogle Scholar |
Vaudo, JJ, Wetherbee, BM, Harvey, G, Nemeth, RS, Aming, C, Burnie, N, Howey-Jordan, LA, and Shivji, MS (2014). Intraspecific variation in vertical habitat use by tiger sharks (Galeocerdo cuvier) in the western North Atlantic. Ecology and Evolution 4, 1768–1786.
| Intraspecific variation in vertical habitat use by tiger sharks (Galeocerdo cuvier) in the western North Atlantic.Crossref | GoogleScholarGoogle Scholar |
Votier, SC, Bearhop, S, Witt, MJ, Inger, R, Thompson, D, and Newton, J (2010). Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. Journal of Applied Ecology 47, 487–497.
| Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems.Crossref | GoogleScholarGoogle Scholar |
Wearmouth, VJ, and Sims, DW (2008). Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Advances in Marine Biology 54, 107–170.
| Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Wickham H (2016) ‘ggplot2: Elegant Graphics for Data Analysis.’ (Springer-Verlag: New York, NY, USA)
Williams, JJ, Papastamatiou, YP, Caselle, JE, Bradley, D, and Jacoby, DMP (2018). Mobile marine predators: an understudied source of nutrients to coral reefs in an unfished atoll. Proceedings of the Royal Society of London – B. Biological Sciences 285, 20172456.
| Mobile marine predators: an understudied source of nutrients to coral reefs in an unfished atoll.Crossref | GoogleScholarGoogle Scholar |


